Hemodynamic Profiles and Their Prognostic Relevance in Cardiac Amyloidosis
Abstract
1. Introduction
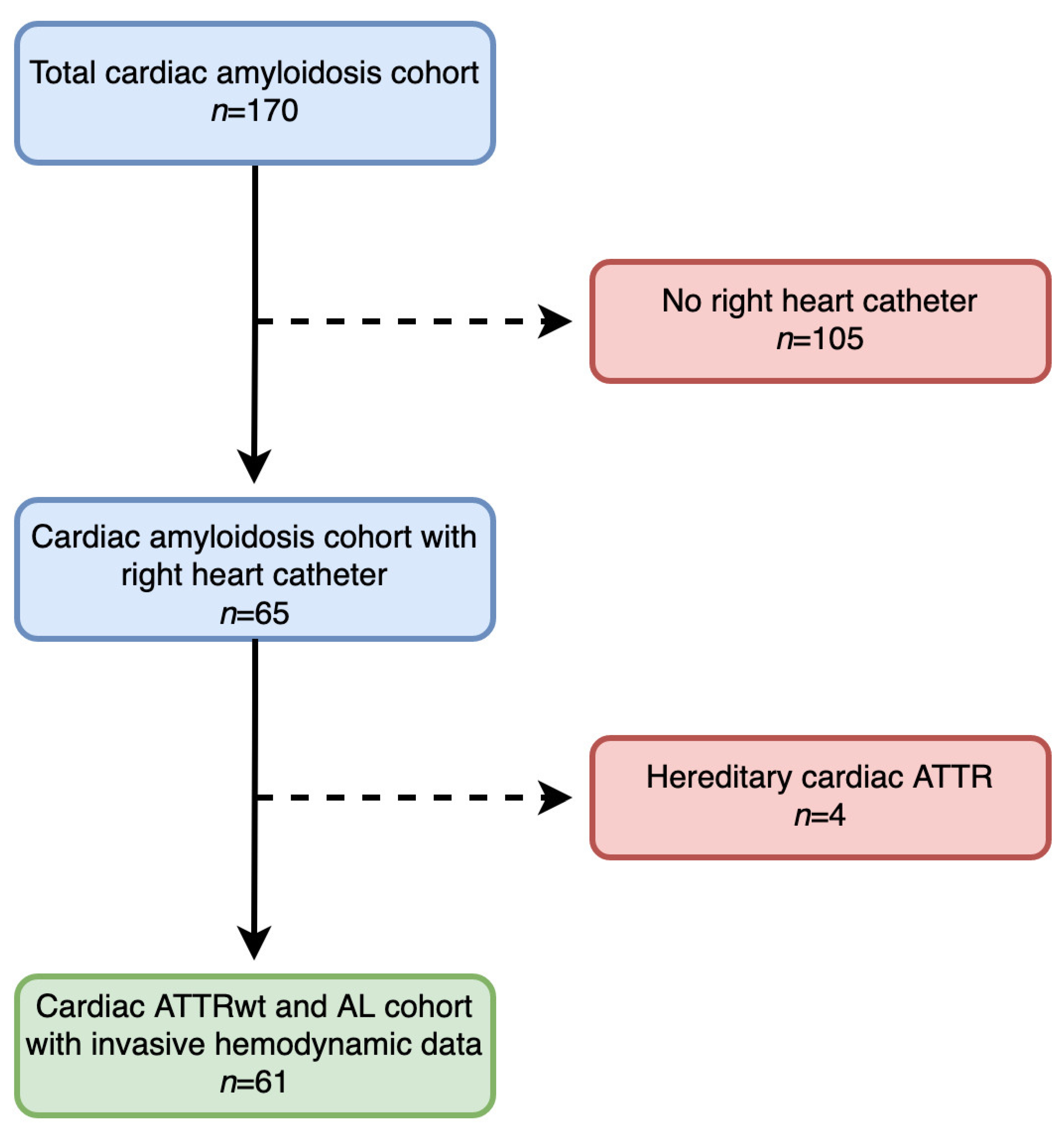
2. Materials and Methods
2.1. Setting and Study Design
2.2. Clinical Definitions
2.2.1. Diagnosis of Cardiac Transthyretin Amyloidosis
2.2.2. Diagnosis of Cardiac Light-Chain Amyloidosis
2.2.3. Obtaining and Histological Analysis of Biopsy Samples
2.3. Cardiac Magnetic Resonance Imaging
2.3.1. Transthorathic Echocardiography
2.3.2. Invasive Hemodynamic Assessment
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Clinical Presentation of the Overall Cohort
3.2. Differences Between Cardiac Wild-Type Transthyretin and Light-Chain Amyloidosis Patients
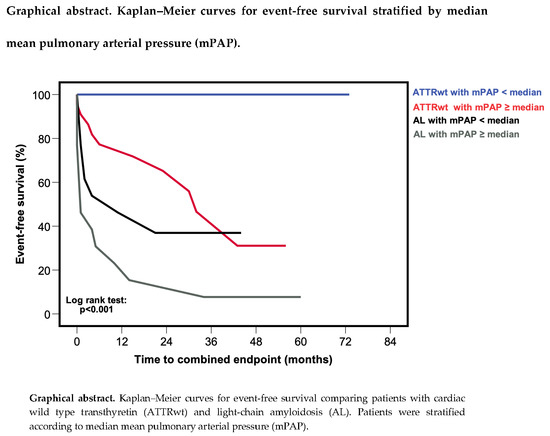
3.3. Patient Outcomes
3.4. Predictors of Outcome in Cardiac Wild-Type Transthyretin Amyloidosis Patients
3.5. Predictors of Outcome in Cardiac Light-Chain Amyloidosis Patients
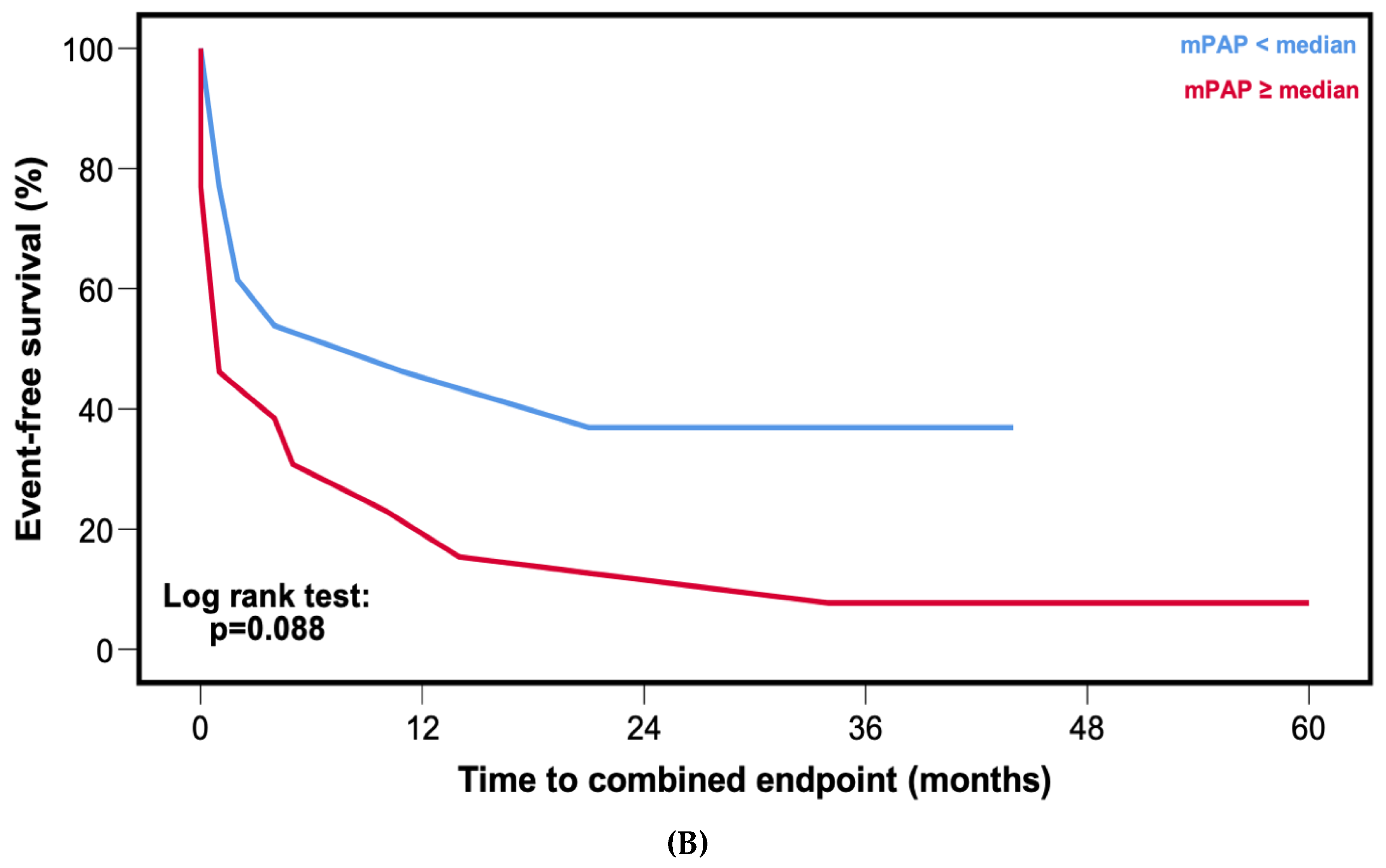
3.6. Comparison of Outcomes in Cardiac Wild-Type Transthyretin and Light-Chain Amyloidosis
3.7. Association of Pulmonary Arterial Pressures with Valvular Heart Disease and Diuretics
4. Discussion
4.1. Hemodynamic Profiles in Cardiac Amyloidosis
4.2. Pulmonary Arterial Pressures as a Potential Therapeutic Target in Cardiac Amyloidosis
4.3. Association of Diuretics with Pulmonary Arterial Pressures and Outcome
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pieske, B.; Tschöpe, C.; De Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Carli, M.F.D.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis-Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Failure 2019, 12, e005407. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; Hajj, S.E.; Kueffer, F.J.; Baicu, C.F.; Abraham, W.T.; Bourge, R.C.; Stevenson, L.W. Intracardiac Pressures Measured Using an Implantable Hemodynamic Monitor. Circ. Heart Fail. 2017, 10, e003594. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Russo, C.; Green, P.; Maurer, M. The prognostic significance of central hemodynamics in patients with cardiac amyloidosis. Amyloid 2013, 20, 199–203. [Google Scholar] [CrossRef]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic Cardiac Amyloidoses. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. A combined elastic, fibrin and collagen stain. Stain Technol. 1987, 62, 365–368. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC: Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef]
