Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Imaging
2.3. Evaluation
2.4. Statistical Analysis
3. Results
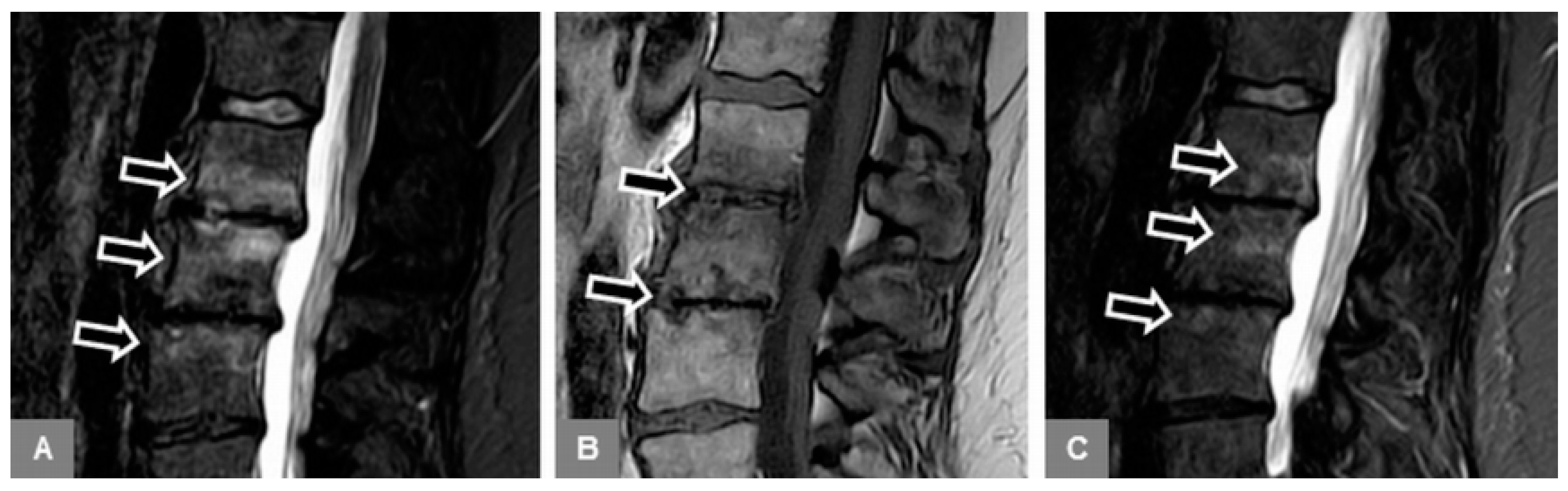
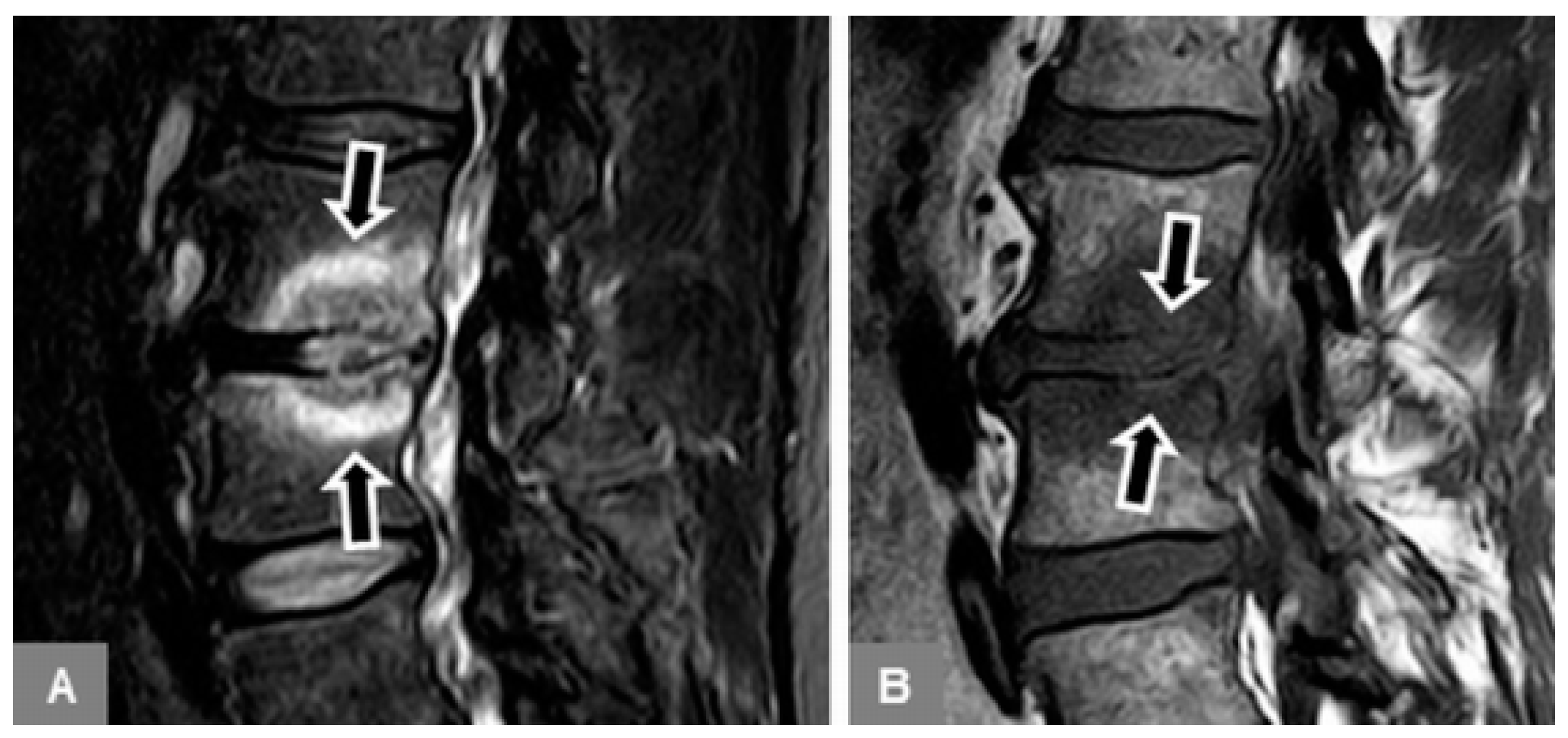
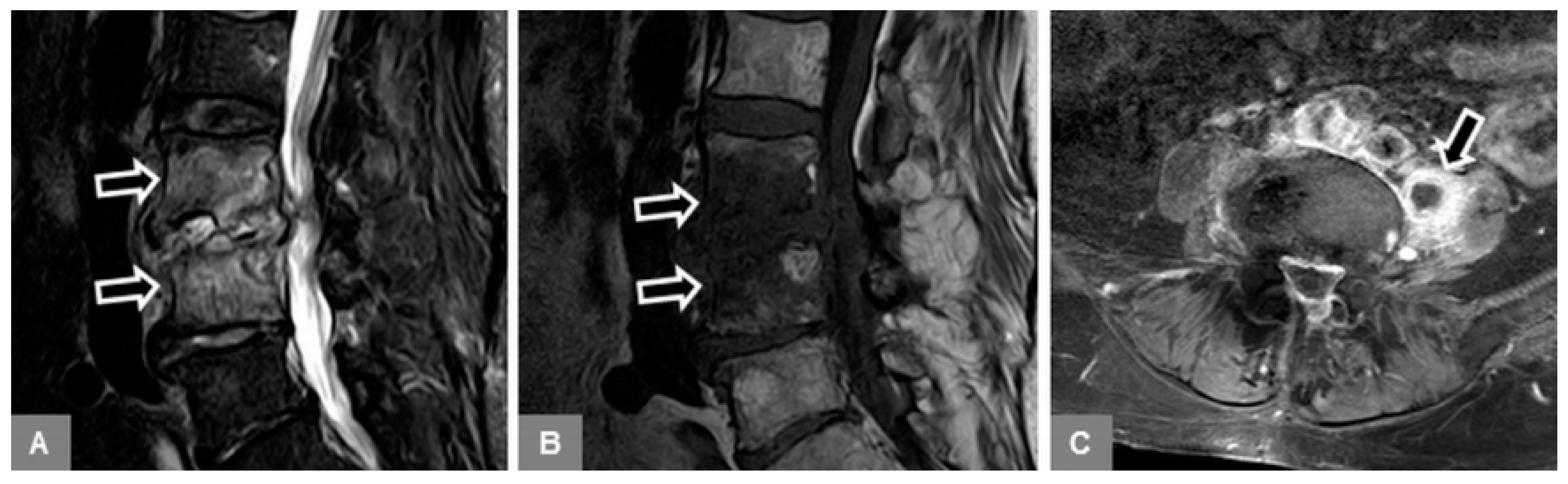
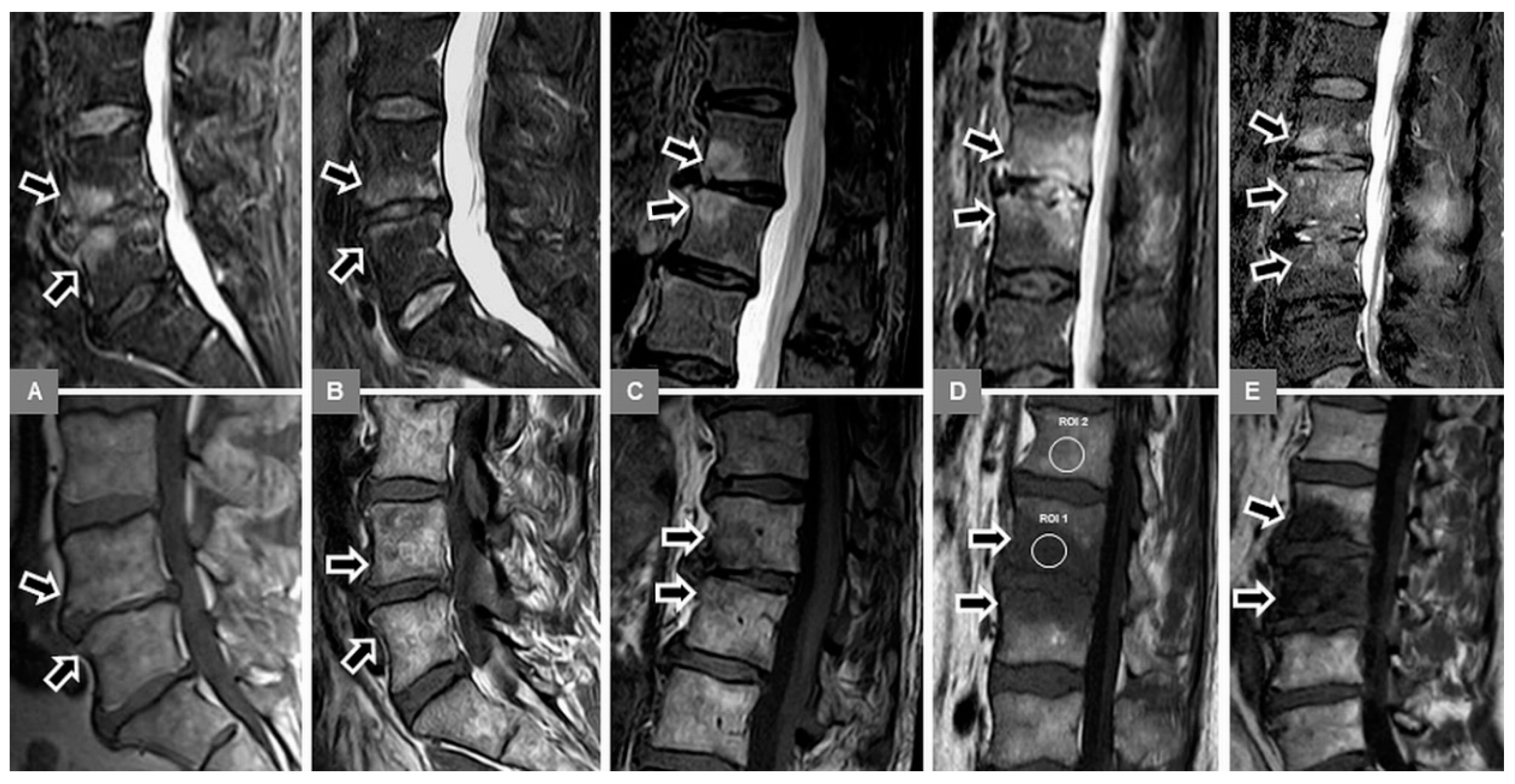
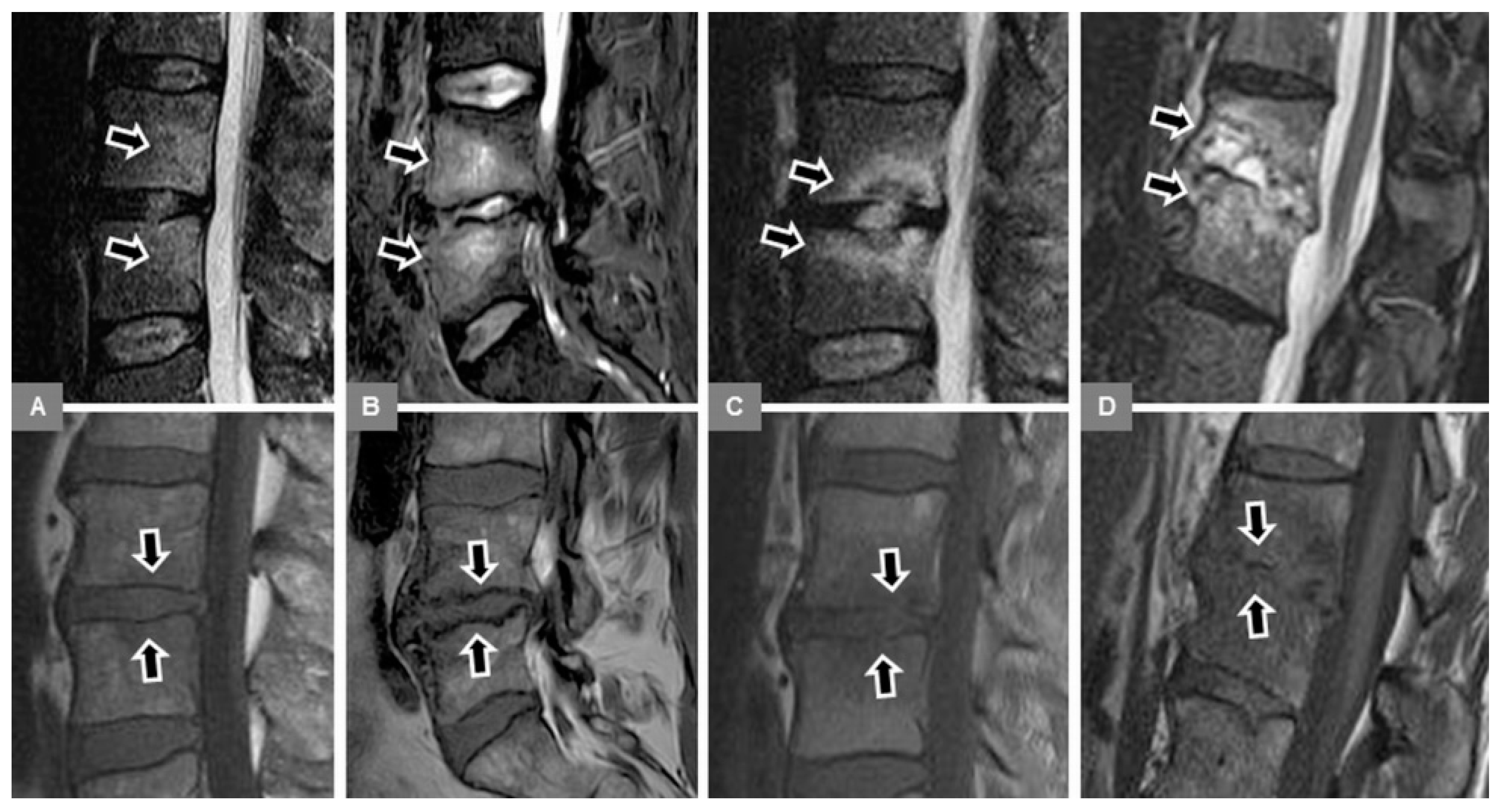
3.1. Bone Marrow Edema Extent, T1-w Signal Ratios of Edema to Unaffected Bone, and Visual T1-w Signal Assessment
3.2. Vertebral Endplate Contour
3.3. Diagnostic Accuracy in the Differentiation Between MT1 and Spondylodiscitis
3.4. Site of Vertebral Body Involvement
3.5. Appearance of the Intervertebral Disc
3.6. Modic Type 2 and 3 Endplate Changes
3.7. Reader Agreement
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Crockett, M.T.; Kelly, B.S.; van Baarsel, S.; Kavanagh, E.C. Modic type 1 vertebral endplate changes: Injury, inflammation, or infection? AJR Am. J. Roentgenol. 2017, 209, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Rahme, R.; Moussa, R. The modic vertebral endplate and marrow changes: Pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am. J. Neuroradiol. 2008, 29, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.B.; Manniche, C. Modic changes following lumbar disc herniation. Eur. Spine J. 2007, 16, 977–982. [Google Scholar] [CrossRef]
- Kerttula, L.; Luoma, K.; Vehmas, T.; Grönblad, M.; Kääpä, E. Modic type I change may predict rapid progressive, deforming disc degeneration: A prospective 1-year follow-up study. Eur. Spine J. 2012, 21, 1135–1142. [Google Scholar] [CrossRef]
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Lu, B.; Lauricella, M.; Lotz, J.C. Modic type 1 change is an autoimmune response that requires a proinflammatory milieu provided by the ‘Modic disc’. Spine J. 2018, 18, 831–844. [Google Scholar] [CrossRef]
- Nguyen, C.; Poiraudeau, S.; Rannou, F. From Modic 1 vertebral-endplate subchondral bone signal changes detected by MRI to the concept of ‘active discopathy’. Ann. Rheum. Dis. 2015, 74, 1488–1494. [Google Scholar] [CrossRef]
- Stäbler, A.; Weiss, M.; Scheidler, J.; Krödel, A.; Seiderer, M.; Reiser, M. Degenerative disk vascularization on MRI: Correlation with clinical and histopathologic findings. Skelet. Radiol. 1996, 25, 119–126. [Google Scholar] [CrossRef]
