Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness
Abstract
1. Introduction
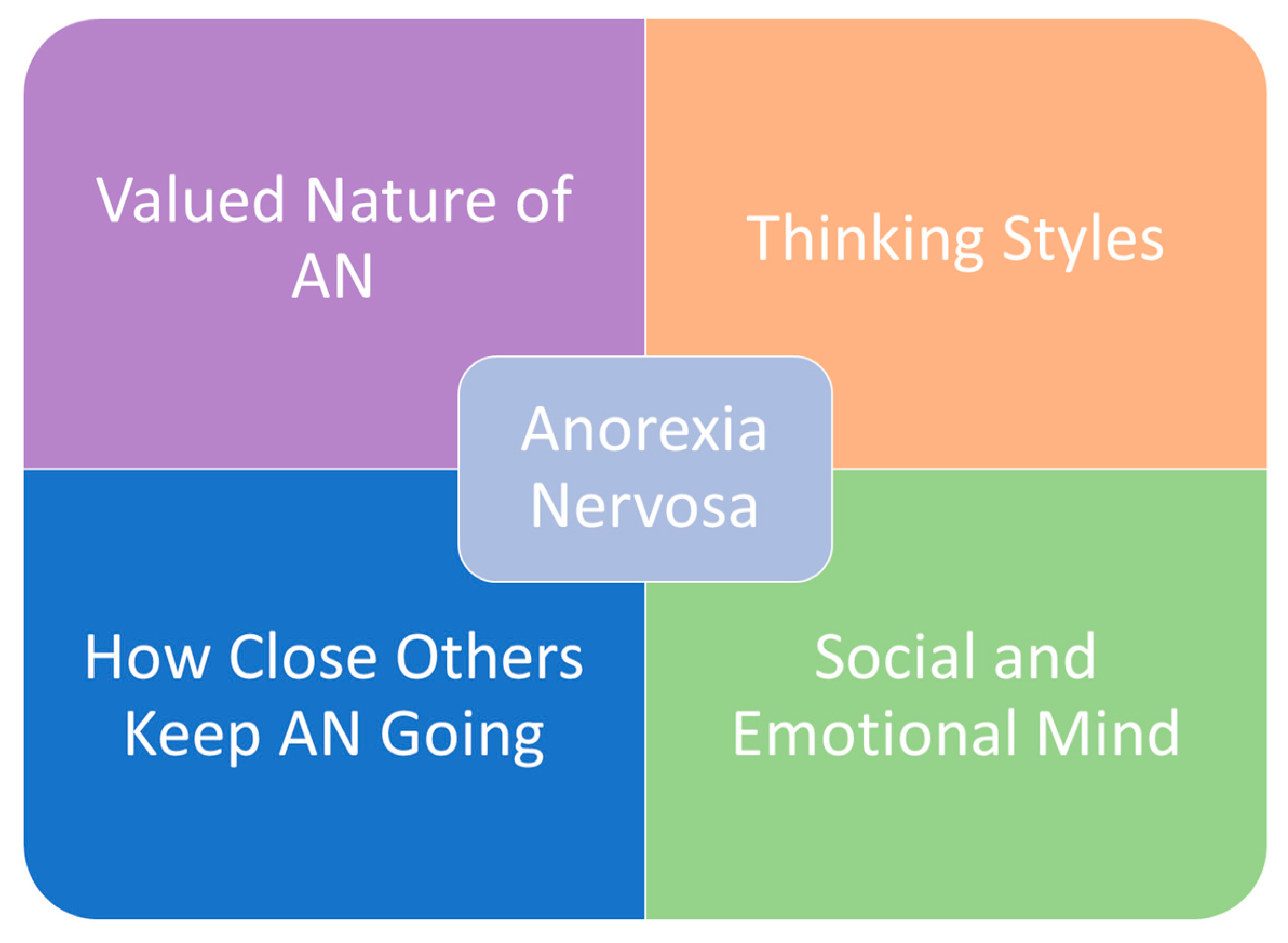
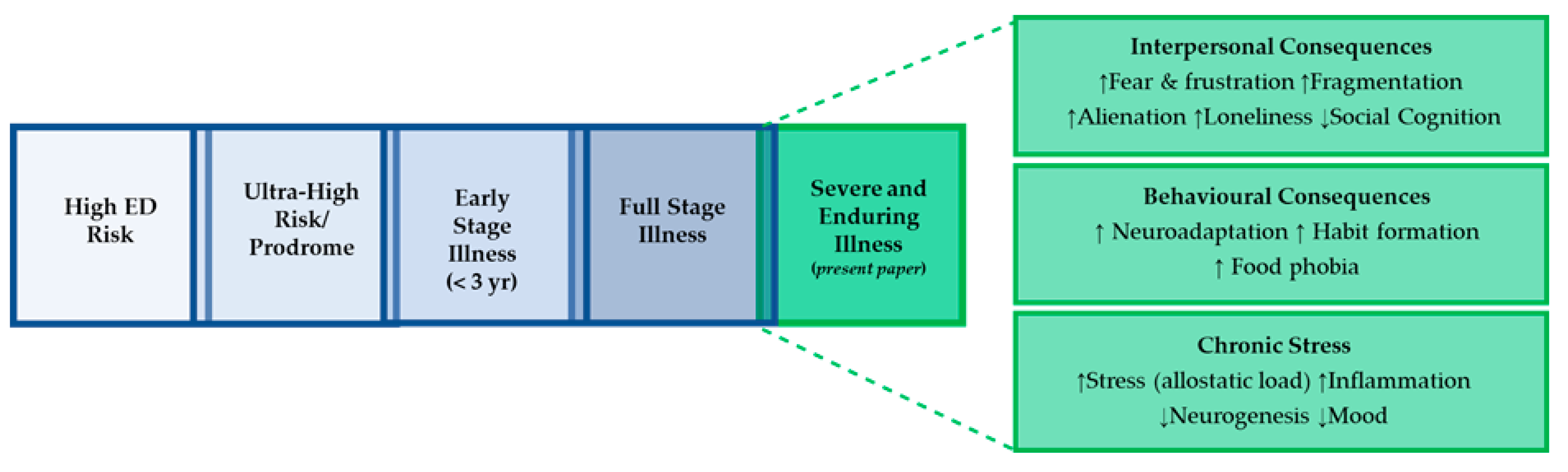
2. Staging Models of Anorexia Nervosa: the Enhanced Cognitive Interpersonal Maintenance Model
3. Social Risk and Maintaining Factors
3.1. Interpersonal Reactions to Living with Anorexia Nervosa
3.2. Targeting Stressful Interpersonal Reactions
4. Behavioural Consequences of Anorexia Nervosa
5. Chronic Stress Response: Co-Morbidity with Depression and Anxiety
Treatment-Resistant Depression and other Co-Morbidities as Maintaining Factors: Implications for Treatment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Treasure, J. Anorexia nervosa: Valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br. J. Clin. Psychol. 2006, 45, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Schmidt, U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: A summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J. Eat. Disord. 2013, 1, 13. [Google Scholar] [CrossRef]
- Farstad, S.M.; McGeown, L.M.; von Ranson, K.M. Eating disorders and personality, 2004–2016: A systematic review and meta-analysis. Clin. Psychol. Rev. 2016, 46, 91–105. [Google Scholar] [CrossRef]
- Miller, J.L.; Schmidt, L.A.; Vaillancourt, T.; McDougall, P.; Laliberte, M. Neuroticism and introversion: A risky combination for disordered eating among a non-clinical sample of undergraduate women. Eat. Behav. 2006, 7, 69–78. [Google Scholar] [CrossRef]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.; Gaspar, H.A.; Medland, S.E. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Guisinger, S. Adapted to flee famine: Adding an evolutionary perspective on anorexia nervosa. Psychol. Rev. 2003, 110, 745. [Google Scholar] [CrossRef]
- Treasure, J.; Stein, D.; Maguire, S. Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Interv. Psychiatry 2015, 9, 173–184. [Google Scholar] [CrossRef]
- Goddard, E.; Treasure, J. Anxiety and social-emotional processing in eating disorders: Examination of family trios. Cogn. Ther. Res. 2013, 37, 890–904. [Google Scholar] [CrossRef]
- Rhind, C.; Bonfioli, E.; Hibbs, R.; Goddard, E.; Macdonald, P.; Gowers, S.; Treasure, J. An examination of autism spectrum traits in adolescents with anorexia nervosa and their parents. Mol. Autism 2014, 5, 56. [Google Scholar] [CrossRef]
- Nielsen, S.; Anckarsäter, H.; Gillberg, C.; Gillberg, C.; Råstam, M.; Wentz, E. Effects of autism spectrum disorders on outcome in teenage-onset anorexia nervosa evaluated by the Morgan-Russell outcome assessment schedule: A controlled community-based study. Mol. Autism 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Barona, M.; Treasure, J.O.; Micali, N. Social cognition in children at familial high-risk of developing an eating disorder. Front. Behav. Neurosc. 2015, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.G.; Cohen, P.; Kasen, S.; Brook, J.S. Childhood adversities associated with risk for eating disorders or weight problems during adolescence or early adulthood. Am. J. Psychiatry 2002, 159, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Jewell, T.; Collyer, H.; Gardner, T.; Tchanturia, K.; Simic, M.; Fonagy, P.; Eisler, I. Attachment and mentalization and their association with child and adolescent eating pathology: A systematic review. Int. J. Eat. Disord. 2016, 49, 354–373. [Google Scholar] [CrossRef]