- Duca, F.; Kammerlander, A.A.; Zotter-Tufaro, C.; Aschauer, S.; Schwaiger, M.L.; Marzluf, B.A.; Bonderman, D.; Mascherbauer, J. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Kellman, P.; Wilson, J.R.; Xue, H.; Ugander, M.; Arai, A.E. Extracellular volume fraction mapping in the myocardium, part 1: Evaluation of an automated method. J. Cardiovasc. Magn. Reson. 2012, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Picard, M.H.; Adams, D.; Bierig, S.M.; Dent, J.M.; Douglas, P.S.; Gillam, L.D.; Keller, A.M.; Malenka, D.J.; Masoudi, F.A.; McCulloch, M.; et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J. Am. Soc. Echocardiogr. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Aschauer, S.; Kammerlander, A.A.; Zotter-Tufaro, C.; Ristl, R.; Pfaffenberger, S.; Bachmann, A.; Duca, F.; Marzluf, B.A.; Bonderman, D.; Mascherbauer, J. The right heart in heart failure with preserved ejection fraction: Insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur. J. Heart Fail. 2016, 18, 71–80. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef]
- Kristen, A.V.; Scherer, K.; Buss, S.; Aus dem Siepen, F.; Haufe, S.; Bauer, R.; Hinderhofer, K.; Giannitsis, E.; Hardt, S.; Haberkorn, U.; et al. Noninvasive risk stratification of patients with transthyretin amyloidosis. JACC. Cardiovasc. Imaging 2014, 7, 502–510. [Google Scholar] [CrossRef]
- Goliasch, G.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Koell, B.; Kammerlander, A.A.; Ristl, R.; Lang, I.M.; Maurer, G.; Mascherbauer, J.; et al. Outcome in Heart Failure with Preserved Ejection Fraction: The Role of Myocardial Structure and Right Ventricular Performance. PLoS ONE 2015, 10, e0134479. [Google Scholar] [CrossRef]
- Miller, W.L.; Grill, D.E.; Borlaug, B.A. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Heart Fail 2013, 1, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Duca, F.; Stelzer, P.D.; Nitsche, C.; Rettl, R.; Aschauer, S.; Kammerlander, A.A.; Binder, T.; Agis, H.; Kain, R.; et al. Mechanisms of heart failure in transthyretin vs. light chain amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.P.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.-V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef]