- Albert, H.B.; Lambert, P.; Rollason, J.; Sorensen, J.S.; Worthington, T.; Pedersen, M.B.; Nørgaard, H.S.; Vernallis, A.; Busch, F.; Manniche, C.; et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur. Spine J. 2013, 22, 690–696. [Google Scholar] [CrossRef]
- Aghazadeh, J.; Salehpour, F.; Ziaeii, E.; Javanshir, N.; Samadi, A.; Sadeghi, J.; Mirzaei, F.; Naseri Alavi, S.A. Modic changes in the adjacent vertebrae due to disc material infection with Propionibacterium acnes in patients with lumbar disc herniation. Eur. Spine J. 2017, 26, 3129–3134. [Google Scholar] [CrossRef]
- Stirling, A.; Worthington, T.; Rafiq, M.; Lambert, P.A.; Elliott, T.S. Association between sciatica and Propionibacterium acnes. Lancet 2001, 357, 2024–2025. [Google Scholar] [CrossRef]
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Miller, S.; Demir-Deviren, S.; Lotz, J.C. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J. Orthop. Res. 2016, 34, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Dudli, S.; Miller, S.; Demir-Deviren, S.; Lotz, J.C. Inflammatory response of disc cells against Propionibacterium acnes depends on the presence of lumbar Modic changes. Eur. Spine J. 2018, 27, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Rigal, J.; Thelen, T.; Byrne, F.; Cogniet, A.; Boissière, L.; Aunoble, S.; Le Huec, J.C. Prospective study using anterior approach did not show association between Modic 1 changes and low grade infection in lumbar spine. Eur. Spine J. 2016, 25, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Wedderkopp, N.; Thomsen, K.; Manniche, C.; Kolmos, H.J.; Secher Jensen, T.; Leboeuf Yde, C. No evidence for presence of bacteria in modic type I changes. Acta Radiol. 2009, 50, 65–70. [Google Scholar] [CrossRef]
- Dudli, S.; Fields, A.J.; Samartzis, D.; Karppinen, J.; Lotz, J.C. Pathobiology of Modic changes. Eur. Spine J. 2016, 25, 3723–3734. [Google Scholar] [CrossRef]
- Albert, H.B.; Kjaer, P.; Jensen, T.S.; Sorensen, J.S.; Bendix, T.; Manniche, C. Modic changes, possible causes and relation to low back pain. Med. Hypotheses 2008, 70, 361–368. [Google Scholar] [CrossRef]
