Effects of Contemporary Irrigant Activation Schemes and Subsequent Placement of an Interim Dressing on Bacterial Presence and Activity in Root Canals Associated with Asymptomatic Apical Periodontitis
Abstract
1. Introduction
2. Materials and Methods
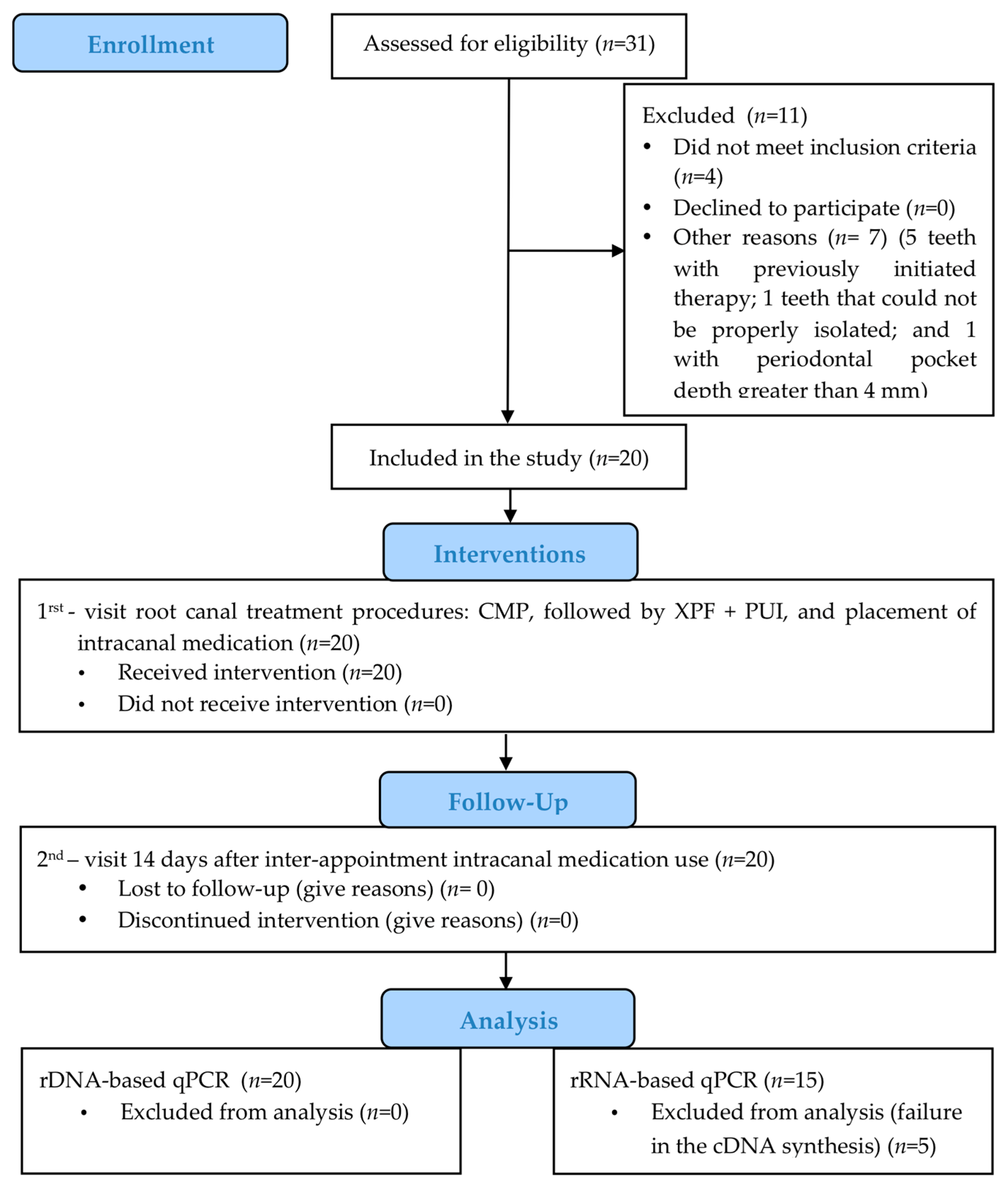
2.1. Patient Selection
2.2. Pilot Study
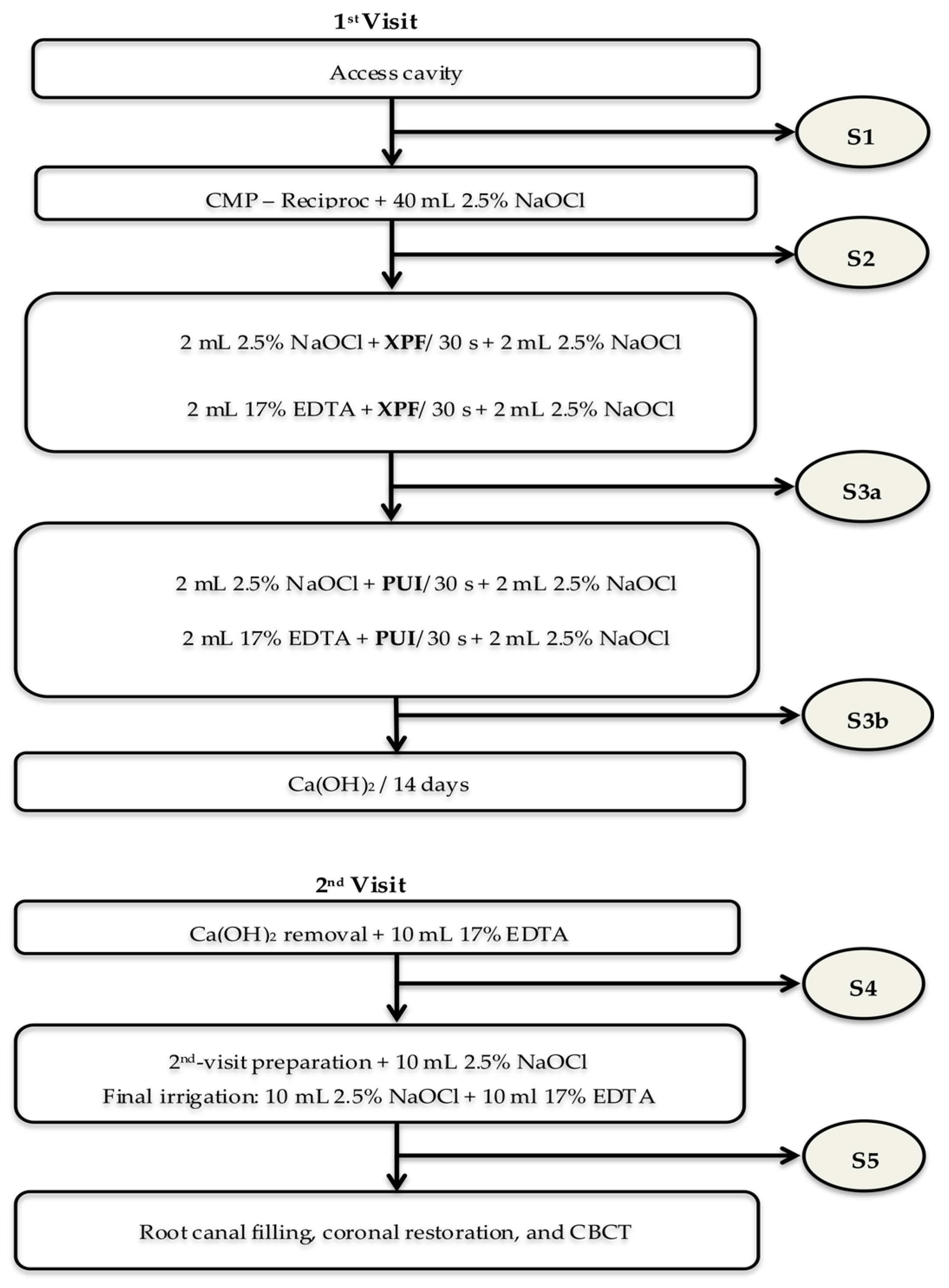
2.3. Clinical Study Design
2.4. Interventions and Microbiologic Samples
2.5. Nucleic Acids Extraction and cDNA Synthesis
2.6. qPCR Assays
2.7. Data Analyses
3. Results
3.1. Bacterial Counts
3.2. Bacterial Activity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Candeiro, G.T.; Teixeira, S.R.; Shin, R.C.; Prado, L.C.; Gavini, G.; Mayer, M.P.A. RNA-based Assay Demonstrated Enterococcus faecalis Metabolic Activity after Chemomechanical Procedures. J. Endod. 2015, 41, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Martellacci, L.; Quaranta, G.; Patini, R.; Isola, G.; Gallenzi, P.; Masucci, L. A Literature Review of Metagenomics and Culturomics of the Peri-implant Microbiome: Current Evidence and Future Perspectives. Materials 2019, 12, 3010. [Google Scholar] [CrossRef]
- Brundin, M.; Figdor, D.; Sundqvist, G.; Sjögren, U. DNA binding to hydroxyapatite: A potential mechanism for preservation of microbial DNA. J. Endod. 2013, 39, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.P.; McCuthan, T.F. Ribosomal RNA: Nature’s own polymerase-amplified target for diagnosis. Parasitol. Today 1990, 6, 56–59. [Google Scholar] [CrossRef]
- Matsuda, K.; Tsuji, H.; Asahara, T.; Kado, Y.; Nomoto, K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 2007, 73, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, T.; Ryu, H.; Elk, M.; Hokajärvi, A.-M.; Siponen, S.; Vepsäläinen, A.; Räsänen, P.; Domingo, J.W.S. Detection of fecal bacteria and source tracking identifiers in environmental waters using rRNA-based RT-qPCR and rDNA-based qPCR assays. Environ. Sci. Technol. 2013, 47, 13611–13620. [Google Scholar] [CrossRef]
- Campbell, B.J.; Yu, L.; Heidelberg, J.F.; Kirchman, D.L. Activity of abundant and rare bacteria in a coastal ocean. Proc. Natl. Acad. Sci. USA 2011, 108, 12776–12781. [Google Scholar] [CrossRef]
- Campbell, B.J.; Kirchman, D.L. Bacterial diversity, community structure and potential growth rates along an estuarine salinity gradient. ISME J. 2013, 7, 210–220. [Google Scholar] [CrossRef]