- Duca, F.; Aschauer, S.; Zotter-Tufaro, C.; Binder, C.; Kammerlander, A.; Börries, B.; Agis, H.; Kain, R.; Hengstenberg, C.; Mascherbauer, J.; et al. EXPRESS: Riociguat for the treatment of transthyretin cardiac amyloidosis - Data from a named patient use program in Austria. Pulm. Circ. 2019. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Escher, F.; Senoner, M.; Doerler, J.; Zaruba, M.M.; Messner, M.; Mussner-Seeber, C.; Ebert, M.; Ensinger, C.; Mair, A.; Kroiss, A.; et al. When and how do patients with cardiac amyloidosis die? Clin. Res. Cardiol. 2020, 109, 78–88. [Google Scholar] [CrossRef]
- Barrett, C.D.; Dobos, K.; Liedtke, M.; Tuzovic, M.; Haddad, F.; Kobayashi, Y.; Lafayette, R.; Fowler, M.B.; Arai, S.; Schrier, S.; et al. A Changing Landscape of Mortality for Systemic Light Chain Amyloidosis. JACC Heart Fail 2019, 7, 958–966. [Google Scholar] [CrossRef]
- González-López, E.; Gagliardi, C.; Dominguez, F.; Quarta, C.C.; De Haro-del Moral, F.J.; Milandri, A.; Salas, C.; Cinelli, M.; Cobo-Marcos, M.; Lorenzini, M.; et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: Disproving myths. Eur. Heart J. 2017, 38, 1895–1904. [Google Scholar] [CrossRef]

| Variable | All Patients (n = 61) | ATTRwt (n = 35) | AL (n = 26) | p Value |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years (IQR) | 74.0 (66.5–78.0) | 75.0 (73.0–82.0) | 68.0 (54.8–74.3) | <0.001 |
| Sex, male gender, n (%) | 38.0 (62.3) | 28.0 (80.0) | 10.0 (38.5) | 0.001 |
| NYHA functional class ≥ III, n (%) | 30.0 (49.2) | 14 (40.0) | 16.0 (61.5) | 0.027 |
| Systolic blood pressure, mmHg (IQR) | 118 (112–139) | 122 (114–135) | 117 (103–142) | 0.431 |
| Diastolic blood pressure, mmHg (IQR) | 68.0 (61.0–78.0) | 67.0 (61.0–74.5) | 70.0 (63.8–78.3) | 0.521 |
| NT-proBNP, pg/mL (IQR) | 3552 (1501–7357) | 2368 (1331–5834) | 4900 (2045–10571) | 0.020 |
| Troponin t, ng/mL (IQR) | 0.07 (0.03–0.12) | 0.07 (0.03–0.10) | 0.07 (0.03–0.21) | 0.611 |