- Cardi, V.; Tchanturia, K.; Treasure, J. Premorbid and illness-related social difficulties in eating disorders: An overview of the literature and treatment developments. Curr. Neuropharmacol. 2018, 16, 1122–1130. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Hayward, L.E.; Smyth, J.M.; Paxton, S.J.; Touyz, S.W. Risk and resiliency factors related to body dissatisfaction and disordered eating: The identity disruption model. Int. J. Eat. Disord. 2018, 51, 322–330. [Google Scholar] [CrossRef]
- Young, J.E.; Klosko, J.S.; Weishaar, M.E. Schema Therapy: A Practitioner’s Guide; Guilford Press: New York, NY, USA, 2003. [Google Scholar]
- Arcelus, J.; Haslam, M.; Farrow, C.; Meyer, C. The role of interpersonal functioning in the maintenance of eating psychopathology: A systematic review and testable model. Clin. Psychol. Rev. 2013, 33, 156–167. [Google Scholar] [CrossRef]
- Oldershaw, A.; Lavender, T.; Sallis, H.; Stahl, D.; Schmidt, U. Emotion generation and regulation in anorexia nervosa: A systematic review and meta-analysis of self-report data. Clin. Psychol. Rev. 2015, 39, 83–95. [Google Scholar] [CrossRef]
- Engel, S.G.; Wonderlich, S.A.; Crosby, R.D.; Mitchell, J.E.; Crow, S.; Peterson, C.B.; Gordon, K.H. The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. J. Abnorm. Psychol. 2013, 122, 709. [Google Scholar] [CrossRef]
- Rapee, R.M.; Oar, E.L.; Johnco, C.J.; Forbes, M.K.; Fardouly, J.; Magson, N.R.; Richardson, C.E. Adolescent development and risk for the onset of social-emotional disorders: A review and conceptual model. Behav. Res. Ther. 2019, 123, 103501. [Google Scholar] [CrossRef]
- Caglar-Nazali, H.P.; Corfield, F.; Cardi, V.; Ambwani, S.; Leppanen, J.; Olabintan, O.; Micali, N. A systematic review and meta-analysis of ‘Systems for Social Processes’ in eating disorders. Neurosci. Biobehav. Rev. 2014, 42, 55–92. [Google Scholar] [CrossRef] [PubMed]
- Cardi, V.; Corfield, F.; Leppanen, J.; Rhind, C.; Deriziotis, S.; Hadjimichalis, A.; Treasure, J. Emotional processing, recognition, empathy and evoked facial expression in eating disorders: An experimental study to map deficits in social cognition. PLoS ONE 2015, 10, e0133827. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Wolz, I.; Leppanen, J.; Fernandez-Aranda, F.; Schmidt, U.; Tchanturia, K. Facial expression to emotional stimuli in non-psychotic disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, A.; DeJong, H.; Hambrook, D.; Schmidt, U. Social attribution in anorexia nervosa. Eur. Eat. Disord. Rev. 2018, 26, 197–206. [Google Scholar] [CrossRef]
- Cardi, V.; Di Matteo, R.; Corfield, F.; Treasure, J. Social reward and rejection sensitivity in eating disorders: An investigation of attentional bias and early experiences. World J. Biol. Psychiatry 2013, 14, 622–633. [Google Scholar] [CrossRef]
- Cardi, V.; Di Matteo, R.; Gilbert, P.; Treasure, J. Rank perception and self-evaluation in eating disorders. Int. J. Eat. Disord. 2014, 47, 543–552. [Google Scholar] [CrossRef]
- Ambwani, S.; Berenson, K.R.; Simms, L.; Li, A.; Corfield, F.; Treasure, J. Seeing things differently: An experimental investigation of social cognition and interpersonal behavior in anorexia nervosa. Int. J. Eat. Disord. 2016, 49, 499–506. [Google Scholar] [CrossRef]
- Szczurek, L.; Monin, B.; Gross, J.J. The Stranger Effect: The Rejection of Affective Deviants. Psychol. Sci. 2012, 23, 1105–1111. [Google Scholar] [CrossRef]
- Hess, U.; Fischer, A. Emotional Mimicry as Social Regulation. Personal. Soc. Psychol. Rev. 2013, 17, 142–157. [Google Scholar] [CrossRef]