- Birkenmaier, C. Should we start treating chronic low back pain with antibiotics rather than with pain medications? Korean J. Pain 2013, 26, 327–335. [Google Scholar] [CrossRef]
- Albert, H.B.; Sorensen, J.S.; Christensen, B.S.; Manniche, C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): A double-blind randomized clinical controlled trial of efficacy. Eur. Spine J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Cai, G.; Laslett, L.L.; Aitken, D.; Halliday, A.; Pan, F.; Otahal, P.; Speden, D.; Winzenberg, T.M.; Jones, G. Effect of zoledronic acid and denosumab in patients with low back pain and Modic change: A proof-of-principle trial. J. Bone Miner. Res. 2018, 33, 773–782. [Google Scholar] [CrossRef]
- Kumar, Y.; Gupta, N.; Chhabra, A.; Fukuda, T.; Soni, N.; Hayashi, D. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: A pictorial review. BMC Musculoskelet. Disord. 2017, 18, 244. [Google Scholar] [CrossRef]
- Morales, H. Infectious spondylitis mimics: Mechanisms of disease and imaging findings. Semin. Ultrasound CT MRI 2018, 39, 587–604. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, J.Y.; Lee, J.W.; Kim, N.R.; Choi, J.A.; Kang, H.S. MR imaging assessment of the spine: Infection or an imitation? Radiographics 2009, 29, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.; Lazzeri, E.; Palestro, C.J. Imaging of spondylodiscitis. Semin. Nucl. Med. 2018, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Sans, N.; Faruch, M.; Lapègue, F.; Ponsot, A.; Chiavassa, H.; Railhac, J.J. Infections of the spinal column--spondylodiscitis. Diagn. Interv. Imaging 2012, 93, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, L.N.; Salzman, K.L.; Shah, L.M. Imaging psoas sign in lumbar spinal infections: Evaluation of diagnostic accuracy and comparison with established imaging characteristics. AJNR Am. J. Neuroradiol. 2016, 37, 736–741. [Google Scholar] [CrossRef]
- Ledermann, H.P.; Schweitzer, M.E.; Morrison, W.B.; Carrino, J.A. MR imaging findings in spinal infections: Rules or myths? Radiology 2003, 228, 506–514. [Google Scholar] [CrossRef]
- Yeom, J.A.; Lee, I.S.; Suh, H.B.; Song, Y.S.; Song, J.W. Magnetic resonance imaging findings of early spondylodiscitis: Interpretive challenges and atypical findings. Korean J. Radiol. 2016, 17, 565–580. [Google Scholar] [CrossRef]