| eGFR, mL/min/1.73 m2 (IQR) | 58.4 (43.8–74.8) | 56.4 (43.5–79.6) | 61.2 (46.2–71.3) | 0.838 |
| Combined endpoint, n (%) | 30.0 (49.2) | 10.0 (28.6) | 20.0 (76.9) | <0.001 |
| Medication | ||||
| Beta Blocker, n (%) | 34.0 (55.7) | 19.0 (54.3) | 15.0 (57.7) | 0.889 |
| ACE inhibitor, n (%) | 14.0 (23.0) | 8.0 (22.9) | 6.0 (23.1) | 0.967 |
| Angiotensin receptor blocker, n (%) | 17.0 (27.9) | 11.0 (31.4) | 6.0 (23.1) | 0.429 |
| Epigallocatechin gallate, n (%) | 20.0 (32.8) | 20.0 (57.1) | 0.0 (0.0) | n.a |
| Tafamidis, n (%) | 2.0 (3.3) | 2.0 (5.7) | 0.0 (0.0) | n.a |
| Daratumumab, n (%) | 5.0 (8.2) | 0.0 (0.0) | 5.0 (19.2) | n.a |
| Lenalidomide, n (%) | 1.0 (1.6) | 0.0 (0.0) | 1.0 (3.8) | n.a |
| Thalidomide, n (%) | 2.0 (3.3) | 0.0 (0.0) | 2.0 (7.7) | n.a |
| Bortezomib, n (%) | 14.0 (23.0) | 0.0 (0.0) | 14.0 (53.8) | n.a |
| Cyclophosphamide, (%) | 8.0 (13.1) | 0.0 (0.0) | 8.0 (30.8) | n.a |
| Dexamethasone, n (%) | 14.0 (23.0) | 0.0 (0.0) | 14.0 (53.8) | n.a |
| Rituximab, n (%) | 1.0 (1.6) | 0.0 (0.0) | 1.0 (3.8) | n.a |
| Bendamustine, n (%) | 1.0 (1.6) | 0.0 (0.0) | 1.0 (3.8) | n.a |
| No diuretic agents, n (%) | 12.0 (19.7) | 8.0 (22.9) | 4.0 (15.4) | 0.434 |
| One diuretic agent, n (%) | 13.0 (21.3) | 10.0 (28.6) | 3.0 (11.5) | 0.096 |
| Two diuretic agents, n (%) | 28.0 (45.9) | 13.0 (37.1) | 15.0 (57.7) | 0.134 |
| Three diuretic agents, n (%) | 7.0 (11.5) | 3 (8.6) | 4.0 (15.4) | 0.433 |
| Invasive hemodynamic parameters | ||||
| Mean pulmonary arterial pressure, mmHg (IQR) | 30.0 (25.5–36.5) | 30.0 (26.0–34.0) | 32.0 (25.0–43.0) | 0.296 |
| Right atrial pressure, mmHg (IQR) | 11.0 (7.3–16.8) | 11.0 (7.8–16.0) | 11.5 (7.0–18.0) | 0.654 |
| Pulmonary artery wedge pressure, mmHg (IQR) | 20.0 (16.5–24.0) | 19.0 (16.0–22.0) | 20.5 (16.8–29.3) | 0.201 |
| Cardiac index, L/min/m2 (IQR) | 2.4 (1.9–2.8) | 2.4 (2.0–2.7) | 2.4 (1.8–3.1) | 0.941 |
| Stroke volume index, mL/m2 (IQR) | 30.7 (25.2–41.6) | 31.4 (24.4–42.3) | 30.3 (25.9–40.2) | 0.835 |
| Pulmonary vascular resistance, dyn·s·cm−5 (IQR) | 180 (129–266) | 181 (128–300) | 166 (126–264) | 0.726 |
| Diastolic pressure gradient, mmHg (IQR) | 1.0 (−1.0–3.8) | 2.0 (−1.0–4.3) | 0.0 (−1.3–3.0) | 0.217 |
| Cardiac magnetic resonance imaging parameters | ||||