- Schneider, K.G.; Hempel, R.J.; Lynch, T.R. That “poker face” just might lose you the game! the impact of expressive suppression and mimicry on sensitivity to facial expressions of emotion. Emotion 2013, 13, 852–866. [Google Scholar] [CrossRef]
- McKnight, R.; Boughton, N. Anorexia Nervosa. Br. Med. J. 2014, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hjern, A.; Lindberg, L.; Lindblad, F. Outcome and prognostic factors for adolescent female in-patients with anorexia nervosa: 9-to 4-year follow-up. Br. J. Psychiatry 2006, 189, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Crane, A.; McKnight, R.; Buchanan, E.; Wolfe, M. First do no harm: Iatrogenic maintaining factors in anorexia nervosa. Eur. Eat. Disord. Rev. 2011, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, D.; Medina-Pradas, C.; Sepulveda, A.R.; Treasure, J. A systematic review of family caregiving in eating disorders. Eat. Behav. 2014, 15, 464–477. [Google Scholar] [CrossRef]
- Konstantakopoulos, G.; Tchanturia, K.; Surguladze, S.A.; David, A.S. Insight in eating disorders: Clinical and cognitive correlates. Psychol. Med. 2011, 41, 1951–1961. [Google Scholar] [CrossRef]
- Gorwood, P.; Duriez, P.; Lengvenyte, A.; Guillaume, S.; Criquillion, S. Clinical insight in anorexia nervosa: Associated and predictive factors. Psychiatry Res. 2019, 281, 112561. [Google Scholar] [CrossRef]
- Treasure, J.; Schmidt, U.; Macdonald, P. (Eds.) The Clinician’s Guide to Collaborative Caring in Eating Disorders: The New Maudsley Method; Routledge: London, UK, 2009. [Google Scholar]
- Treasure, J.; Smith, G.; Crane, A. Skills-Based Caring for a Loved One With an Eating Disorder: The New Maudsley Method; Routledge: London, UK, 2016. [Google Scholar]
- Sepulveda, A.R.; Kyriacou, O.; Treasure, J. Development and validation of the accommodation and enabling scale for eating disorders (AESED) for caregivers in eating disorders. BMC Health Serv. Res. 2009, 9, 171. [Google Scholar] [CrossRef]
- Salerno, L.; Rhind, C.; Hibbs, R.; Micali, N.; Schmidt, U.; Gowers, S.; Treasure, J. An examination of the impact of care giving styles (accommodation and skilful communication and support) on the one year outcome of adolescent anorexia nervosa: Testing the assumptions of the cognitive interpersonal model in anorexia nervosa. J. Affect. Disord. 2016, 191, 230–236. [Google Scholar] [CrossRef]
- Dimitropoulos, G.; Klopfer, K.; Lazar, L.; Schacter, R. Caring for a sibling with anorexia nervosa: A qualitative study. Eur. Eat. Disord. Rev. Prof. J. Eat. Disord. Assoc. 2009, 17, 350–365. [Google Scholar] [CrossRef]
- Dimitropoulos, G.; Freeman, V.E.; Bellai, K.; Olmsted, M. Inpatients with severe anorexia nervosa and their siblings: Non-shared experiences and family functioning. Eur. Eat. Disord. Rev. 2013, 21, 284–293. [Google Scholar] [CrossRef]
- House of Commons Health Committee. Suicide Prevention: Interim Report. Fourth Report of Session; House of Commons: London, UK, 2016.
- Treasure, J.; Nazar, B.P. Interventions for the carers of patients with eating disorders. Curr. Psychiatry Rep. 2016, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.; Treasure, J.; Todd, G. Caring for a Loved One with an Eating Disorder: The New Maudsley Skills-Based Training Manual; Routledge: London, UK, 2018. [Google Scholar]
- Treasure, J.; Murphy, T.; Szmukler, T.; Todd, G.; Gavan, K.; Joyce, J. The experience of caregiving for severe mental illness: A comparison between anorexia nervosa and psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2001, 36, 343–347. [Google Scholar] [CrossRef]