- Vianna, M.E.; Horz, H.P.; Gomes, B.P.; Conrads, G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int. Endod. J. 2006, 39, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Provenzano, J.C.; Rôças, I.N.; Siqueira, J.F., Jr. Clinical antibacterial effectiveness of root canal preparation with reciprocating single-instrument or continuously rotating multi-instrument systems. J. Endod. 2016, 42, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Provenzano, J.C.; Neves, M.A.; Siqueira, J.F., Jr. Disinfecting effects of rotary instrumentation with either 2.5% sodium hypochlorite or 2% chlorhexidine as the main irrigant: A randomized clinical study. J. Endod. 2016, 42, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Optimising single-visit disinfection with supplementary approaches: A quest for predictability. Aust. Endod. J. 2011, 37, 92–98. [Google Scholar] [CrossRef]
- van der Sluis, L.W.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Eneide, C.; Castagnola, R.; Martini, C.; Grande, N.M.; Bugli, F.; Patini, R.; Cordaro, M.; Sanguinetti, M.; Olivi, G.; Isola, G.; et al. Antibiofilm Activity of Three Different Irrigation Techniques: An in Vitro Study. Antibiotics 2019, 8, 112. [Google Scholar] [CrossRef]
- Debelian, G.; Trope, M. Cleaning the third dimension. Endod. Pract. 2015, 8, 18–21. [Google Scholar]
- Bao, P.; Shen, Y.; Lin, J.; Haapasalo, M. In vitro efficacy of XP-endo Finisher with 2 different protocols on biofilm removal from apical root canals. J. Endod. 2017, 43, 321–325. [Google Scholar] [CrossRef]
- Alves, F.R.; Andrade-Junior, C.V.; Marceliano-Alves, M.F.V.; Pérez, A.R.; Rôças, I.N.; Versiani, M.A.; Sousa-Neto, M.D.; Provenzano, J.C.; Siqueira, J.F. Adjunctive Steps for Disinfection of the Mandibular Molar Root Canal System: A Correlative Bacteriologic, Micro–Computed Tomography, and Cryopulverization Approach. J. Endod. 2016, 42, 1667–1672. [Google Scholar] [CrossRef]
- Bergenholtz, G.; Spangberg, L. Controversies in endodontics. Crit. Rev. Oral Biol. Med. 2004, 15, 99–114. [Google Scholar] [CrossRef]