- Dunbar, J.A.; Sandoe, J.A.; Rao, A.S.; Crimmins, D.W.; Baig, W.; Rankine, J.J. The MRI appearances of early vertebral osteomyelitis and discitis. Clin. Radiol. 2010, 65, 974–981. [Google Scholar] [CrossRef]
- Stäbler, A.; Baur, A.; Krüger, A.; Weiss, M.; Helmberger, T.; Reiser, M. Differential diagnosis of erosive osteochondrosis and bacterial spondylitis: Magnetic resonance tomography (MRT). Rofo 1998, 168, 421–428. [Google Scholar] [CrossRef]
- Shrot, S.; Sayah, A.; Berkowitz, F. Can the pattern of vertebral marrow oedema differentiate intervertebral disc infection from degenerative changes? Clin. Radiol. 2017, 72, e7–e613. [Google Scholar] [CrossRef] [PubMed]
- Stäbler, A. Degenerative diseases of the spine. In Musculoskeletal Diseases 2009–2012; Hodler, J., Zollikofer, C.L., von Schulthess, G.K., Eds.; Springer: Milano, Italy, 2009; pp. 132–136. [Google Scholar]
- Kowalski, T.J.; Layton, K.F.; Berbari, E.F.; Steckelberg, J.M.; Huddleston, P.M.; Wald, J.T.; Osmon, D.R. Follow-up MR imaging in patients with pyogenic spine infections: Lack of correlation with clinical features. AJNR Am. J. Neuroradiol. 2007, 28, 693–699. [Google Scholar] [PubMed]
- Foreman, S.C.; Schwaiger, B.J.; Gempt, J.; Jungmann, P.M.; Kehl, V.; Delbridge, C.; Wantia, N.; Zimmer, C.; Kirschke, J.S. MR and CT imaging to optimize CT-guided biopsies in suspected spondylodiscitis. World Neurosurg. 2017, 99, 726–734.e7. [Google Scholar] [CrossRef]
- Finkenstaedt, T.; Del Grande, F.; Bolog, N.; Ulrich, N.; Tok, S.; Kolokythas, O.; Steurer, J.; Andreisek, G.; Winklhofer, S.; LSOS Study Group. Modic type 1 changes: Detection performance of fat-suppressed fluid-sensitive MRI sequences. Rofo 2018, 190, 152–160. [Google Scholar] [CrossRef]
- Kwon, J.W.; Yoon, Y.C.; Choi, S.H.; Jung, J.Y.; Choe, B.K. MR imaging for the differentiation of early infectious spondylitis and Modic type I change in the lumbar spine. J. Korean Soc. Radiol. 2010, 62, 563–570. [Google Scholar] [CrossRef]