- Magill, N.; Rhind, C.; Hibbs, R.; Goddard, E.; Macdonald, P.; Arcelus, J.; Treasure, J. Two-year follow-up of a pragmatic randomised controlled trial examining the effect of adding a carer’s skill training intervention in inpatients with anorexia nervosa. Eur. Eat. Disord. Rev. 2016, 24, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, R.; Magill, N.; Goddard, E.; Rhind, C.; Raenker, S.; Macdonald, P.; Schmidt, U. Clinical effectiveness of a skills training intervention for caregivers in improving patient and caregiver health following in-patient treatment for severe anorexia nervosa: Pragmatic randomised controlled trial. BJPsych Open 2015, 1, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hodsoll, J.; Rhind, C.; Micali, N.; Hibbs, R.; Goddard, E.; Nazar, B.P.; Landau, S. A pilot, multicentre pragmatic randomised trial to explore the impact of carer skills training on carer and patient behaviours: Testing the cognitive interpersonal model in adolescent anorexia nervosa. Eur. Eat. Disord. Rev. 2017, 25, 551–561. [Google Scholar] [CrossRef]
- Adamson, J.; Cardi, V.; Kan, C.; Harrison, A.; Macdonald, P.; Treasure, J. Evaluation of a novel transition support intervention in an adult eating disorders service: ECHOMANTRA. Int. Rev. Psychiatry 2019, 31, 382–390. [Google Scholar] [CrossRef]
- Pépin, G.; King, R. Collaborative care skills training workshops: Helping carers cope with eating disorders from the UK to Australia. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 805–812. [Google Scholar] [CrossRef]
- Quiles, M.Y.; Quiles, S.M.; Escolano, H.M.; Sanmartín, R.; Treasure, J. Testing carer skill training programs in Spanish carers of patients with eating disorders. Psicothema 2018, 30, 295. [Google Scholar]
- Schmidt, U.; Wade, T.D.; Treasure, J. The Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA): Development, key features, and preliminary evidence. J. Cog. Psychother. 2014, 28, 48–71. [Google Scholar] [CrossRef]
- Schmidt, U.; Startup, H.; Treasure, J. A Cognitive-Interpersonal Therapy Workbook for Treating Anorexia Nervosa: The Maudsley Model; Routledge: London, UK, 2018. [Google Scholar]
- Walsh, B.T. The enigmatic persistence of anorexia nervosa. Am. J. Psychiatry 2013, 170, 477–484. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Walsh, B.T. Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Uniacke, B.; Walsh, B.T.; Foerde, K.; Steinglass, J. The role of habits in anorexia nervosa: Where we are and where to go from here? Curr. Psychiatry Rep. 2018, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.E.; Berner, L.A.; Attia, E. Cognitive neuroscience of eating disorders. Psychiatr. Clin. 2019, 42, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.E.; Glasofer, D.R.; Walsh, E.; Guzman, G.; Peterson, C.B.; Walsh, B.T.; Wonderlich, S.A. Targeting habits in anorexia nervosa: A proof-of-concept randomized trial. Psychol. Med. 2018, 48, 2584–2591. [Google Scholar] [CrossRef]
- Levinson, C.A.; Brosof, L.C.; Ma, J.; Fewell, L.; Lenze, E.J. Fear of food prospectively predicts drive for thinness in an eating disorder sample recently discharged from intensive treatment. Eat. Behav. 2017, 27, 45–51. [Google Scholar] [CrossRef]
- Lambert, E.; Purves, K.; McGregor, T.; Treasure, J.; Cardi, V. Fear conditioning in women with a lifetime diagnosis of anorexia nervosa and healthy controls. In Preparation.