- Paiva, S.S.M.; Siqueira, J.F., Jr.; Rôças, I.N.; Carmo, F.L.; Leite, D.C.A.; Ferreira, D.D.C.; Rachid, C.T.C.D.C.; Rosado, A.S. Clinical antimicrobial efficacy of NiTi rotary instrumentation with NaOCl irrigation, final rinse with chlorhexidine and interappointment medication: A molecular study. Int. Endod. J. 2012, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, V.C.; Pinheiro, E.T.; Prado, L.C.; Silveira, A.C.; Carvalho, A.P.L.; Mayer, M.P.A.; Gavini, G. Effect of ultrasonic activation on the reduction of bacteria and endotoxins in root canals: A randomized clinical trial. Int. Endod. J. 2017, 51, e12–e22. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Siqueira, J.F., Jr.; Rôças, I.N.; Benno, Y. Bacterial reduction and persistence after endodontic treatment procedures. Oral Microbiol. Immunol. 2007, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Manso, M.C.; Loureiro, S.; Silva, R.; Madeira, I.G.C.; Pina, C.; Cabeda, J.M. Effectiveness of two intracanal dressings in adult Portuguese patients: A qPCR and anaerobic culture assessment. Int. Endod. J. 2013, 47, 32–40. [Google Scholar] [CrossRef]
- Zandi, H.; Rodrigues, R.C.; Kristoffersen, A.K.; Enersen, M.; Mdala, I.; Ørstavik, D.; Rôças, I.N.; Siqueira, J.F. Antibacterial Effectiveness of 2 Root Canal Irrigants in Root-filled Teeth with Infection: A Randomized Clinical Trial. J. Endod. 2016, 42, 1307–1313. [Google Scholar] [CrossRef]
- Shelburne, C.E.; Prabhu, A.; Gleason, R.M.; Mullally, B.H.; Coulter, W.A. Quantitation of Bacteroides forsythus in subgingival plaque comparison of immunoassay and quantitative polymerase chain reaction. J. Microbiol. Methods 2000, 39, 97–107. [Google Scholar] [CrossRef]
- Paiva, S.S.; Siqueira, J.F., Jr.; Rôças, I.N.; Carmo, F.L.; Leite, D.C.; Ferreira, D.C.; Rachid, C.T.; Rosado, A.S. Molecular microbiological evaluation of passive ultrasonic activation as a supplementary disinfecting step: A clinical study. J. Endod. 2013, 39, 190–194. [Google Scholar] [CrossRef]
- Gazzaneo, I.; Vieira, G.C.; Pérez, A.R.; Marceliano-Alves, M.F.V.; Gonçalves, L.; Mdala, I.; Siqueira, J.F.; Rôças, I.N. Root Canal Disinfection by Single- and Multiple-instrument Systems: Effects of Sodium Hypochlorite Volume, Concentration, and Retention Time. J. Endod. 2019, 45, 736–741. [Google Scholar] [CrossRef]