- Johnson, P.W.; Collins, M.S.; Wenger, D.E. Diagnostic utility of T1-weighted MRI characteristics in evaluation of osteomyelitis of the foot. AJR Am. J. Roentgenol. 2009, 192, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chu, B.; Feng, Y.; Xu, F.; Zou, Y.F. Modic changes in lumbar spine: Prevalence and distribution patterns of end plate oedema and end plate sclerosis. Br. J. Radiol. 2016, 89, 20150650. [Google Scholar] [CrossRef] [PubMed]
- Wolansky, L.J.; Heary, R.F.; Patterson, T.; Friedenberg, J.S.; Tholany, J.; Chen, J.K.; Patel, N.; Doddakashi, S. Pseudosparing of the endplate: A potential pitfall in using MR imaging to diagnose infectious spondylitis. AJR Am. J. Roentgenol. 1999, 172, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Schweitzer, M.E. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 2010, 30, 723–736. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Xu, H.C.; Lei, T.; Li, Q.L.; Wu, A.M.; Ni, W.F. The location of Modic changes in the lumbar spine: A meta-analysis. Eur. Spine J. 2016, 25, 3746–3759. [Google Scholar] [CrossRef]
- Kim, N.R.; Choi, J.Y.; Hong, S.H.; Jun, W.S.; Lee, J.W.; Choi, J.A.; Kang, H.S. “MR corner sign”: Value for predicting presence of ankylosing spondylitis. AJR Am. J. Roentgenol. 2008, 191, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Ding, W.Y.; Shen, Y.; Zhang, Y.Z.; Guo, J.K.; Sun, Y.P.; Cao, L.Z. Prevalence of vertebral endplate modic changes in degenerative lumbar scoliosis and its associated factors analysis. Spine 2012, 37, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Granata, F.; Ricciardi, K.; Gaeta, M.; Blandino, A. Contrast-enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur. Radiol. 2003, 13, 626–637. [Google Scholar] [CrossRef]
- D’Anastasi, M.; Birkenmaier, C.; Schmidt, G.P.; Wegener, B.; Reiser, M.F.; Baur-Melnyk, A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am. J. Roentgenol. 2011, 197, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.E.; el-Noueam, K.I. Vacuum disc: Frequency of high signal intensity on T2-weighted MR images. Skelet. Radiol. 1998, 27, 83–86. [Google Scholar] [CrossRef]
- Rausch, V.H.; Bannas, P.; Schoen, G.; Froelich, A.; Well, L.; Regier, M.; Adam, G.; Henes, F.O. Diagnostic yield of multidetector computed tomography in patients with acute spondylodiscitis. Rofo 2017, 189, 339–346. [Google Scholar] [CrossRef]