- Cardi, V.; Leppanen, J.; Mataix-Cols, D.; Campbell, I.C.; Treasure, J. A case series to investigate food-related fear learning and extinction using in vivo food exposure in anorexia nervosa: A clinical application of the inhibitory learning framework. Eur. Eat. Disord. Rev. 2019, 27, 173–181. [Google Scholar] [CrossRef]
- Ramjan, L.M. Nurses and the ‘therapeutic relationship’: Caring for adolescents with anorexia nervosa. J. Adv. Nurs. 2004, 45, 495–503. [Google Scholar] [CrossRef]
- Kimber, M.; McTavish, J.R.; Couturier, J.; Le Grange, D.; Lock, J.; MacMillan, H.L. Identifying and responding to child maltreatment when delivering family-based treatment—A qualitative study. Int. J. Eat. Disord. 2019, 52, 292–298. [Google Scholar] [CrossRef]
- Den, M.L.; Graham, B.M.; Newall, C.; Richardson, R. Teens that fear screams: A comparison of fear conditioning, extinction, and reinstatement in adolescents and adults. Dev. Psychobiol. 2015, 57, 818–832. [Google Scholar] [CrossRef]
- Koskina, A.; Campbell, I.C.; Schmidt, U. Exposure therapy in eating disorders revisited. Neurosci. Biobehav. Rev. 2013, 37, 193–208. [Google Scholar] [CrossRef]
- Murray, S.B.; Treanor, M.; Liao, B.; Loeb, K.L.; Griffiths, S.; Le Grange, D. Extinction theory & anorexia nervosa: Deepening therapeutic mechanisms. Behav. Res. Ther. 2016, 87, 1–10. [Google Scholar] [PubMed]
- Cardi, V.; Esposito, M.; Clarke, A.; Schifano, S.; Treasure, J. The impact of induced positive mood on symptomatic behaviour in eating disorders. An experimental, AB/BA crossover design testing a multimodal presentation during a test-meal. Appetite 2015, 87, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.; Sysko, R.; Schebendach, J.; Broft, A.; Strober, M.; Walsh, B.T. The application of exposure therapy and D-cycloserine to the treatment of anorexia nervosa: A preliminary trial. J. Psychiatr. Pract. 2007, 13, 238. [Google Scholar] [CrossRef]
- Levinson, C.A.; Rodebaugh, T.L.; Fewell, L.; Kass, A.E.; Riley, E.N.; Stark, L.; Lenze, E.J. D-Cycloserine facilitation of exposure therapy improves weight regain in patients with anorexia nervosa: A pilot randomized controlled trial. J. Clin. Psychiatry 2015, 76, e787. [Google Scholar] [CrossRef]
- Cardi, V.; Krug, I.; Perpiñá, C.; Mataix-Cols, D.; Roncero, M.; Treasure, J. The use of a nonimmersive virtual reality programme in anorexia nervosa: A single case-report. Eur. Eat. Disord. Rev. 2012, 20, 240–245. [Google Scholar] [CrossRef]
- Tchanturia, K.; Davies, H.; Reeder, C.; Wykes, T. Cognitive Remediation Therapy for AN Therapist Manual. 2010. Available online: http://www.katetchanturia.com/publications/c1y51 (accessed on 26 February 2020).