- Beus, C.; Safavi, K.; Stratton, J.; Kaufman, B. Comparison of the Effect of Two Endodontic Irrigation Protocols on the Elimination of Bacteria from Root Canal System: A Prospective, Randomized Clinical Trial. J. Endod. 2012, 38, 1479–1483. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E.; Bergenholtz, G.; Dahlén, G.; Svensäter, G. Response to alkaline stress by root canal bacteria in biofilms. Int. Endod. J. 2007, 40, 344–355. [Google Scholar] [CrossRef]
- Waltimo, T.; Trope, M.; Haapasalo, M.; Ørstavik, D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J. Endod. 2005, 31, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F.; Ørstavik, D. Outcome of endodontic retreatment using 2 root canal irrigants and influence of infection on healing as determined by a molecular method: A randomized clinical trial. J. Endod. 2019, 45, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Prado, L.C.; Gavini, G.; Silveira, A.D.C.; Nakamura, V.C.; Mayer, M.P.A.; Pinheiro, E.T. Comparison of rRNA-based reverse transcription PCR and rDNA-based PCR for the detection of streptococci in root canal infections. J. Appl. Oral Sci. 2019, 29, e20180256. [Google Scholar] [CrossRef] [PubMed]
| Clinical Features | |||
|---|---|---|---|
| n | % | ||
| Age | 20–40 | 8 | 40 |
| >40 | 12 | 60 | |
| Gender | Male | 9 | 45 |
| Female | 11 | 55 | |
| Tooth type | Anterior | 11 | 55 |
| Premolar | 9 | 45 | |
| Root canal preparation | Reciproc instrument R40 (0.40 mm) | 12 | 60 |
| Reciproc instrument R50 (0.50 mm) | 8 | 40 |
| rDNA Levels | Root Canal Samples | |||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3a | S3b | S4 | S5 | |
| Median (n = 20) | 1.79 × 105 a | 7.58 × 103 b | 6.40 × 103 b | 3.82 × 103 b | 1.56 × 104 b | 0 c |
| Range | (6.21 × 103–2.52 × 106) | (0–8.35 × 105) | (0–7.37 × 105) | (0–1.84 × 105) | (0–2.09 × 105) | (0–9.62 × 104) |
| n (%) | 20 (100%) | 12 (60%) | 11 (55%) | 10 (50%) | 14 (70%) | 6 (30%) |
| Samples | Detection Rates | p-Value * | |||
|---|---|---|---|---|---|
| rDNA | |||||
| + | − | ||||
| S1 | rRNA | + | 15 | 0 | 1.00 |
| − | 0 | 0 | |||
| + | − | ||||
| S2 | rRNA | + | 10 | 5 | 0.06 |
| − | 0 | 0 | |||
| + | − | ||||
| S3a | rRNA | + | 9 | 4 | 0.12 |
| − | 0 | 2 | |||
| + | − | ||||
| S3b | rRNA | + | 9 | 5 | 0.06 |
| − | 0 | 1 | |||
| + | − | ||||
| S4 | rRNA | + | 12 | 3 | 0.50 |
| − | 0 | 0 | |||
| + | − | ||||
| S5 | rRNA | + | 4 | 9 | 0.0039 * |
| − | 0 | 2 | |||
| n | rDNA | rRNA | p Value * | |
|---|---|---|---|---|
| S1 | 15 | 1.62 × 105 (6.21 × 103–2.52 × 106) | 4.08 × 105 (6.27 × 104–8.75 × 106) | 0.0007 * |