- Braun, A.; Germann, T.; Wünnemann, F.; Weber, M.A.; Schiltenwolf, M.; Akbar, M.; Burkholder, I.; Kauczor, H.U.; Rehnitz, C. Impact of MRI, CT, and clinical characteristics on microbial pathogen detection using CT-guided biopsy for suspected spondylodiscitis. J. Clin. Med. 2019, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Poplawski, M.M.; Pawha, P.S.; Naidich, T.P.; Tanenbaum, L.N. Diffusion-weighted MRI “claw sign” improves differentiation of infectious from degenerative Modic type 1 signal changes of the spine. AJNR Am. J. Neuroradiol. 2014, 35, 1647–1652. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B.; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]

| Percentage Edema Extent (%) | T1-w Signal Ratios | ||
|---|---|---|---|
| Modic type 1 | Mean | 31.83 | 0.82 |
| Median | 30.00 | 0.82 | |
| Standard deviation | 23.66 | 0.14 | |
| Minimum | 5.00 | 0.53 | |
| Maximum | 100.00 | 1.22 | |
| Range | 95.00 | 0.70 | |
| Early spondylodiscitis | Mean | 55.61 | 0.59 |
| Median | 60.00 | 0.56 | |
| Standard deviation | 28.18 | 0.14 | |
| Minimum | 5.00 | 0.34 | |
| Maximum | 100.00 | 0.91 | |
| Range | 95.00 | 0.57 | |
| Advanced spondylodiscitis | Mean | 91.60 | 0.61 |
| Median | 100.00 | 0.61 | |
| Standard deviation | 18.26 | 0.13 | |
| Minimum | 10.00 | 0.29 | |
| Maximum | 100.00 | 0.96 | |
| Range | 90.00 | 0.66 |
| T1-w Signal Intensity | |||||||
|---|---|---|---|---|---|---|---|
| Hyperintense | Isointense | Mixed | Hypointense | Marked Hypointense | Total | ||
| Modic type 1 | n | 8 | 8 | 71 | 11 | 0 | 98 |
| % | 8.2% | 8.2% | 72.4% | 11.2% | 0.0% | 100.0% | |
| Early spondylodiscitis | n | 0 | 4 | 4 | 19 | 13 | 40 |
| % | 0.0% | 10.0% | 10.0% | 47.5% | 32.5% | 100.0% | |
| Advanced spondylodiscitis | n | 0 | 4 | 14 | 27 | 23 | 68 |
| % | 0.0% | 5.9% | 20.6% | 39.7% | 33.8% | 100.0% | |
| n | 8 | 16 | 89 | 57 | 36 | 206 | |
| % | 3.9% | 7.8% | 43.2% | 27.7% | 17.5% | 100.0% | |
| Endplate contour | ||||||
|---|---|---|---|---|---|---|
| Normal | Irregular | Blurred | Destructive | Total | ||
| Upper endplate | ||||||
| Modic type 1 | n | 6 | 52 | 1 | 0 | 59 |
| % | 10.2% | 88.1% | 1.7% | 0.0% | 100.0% | |