- Cardi, V.; Esposito, M.; Bird, G.; Rhind, C.; Yiend, J.; Schifano, S.; Treasure, J. A preliminary investigation of a novel training to target cognitive biases towards negative social stimuli in Anorexia Nervosa. J. Affect. Disord. 2015, 188, 188–193. [Google Scholar] [CrossRef]
- Turton, R.; Nazar, B.P.; Burgess, E.E.; Lawrence, N.S.; Cardi, V.; Treasure, J.; Hirsch, C.R. To go or not to go: A proof of concept study testing food-specific inhibition training for women with eating and weight disorders. Eur. Eat. Disord. Rev. 2018, 26, 11–21. [Google Scholar] [CrossRef]
- Werthmann, J.; Simic, M.; Konstantellou, A.; Mansfield, P.; Mercado, D.; van Ens, W.; Schmidt, U. Same, same but different: Attention bias for food cues in adults and adolescents with anorexia nervosa. Int. J. Eat. Disord. 2019, 52, 681–690. [Google Scholar] [CrossRef]
- Mercado, D.; Schmidt, U.; O’Daly, O.; Campbell, I.; Werthmann, J. Food related attention bias modification training for anorexia nervosa and its potential underpinning mechanisms. J. Eat. Disord. 2020, 8, 1. [Google Scholar] [CrossRef]
- Wildes, J.E.; Marcus, M.D.; Bright, A.C.; Dapelo, M.M. Emotion and eating disorder symptoms in patients with anorexia nervosa: An experimental study. Int. J. Eat. Disord. 2012, 45, 876–882. [Google Scholar] [CrossRef]
- Rodgers, R.F.; Paxton, S.J. The impact of indicated prevention and early intervention on co-morbid eating disorder and depressive symptoms: A systematic review. J. Eat. Disord. 2014, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Hoek, H.W.; Brewerton, T.D.; Elzinga, B.M. Childhood maltreatment and eating disorder pathology: A systematic review and dose-response meta-analysis. Psychol. Med. 2017, 47, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, A.M.; Mereu, A.; Cascino, G.; Criscuolo, M.; Castiglioni, M.C.; Pellegrino, F.; Zanna, V. Re-conceptualization of anorexia nervosa psychopathology: A network analysis study in adolescents with short duration of the illness. Int. J. Eat. Disord. 2019, 52, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Sauro, C.L.; Ravaldi, C.; Cabras, P.L.; Faravelli, C.; Ricca, V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology 2008, 57, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.W.; Lewis, G.; Easter, A.; Lewis, G.; Solmi, F. Eighteen-year trajectories of depressive symptoms in mothers with a lifetime eating disorder: Findings from the ALSPAC cohort. Br. J. Psychiatry 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Tomba, E.; Tecuta, L.; Crocetti, E.; Squarcio, F.; Tomei, G. Residual eating disorder symptoms and clinical features in remitted and recovered eating disorder patients: A systematic review with meta-analysis. Int. J. Eat. Disord. 2019, 52, 759–776. [Google Scholar] [CrossRef]
- Godart, N.; Radon, L.; Curt, F.; Duclos, J.; Perdereau, F.; Lang, F.; Corcos, M. Mood disorders in eating disorder patients: Prevalence and chronology of ONSET. J. Affect. Disord. 2015, 185, 115–122. [Google Scholar] [CrossRef]
- Dobrescu, S.R.; Dinkler, L.; Gillberg, C.; Råstam, M.; Gillberg, C.; Wentz, E. Anorexia nervosa: 30-year outcome. Br. J. Psychiatry 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Solmi, M.; Collantoni, E.; Meneguzzo, P.; Tenconi, E.; Favaro, A. Network analysis of specific psychopathology and psychiatric symptoms in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2019, 27, 24–33. [Google Scholar] [CrossRef]
- Voderholzer, U.; Hessler-Kaufmann, J.B.; Lustig, L.; Läge, D. Comparing severity and qualitative facets of depression between eating disorders and depressive disorders: Analysis of routine data. J. Affect. Disord. 2019, 257, 758–764. [Google Scholar] [CrossRef]
- Keski-Rahkonen, A.; Raevuori, A.; Bulik, C.M.; Hoek, H.W.; Rissanen, A.; Kaprio, J. Factors associated with recovery from anorexia nervosa: A population-based study. Int. J. Eat. Disord. 2014, 47, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Franko, D.L.; Tabri, N.; Keshaviah, A.; Murray, H.B.; Herzog, D.B.; Thomas, J.J.; Eddy, K.T. Predictors of long-term recovery in anorexia nervosa and bulimia nervosa: Data from a 22-year longitudinal study. J. Psychiatr. Res. 2018, 96, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.K.; Sebar, B.; Lee, J. Reclamation of power and self: A meta-synthesis exploring the process of recovery from anorexia nervosa. Adv. Eat. Disord. Theory Res. Pract. 2015, 3, 177–190. [Google Scholar] [CrossRef]
- de Vos, J.A.; LaMarre, A.; Radstaak, M.; Bijkerk, C.A.; Bohlmeijer, E.T.; Westerhof, G.J. Identifying fundamental criteria for eating disorder recovery: A systematic review and qualitative meta-analysis. J. Eat. Disord. 2017, 5, 34. [Google Scholar] [CrossRef]