| S2 | 10 | 4.84 × 104 (8.28 × 103–8.35 × 105) | 8.30 × 104 (7.37 × 103–6.80 × 105) | 0.5076 |
| S3a | 9 | 5.48 × 104 (7.03 × 103–7.37 × 105) | 5.50 × 104 (1.06 × 104–5.84 × 105) | 0.5940 |
| S3b | 9 | 4.48 × 104 (7.63 × 103–1.84 × 105) | 6.26 × 104 (4.63 × 103–3.66 × 105) | 0.2135 |
| S4 | 12 | 7.58 × 104 (7.40 × 103–1.92 × 105) | 9.48 × 104 (6.63 × 103–4.41 × 105) | 0.0499 * |
| S5 | 4 | 8.91 × 103 (4.63 × 103–1.36 × 104) | 3.09 × 104 (8.63 × 103–5.97 × 104) | N.A. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.P.L.; Nardello, L.C.L.; Fernandes, F.S.; Bruno, F.P.; Paz, L.R.; Iglecias, E.F.; Honório, H.M.; Mayer, M.P.A.; Gavini, G.; Pinheiro, E.T. Effects of Contemporary Irrigant Activation Schemes and Subsequent Placement of an Interim Dressing on Bacterial Presence and Activity in Root Canals Associated with Asymptomatic Apical Periodontitis. J. Clin. Med. 2020, 9, 854. https://doi.org/10.3390/jcm9030854
Carvalho APL, Nardello LCL, Fernandes FS, Bruno FP, Paz LR, Iglecias EF, Honório HM, Mayer MPA, Gavini G, Pinheiro ET. Effects of Contemporary Irrigant Activation Schemes and Subsequent Placement of an Interim Dressing on Bacterial Presence and Activity in Root Canals Associated with Asymptomatic Apical Periodontitis. Journal of Clinical Medicine. 2020; 9(3):854. https://doi.org/10.3390/jcm9030854
Chicago/Turabian StyleCarvalho, Alexandre P. L., Laura C. L. Nardello, Fernanda S. Fernandes, Fernanda P. Bruno, Luiza R. Paz, Elaine F. Iglecias, Heitor M. Honório, Márcia P. A. Mayer, Giulio Gavini, and Ericka T. Pinheiro. 2020. "Effects of Contemporary Irrigant Activation Schemes and Subsequent Placement of an Interim Dressing on Bacterial Presence and Activity in Root Canals Associated with Asymptomatic Apical Periodontitis" Journal of Clinical Medicine 9, no. 3: 854. https://doi.org/10.3390/jcm9030854
APA StyleCarvalho, A. P. L., Nardello, L. C. L., Fernandes, F. S., Bruno, F. P., Paz, L. R., Iglecias, E. F., Honório, H. M., Mayer, M. P. A., Gavini, G., & Pinheiro, E. T. (2020). Effects of Contemporary Irrigant Activation Schemes and Subsequent Placement of an Interim Dressing on Bacterial Presence and Activity in Root Canals Associated with Asymptomatic Apical Periodontitis. Journal of Clinical Medicine, 9(3), 854. https://doi.org/10.3390/jcm9030854