| MOLLI-ECV, % (IQR) | 47.2 (41.0–55.9) | 48.0 (41.1–55.6) | 45.8 (39.6–65.4) | 0.860 |
| Left atrial area, cm2 (IQR) | 31.5 (26.0–37.3) | 32.5 (27.8–38.8) | 30.0 (24.0–31.0) | 0.185 |
| Right atrial area, cm2 (IQR) | 29.0 (24.0–38.0) | 33.0 (27.8–39.5) | 25.5 (24.0–31.0) | 0.010 |
| Left ventricular ejection fraction, % (IQR) | 57.5 (50.0–66.3) | 55.6 (49.0–60.5) | 62.5 (52.3–67.0) | 0.077 |
| Left ventricular end-diastolic volume index, ml/m2 (IQR) | 66.1 (56.5–85.0) | 81.6 (64.9–91.5) | 60.1 (45.9–72.3) | 0.001 |
| Interventricular septum, mm (IQR) | 19.0 (15.5–22.0) | 20.0 (16.0–23.0) | 17.0 (13.0–20.0) | 0.040 |
| Right ventricular ejection fraction, % (IQR) | 48.0 (41.0–61.5) | 48.0 (38.0–60.5) | 52.0 (42.0–62.0) | 0.629 |
| Right ventricular end-diastolic volume index, mL/m2 (IQR) | 78.5 (64.0–96.7) | 82.8 (66.7–100) | 72.3 (62.1–94.0) | 0.133 |
| Transthorathic echocardiography parameters | ||||
| Significant aortic valve stenosis, n (%) | 1.0 (1.6) | 1.0 (2.9) | 0.0 (0.0) | 0.378 |
| Significant aortic valve regurgitation, n (%) | 1.0 (1.6) | 1.0 (2.9) | 0.0 (0.0) | 0.378 |
| Significant mitral valve stenosis, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | n.a |
| Significant mitral valve regurgitation, n (%) | 20.0 (32.8) | 12.0 (34.3) | 8.0 (30.8) | 0.713 |
| Variable | Crude Hazard Ratio | 95% Confidence Interval | p Value | Adjusted Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|
| Univariable Regression | Multivariable Regression | |||||
| Clinical parameters | ||||||
| Age, years | 1.143 | 1.021–1.279 | 0.020 | 1.255 | 1.055–1.493 | 0.010 |
| Sex, male gender | 2.252 | 0.284–17.843 | 0.442 | 0.387 | 0.025–5.987 | 0.497 |
| NYHA functional class ≥ III | 1.441 | 0.379–5.485 | 0.592 | 2.927 | 0.662–12.931 | 0.157 |
| Systolic blood pressure, mmHg | 0.990 | 0.955–1.027 | 0.602 | 0.985 | 0.954–1.017 | 0.353 |
| Diastolic blood pressure, mmHg | 0.966 | 0.906–1.029 | 0.283 | 0.897 | 0.828–0.971 | 0.008 |
| NT-proBNP, pg/mL* | 1.383 | 0.769–2.487 | 0.279 | 1.181 | 0.641–2.176 | 0.593 |
| Troponin t, ng/mL † | 2.499 | 1.231–5.072 | 0.011 | 2.466 | 1.187–5.124 | 0.016 |
| eGFR, mL/min/1.73 m2 | 0.989 | 0.962–1.017 | 0.442 | 0.991 | 0.957–1.027 | 0.630 |