- Measelle, J.R.; Stice, E.; Hogansen, J.M. Developmental trajectories of co-occurring depressive, eating, antisocial, and substance abuse problems in female adolescents. J. Abnorm. Psychol. 2006, 115, 524. [Google Scholar] [CrossRef]
- Degortes, D.; Zanetti, T.; Tenconi, E.; Santonastaso, P.; Favaro, A. Childhood obsessive–compulsive traits in anorexia nervosa patients, their unaffected sisters and healthy controls: A retrospective study. Eur. Eat. Disord. Rev. 2014, 22, 237–242. [Google Scholar] [CrossRef]
- Lilenfeld, L.R.; Kaye, W.H.; Greeno, C.G.; Merikangas, K.R.; Plotnicov, K.; Pollice, C.; Nagy, L. A controlled family study of anorexia nervosa and bulimia nervosa: Psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch. Gen. Psychiatry 1998, 55, 603–610. [Google Scholar] [CrossRef]
- Cederlöf, M.; Thornton, L.M.; Baker, J.; Lichtenstein, P.; Larsson, H.; Rück, C.; Mataix-Cols, D. Etiological overlap between obsessive-compulsive disorder and anorexia nervosa: A longitudinal cohort, multigenerational family and twin study. World Psychiatry 2015, 14, 333–338. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Halvorsen, M.; Bryois, J.; Yu, D.; Thornton, L.M.; Zerwas, S.; Erdman, L. Examination of the shared genetic basis of anorexia nervosa and obsessive–compulsive disorder. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Forrest, L.N.; Jones, P.J.; Ortiz, S.N.; Smith, A.R. Core psychopathology in anorexia nervosa and bulimia nervosa: A network analysis. Int. J. Eat. Disord. 2018, 51, 668–679. [Google Scholar] [CrossRef]
- Montigny, C.; Castellanos-Ryan, N.; Whelan, R.; Banaschewski, T.; Barker, G.J.; Büchel, C.; Nees, F. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS ONE 2013, 8, e80151. [Google Scholar] [CrossRef] [PubMed]
- Fonville, L.; Giampietro, V.; Williams, S.C.R.; Simmons, A.; Tchanturia, K. Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychol. Med. 2014, 44, 1965–1975. [Google Scholar] [CrossRef]
- Connan, F.; Treasure, J. Stress, eating and neurobiology. In Neurobiology in the Treatment of Eating Disorders; Wiley: Chichester, UK, 1998; pp. 211–236. [Google Scholar]
- Myrvang, A.D.; Vangberg, T.R.; Stedal, K.; Rø, Ø.; Endestad, T.; Rosenvinge, J.H.; Aslaksen, P.M. Hippocampal subfields in adolescent anorexia nervosa. Psychiatry Res. Neuroimaging 2018, 282, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.; Joos, A.; Tebartz van Elst, L.; Matthis, J.; Holovics, L.; Endres, D.; Maier, S. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int. J. Eat. Disord. 2018, 51, 1056–1069. [Google Scholar] [CrossRef]
- Chui, H.T.; Christensen, B.K.; Zipursky, R.B.; Richards, B.A.; Hanratty, M.K.; Kabani, N.J.; Katzman, D.K. Cognitive function and brain structure in females with a history of adolescent-onset anorexia nervosa. Paediatrics 2008, 122, e426–e437. [Google Scholar] [CrossRef] [PubMed]
- Fladung, A.K.; Grön, G.; Grammer, K.; Herrnberger, B.; Schilly, E.; Grasteit, S.; von Wietersheim, J. A neural signature of anorexia nervosa in the ventral striatal reward system. Am. J. Psychiatry 2009, 167, 206–212. [Google Scholar] [CrossRef]
- Fladung, A.K.; Schulze, U.M.E.; Schöll, F.; Bauer, K.; Grön, G. Role of the ventral striatum in developing anorexia nervosa. Transl. Psychiatry 2013, 3, e315. [Google Scholar] [CrossRef]
- Chami, R.; Treasure, J. The Neurobiology of Trauma and Eating Disorders. Trauma-Informed Approaches to Eating Disorders; Springer Publishing Company: New York, NY, USA, 2018. [Google Scholar]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Nestler, E.J. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 2013, 170, 1114–1133. [Google Scholar] [CrossRef]
- Putignano, P.; Dubini, A.; Toja, P.; Invitti, C.; Bonfanti, S.; Redaelli, G.; Cavagnini, F. Salivary cortisol measurement in normal-weight, obese and anorexic women: Comparison with plasma cortisol. Eur. J. Endocrinol. 2001, 145, 165–171. [Google Scholar] [CrossRef]
- Misra, M.; Klibanski, A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014, 2, 581–592. [Google Scholar] [CrossRef]
- Chami, R.; Monteleone, A.M.; Treasure, J.; Monteleone, P. Stress hormones and eating disorders. Mol. Cell. Endocrinol. 2018, 497, 110349. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.; Campbell, I.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. Inflammatory Markers in Anorexia Nervosa: An Exploratory Study. Nutrients 2018, 10, 1573. [Google Scholar] [CrossRef]