| Early spondylodiscitis | n | 4 | 3 | 15 | 0 | 22 |
| % | 18.2% | 13.6% | 68.2% | 0.0% | 100.0% | |
| Advanced spondylodiscitis | n | 1 | 1 | 24 | 9 | 35 |
| % | 2.9% | 2.9% | 68.6% | 25.7% | 100.0% | |
| n | 11 | 56 | 40 | 9 | 116 | |
| % | 9.5% | 48.3% | 34.5% | 7.8% | 100.0% | |
| Lower endplate | ||||||
| Modic type 1 | n | 2 | 56 | 1 | 0 | 59 |
| % | 3.4% | 94.9% | 1.7% | 0.0% | 100.0% | |
| Early spondylodiscitis | n | 4 | 3 | 15 | 0 | 22 |
| % | 18.2% | 13.6% | 68.2% | 0.0% | 100.0% | |
| Advanced spondylodiscitis | n | 2 | 1 | 23 | 9 | 35 |
| % | 5.7% | 2.9% | 65.7% | 25.7% | 100.0% | |
| n | 8 | 60 | 39 | 9 | 116 | |
| % | 6.9% | 51.7% | 33.6% | 7.8% | 100.0% | |
| AUC | Standard Error | Significance p-Value | Confidence Interval | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Percentage of the extent of edema | 0.882 | 0.036 | 0.000 | 0.812 | 0.953 |
| T1-w signal ratio of edema to unaffected bone | 0.920 | 0.027 | 0.000 | 0.867 | 0.974 |
| Modic Type 1 | Early Spondylodiscitis | Advanced Spondylodiscitis | Total | ||
|---|---|---|---|---|---|
| Modic type 1 | n | 39 | 0 | 0 | 39 |
| % | 100.0% | 0.0% | 0.0% | 100.0% | |
| Early spondylodiscitis | n | 2 | 12 | 5 | 19 |
| % | 10.5% | 63.2% | 26.3% | 100.0% | |
| Advanced spondylodiscitis | n | 1 | 3 | 29 | 33 |
| % | 3.0% | 9.1% | 87.9% | 100.0% | |
| n | 42 | 15 | 34 | 91 | |
| % | 46.2% | 16.5% | 37.4% | 100.0% |
| Vertebral Body | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Th12 | L1 | L2 | L3 | L4 | L5 | S1 | Total | ||
| Modic type 1 | n | 1 | 6 | 11 | 17 | 23 | 28 | 12 | 98 |
| % | 1.0% | 6.1% | 11.2% | 17.3% | 23.5% | 28.6% | 11.2% | 100.0% | |
| Early spondylodiscitis | n | 1 | 3 | 6 | 8 | 10 | 8 | 4 | 40 |
| % | 2.5% | 7.5% | 15.0% | 20.0% | 25.0% | 20.0% | 10.0% | 100.0% | |
| Advanced spondylodiscitis | n | 2 | 6 | 10 | 14 | 15 | 15 | 6 | 68 |
| % | 2.9% | 8.8% | 14.7% | 20.6% | 22.1% | 22.1% | 8.8% | 100.0% | |
| n | 4 | 15 | 27 | 39 | 48 | 51 | 22 | 206 | |
| % | 1.9% | 7.3% | 13.1% | 18.9% | 23.3% | 24.8% | 10.7% | 100.0% | |
| A | Intervertebral Disc Appearance | |||||||
| Vacuum Phenomenon | STIR Signal Hyperintensity | |||||||
| Absent | Present | Total | Absent | Present | Total | |||