| Concomitant medication | ||||||
| Beta Blocker | 0.716 | 0.187–2.733 | 0.625 | 0.607 | 0.110–3.340 | 0.566 |
| ACE inhibitor | 0.868 | 0.173–4.347 | 0.863 | 1.024 | 0.180–5.816 | 0.979 |
| Angiotensin receptor blocker | 1.044 | 0.260–4.184 | 0.952 | 0.1756 | 0.391–7.893 | 0.463 |
| Number of diuretic agents | 1.276 | 0.619–2.630 | 0.509 | 5.932 | 1.217–28.906 | 0.028 |
| Invasive hemodynamic parameters | ||||||
| Mean pulmonary arterial pressure, mmHg | 1.177 | 1.049–1.321 | 0.005 | 1.130 | 1.006–1.269 | 0.040 |
| Right atrial pressure, mmHg | 1.124 | 0.992–1.273 | 0.067 | 1.057 | 0.926–1.205 | 0.411 |
| Pulmonary artery wedge pressure, mmHg | 1.116 | 0.995–1.253 | 0.061 | 1.045 | 0.924–1.182 | 0.484 |
| Cardiac index, L/min/m2 | 0.920 | 0.338–2.502 | 0.870 | 0.656 | 0.248–1.739 | 0.397 |
| Stroke volume index, mL/m2 | 0.974 | 0.920–1.031 | 0.369 | 0.950 | 0.890–1.014 | 0.124 |
| Pulmonary vascular resistance, dyn·s·cm−5 | 1.005 | 0.999–1.010 | 0.086 | 1.010 | 1.000–1.020 | 0.046 |
| Diastolic pressure gradient, mmHg | 0.948 | 0.795–1.130 | 0.552 | 1.036 | 0.851–1.260 | 0.727 |
| Cardiac magnetic resonance imaging parameters | ||||||
| MOLLI-ECV, % | 1.064 | 1.001–1.131 | 0.045 | 1.044 | 0.980–1.111 | 0.181 |
| Left atrial area, cm2 | 1.120 | 0.993–1.264 | 0.065 | 1.081 | 0.940–1.241 | 0.274 |
| Right atrial area, cm2 | 1.012 | 0.927–1.104 | 0.792 | 1.010 | 0.901–1.132 | 0.870 |
| Left ventricular ejection fraction, % | 0.981 | 0.921–1.045 | 0.554 | 1.031 | 0.939–1.133 | 0.524 |
| Left ventricular end-diastolic volume index, mL/m2 (IQR) | 1.016 | 0.974–1.060 | 0.468 | 0.984 | 0.932–1.040 | 0.569 |
| Interventricular septum, mm | 1.058 | 0.884–1.267 | 0.536 | 1.005 | 0.851–1.188 | 0.950 |
| Right ventricular ejection fraction, % | 0.973 | 0.924–1.025 | 0.297 | 0.990 | 0.931–1.053 | 0.757 |
| Right ventricular end-diastolic volume index, mL/m2 (IQR) | 1.009 | 0.993–1.026 | 0.260 | 1.022 | 0.975–1.071 | 0.370 |
| Transthorathic echocardiography parameters | ||||||
| Significant aortic valve stenosis | 1.664 | 0.191–14.515 | 0.645 | 5.781 | 0.417–80.052 | 0.191 |
| Significant aortic valve regurgitation | 1.664 | 0.191–14.515 | 0.645 | 5.781 | 0.417–80.052 | 0.191 |
| Significant mitral valve regurgitation | 1.118 | 0.314–3.982 | 0.864 | 0.230 | 0.044–1.197 | 0.081 |
| Variable | Crude Hazard Ratio | 95% Confidence Interval | p Value | Adjusted Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|
| Univariable Regression | Multivariable Regression | |||||
| Clinical parameters | ||||||
| Age, years | 0.983 | 0.942–1.026 | 0.432 | 0.972 | 0.929–1.018 | 0.233 |
| Sex, male gender | 0.632 | 0.249–1.603 | 0.334 | 0.491 | 0.173–1.398 | 0.183 |
| NYHA functional class ≥ III | 14.201 | 1.833–110.010 | 0.011 | 7.763 | 0.729–82.669 | 0.090 |
| Systolic blood pressure, mmHg | 0.981 | 0.962–0.999 | 0.042 | 0.992 | 0.971–1.013 | 0.441 |
| Diastolic blood pressure, mmHg | 0.988 | 0.949–1.029 | 0.568 | 0.983 | 0.949–1.018 | 0.328 |
| NT-proBNP, pg/mL * | 3.131 | 1.619–6.778 | 0.001 | 2.856 | 1.277–6.388 | 0.011 |
| Troponin t, ng/mL † | 1.894 | 1.171–2.778 | 0.007 | 1.180 | 0.740–1.880 | 0.487 |
| eGFR, mL/min/1.73 m2 | 0.994 | 0.974–1.015 | 0.600 | 0.978 | 0.941–1.016 | 0.258 |
| Concomitant medication | ||||||
| Beta Blocker | 1.106 | 0.444–2.756 | 0.828 | 1.132 | 0.408–3.139 | 0.811 |
| ACE inhibitor | 1.540 | 0.554–4.279 | 0.408 | 3.039 | 0.826–11.178 | 0.094 |
| Angiotensin receptor blocker | 0.660 | 0.220–1.977 | 0.457 | 1.125 | 0.351–3.608 | 0.843 |
| Number of diuretic agents | 1.185 | 0.759–1.850 | 0.455 | 1.456 | 0.777–2.728 | 0.241 |
| Invasive hemodynamic parameters | ||||||
| Mean pulmonary arterial pressure, mmHg | 1.027 | 0.991–1.063 | 0.146 | 0.999 | 0.962–1.038 | 0.970 |
| Right atrial pressure, mmHg | 1.027 | 0.971–1.087 | 0.348 | 1.020 | 0.956–1.089 | 0.553 |
| Pulmonary artery wedge pressure, mmHg | 1.060 | 0.993–1.131 | 0.081 | 1.039 | 0.966–1.118 | 0.302 |
| Cardiac index, L/min/m2 | 0.608 | 0.329–1.125 | 0.113 | 0.859 | 0.408–1.809 | 0.690 |
| Stroke volume index, mL/m2 | 0.958 | 0.920–0.997 | 0.034 | 0.987 | 0.944–1.032 | 0.579 |
| Pulmonary vascular resistance, dyn·s·cm−5 | 1.001 | 0.999–1.003 | 0.263 | 1.000 | 0.997–1.002 | 0.645 |
| Diastolic pressure gradient, mmHg | 1.042 | 0.965–1.126 | 0.292 | 0.978 | 0.891–1.073 | 0.635 |
| Cardiac magnetic resonance imaging parameters | ||||||
| MOLLI-ECV, % | 1.042 | 1.011–1.073 | 0.007 | 1.012 | 0.970–1.055 | 0.590 |