| Modic type 1 | n | 44 | 15 | 59 | 47 | 12 | 59 | |
| % | 74.6% | 25.4% | 100% | 79.7% | 20.3% | 100.0% | ||
| Early spondylodiscitis | n | 21 | 1 | 22 | 4 | 18 | 22 | |
| % | 95.5% | 4.5% | 100% | 18.2% | 81.8% | 100.0% | ||
| Advanced spondylodiscitis | n | 35 | 0 | 35 | 4 | 31 | 35 | |
| % | 100.0% | 0.0% | 100% | 11.4% | 88.6% | 100.0% | ||
| n | 100 | 16 | 116 | 55 | 61 | 116 | ||
| % | 86.2% | 13.8% | 100% | 47.4% | 52.6% | 100.0% | ||
| B | Vertebral Endplate | |||||||
| Modic type 2 | Modic type 3 | |||||||
| Absent | Present | Total | Absent | Present | Total | |||
| Modic type 1 | n | 39 | 20 | 59 | 41 | 18 | 59 | |
| % | 66.1% | 33.9% | 100% | 69.5 | 30.5 | 100% | ||
| Early spondylodiscitis | n | 22 | 0 | 22 | 22 | 0 | 22 | |
| % | 100.0% | 0.0% | 100% | 100% | 0.0% | 100% | ||
| Advanced spondylodiscitis | n | 34 | 1 | 35 | 35 | 0 | 35 | |
| % | 97.1% | 2.9% | 100% | 100% | 0.0% | 100% | ||
| n | 95 | 21 | 116 | 98 | 18 | 116 | ||
| % | 81.9% | 18.1% | 100% | 84.5% | 15.5% | 100% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz-Nemec, U.; Friedrich, K.M.; Stihsen, C.; Schwarz, F.K.; Trattnig, S.; Weber, M.; Grohs, J.G.; Nemec, S.F. Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis. J. Clin. Med. 2020, 9, 826. https://doi.org/10.3390/jcm9030826
Schwarz-Nemec U, Friedrich KM, Stihsen C, Schwarz FK, Trattnig S, Weber M, Grohs JG, Nemec SF. Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis. Journal of Clinical Medicine. 2020; 9(3):826. https://doi.org/10.3390/jcm9030826
Chicago/Turabian StyleSchwarz-Nemec, Ursula, Klaus M. Friedrich, Christoph Stihsen, Felix K. Schwarz, Siegfried Trattnig, Michael Weber, Josef G. Grohs, and Stefan F. Nemec. 2020. "Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis" Journal of Clinical Medicine 9, no. 3: 826. https://doi.org/10.3390/jcm9030826
APA StyleSchwarz-Nemec, U., Friedrich, K. M., Stihsen, C., Schwarz, F. K., Trattnig, S., Weber, M., Grohs, J. G., & Nemec, S. F. (2020). Vertebral Bone Marrow and Endplate Assessment on MR Imaging for the Differentiation of Modic Type 1 Endplate Changes and Infectious Spondylodiscitis. Journal of Clinical Medicine, 9(3), 826. https://doi.org/10.3390/jcm9030826