| Left atrial area, cm2 | 0.956 | 0.902–1.012 | 0.123 | 1.000 | 0.894–1.118 | 0.993 |
| Right atrial area, cm2 | 0.943 | 0.876–1.016 | 0.122 | 1.004 | 0.919–1.097 | 0.922 |
| Left ventricular ejection fraction, % | 1.013 | 0.975–1.054 | 0.504 | 1.018 | 0.974–1.065 | 0.425 |
| Left ventricular end-diastolic volume index, mL/m2 (IQR) | 0.977 | 0.955–0.999 | 0.041 | 0.975 | 0.946–1.004 | 0.090 |
| Interventricular septum, mm | 1.209 | 1.057–1.384 | 0.006 | 1.099 | 0.948–1.273 | 0.211 |
| Right ventricular ejection fraction, % | 1.003 | 0.964–1.044 | 0.879 | 1.027 | 0.978–1.078 | 0.287 |
| Right ventricular end-diastolic volume index, mL/m2 (IQR) | 0.980 | 0.955–1.006 | 0.133 | 0.989 | 0.962–1.016 | 0.428 |
| Transthorathic echocardiography parameters | ||||||
| Significant mitral valve regurgitation | 1.477 | 0.572–3.812 | 0.420 | 1.117 | 0.374–3.332 | 0.843 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duca, F.; Snidat, A.; Binder, C.; Rettl, R.; Dachs, T.-M.; Seirer, B.; Camuz-Ligios, L.; Dusik, F.; Capelle, C.D.J.; Hong, Q.; et al. Hemodynamic Profiles and Their Prognostic Relevance in Cardiac Amyloidosis. J. Clin. Med. 2020, 9, 1093. https://doi.org/10.3390/jcm9041093
Duca F, Snidat A, Binder C, Rettl R, Dachs T-M, Seirer B, Camuz-Ligios L, Dusik F, Capelle CDJ, Hong Q, et al. Hemodynamic Profiles and Their Prognostic Relevance in Cardiac Amyloidosis. Journal of Clinical Medicine. 2020; 9(4):1093. https://doi.org/10.3390/jcm9041093
Chicago/Turabian StyleDuca, Franz, Amir Snidat, Christina Binder, René Rettl, Theresa-Marie Dachs, Benjamin Seirer, Luciana Camuz-Ligios, Fabian Dusik, Christophe Denis Josef Capelle, Qin Hong, and et al. 2020. "Hemodynamic Profiles and Their Prognostic Relevance in Cardiac Amyloidosis" Journal of Clinical Medicine 9, no. 4: 1093. https://doi.org/10.3390/jcm9041093
APA StyleDuca, F., Snidat, A., Binder, C., Rettl, R., Dachs, T.-M., Seirer, B., Camuz-Ligios, L., Dusik, F., Capelle, C. D. J., Hong, Q., Agis, H., Kain, R., Mascherbauer, J., Hengstenberg, C., Badr Eslam, R., & Bonderman, D. (2020). Hemodynamic Profiles and Their Prognostic Relevance in Cardiac Amyloidosis. Journal of Clinical Medicine, 9(4), 1093. https://doi.org/10.3390/jcm9041093