- Dalton, B.; Leppanen, J.; Campbell, I.C.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav. Immun. 2019. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B.; Seitz, J.; Baines, J. Food matters: How the microbiome and gut–brain interaction might impact the development and course of anorexia nervosa. Eur. Child Adolesc. Psychiatry 2017, 26, 1031–1041. [Google Scholar] [CrossRef]
- Seitz, J.; Trinh, S.; Herpertz-Dahlmann, B. The microbiome and eating disorders. Psychiatr. Clin. 2019, 42, 93–103. [Google Scholar] [CrossRef]
- Barona, M.; Brown, M.; Clark, C.; Frangou, S.; White, T.; Micali, N. White matter alterations in anorexia nervosa: Evidence from a voxel-based meta-analysis. Neurosc. Biobehav. Rev. 2019, 100, 285–295. [Google Scholar] [CrossRef]
- Connan, F.; Murphy, F.; Connor, S.E.; Rich, P.; Murphy, T.; Bara-Carill, N.; Morris, R.G. Hippocampal volume and cognitive function in anorexia nervosa. Psychiatry Res. Neuroimaging 2006, 146, 117–125. [Google Scholar] [CrossRef]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.H.; Russo, S.J. Neurobiology of resilience: Interface between mind and body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef]
- Collo, G.; Pich, E.M. A human translational model based on neuroplasticity for pharmacological agents potentially effective in Treatment-Resistant Depression: Focus on dopaminergic system. Neural Regen. Res. 2020, 15, 1027. [Google Scholar] [CrossRef]
- Schmidt, U.; Oldershaw, A.; Jichi, F.; Sternheim, L.; Startup, H.; McIntosh, V.; Landau, S. Out-patient psychological therapies for adults with anorexia nervosa: Randomised controlled trial. Br. J. Psychiatry 2012, 201, 392–399. [Google Scholar] [CrossRef]
- Albano, G.; Hodsoll, J.; Kan, C.; Lo Coco, G.; Cardi, V. Task-sharing interventions for patients with anorexia nervosa or their carers: A systematic evaluation of the literature and meta-analysis of outcomes. Int. Rev. Psychiatry 2019, 31, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Magill, N.; Renwick, B.; Keyes, A.; Kenyon, M.; Dejong, H.; Watson, C. The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): Comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: A randomized controlled trial. J. Consult. Clin. Psychol. 2015, 83, 796. [Google Scholar] [PubMed]
- Lipsman, N.; Woodside, D.B.; Giacobbe, P.; Hamani, C.; Carter, J.C.; Norwood, S.J.; Smith, G.S. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: A phase 1 pilot trial. Lancet 2013, 381, 1361–1370. [Google Scholar] [CrossRef]
- McClelland, J.; Kekic, M.; Bozhilova, N.; Nestler, S.; Dew, T.; Van den Eynde, F.; Schmidt, U. A randomised controlled trial of neuronavigated repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PLoS ONE 2016, 11, e0148606. [Google Scholar] [CrossRef]
- Dalton, B.; Bartholdy, S.; McClelland, J.; Kekic, M.; Rennalls, S.J.; Werthmann, J.; Glennon, D. Randomised controlled feasibility trial of real versus sham repetitive transcranial magnetic stimulation treatment in adults with severe and enduring anorexia nervosa: The TIARA study. BMJ Open 2018, 8, e021531. [Google Scholar] [CrossRef]
- Knyahnytska, Y.O.; Blumberger, D.M.; Daskalakis, Z.J.; Zomorrodi, R.; Kaplan, A.S. Insula H-coil deep transcranial magnetic stimulation in severe and enduring anorexia nervosa (SE-AN): A pilot study. Neuropsychiatr. Dis. Treat. 2019, 15, 2247. [Google Scholar] [CrossRef]
- Lipsman, N.; Lam, E.; Volpini, M.; Sutandar, K.; Twose, R.; Giacobbe, P.; Lozano, A.M. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry 2017, 4, 285–294. [Google Scholar] [CrossRef]
- Himmerich, H.; Treasure, J. Psychopharmacological advances in eating disorders. Expert Rev. Clin. Pharmacol. 2018, 11, 95–108. [Google Scholar] [CrossRef]
- Dold, M.; Aigner, M.; Klabunde, M.; Treasure, J.; Kasper, S. Second-generation antipsychotic drugs in anorexia nervosa: A meta-analysis of randomized controlled trials. Psychother. Psychosom. 2015, 84, 110–116. [Google Scholar] [CrossRef]
- Attia, E.; Steinglass, J.E.; Walsh, B.T.; Wang, Y.; Wu, P.; Schreyer, C.; Marcus, M.D. Olanzapine versus placebo in adult outpatients with anorexia nervosa: A randomized clinical trial. Am. J. Psychiatry 2019, 176, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-compulsive disorder: Advances in diagnosis and treatment. JAMA 2017, 317, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.F.; Yücel, M. A Clinical Staging Model for Obsessive–Compulsive Disorder: Is It Ready for Prime Time? EClinicalMedicine 2019, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Rucker, J.J.; Iliff, J.; Nutt, D.J. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [PubMed]
- Mills, I.H.; Park, G.R.; Manara, A.R.; Merriman, R.J. Treatment of compulsive behaviour in eating disorders with intermittent ketamine infusions. QJM Mon. J. Assoc. Physicians 1998, 91, 493–503. [Google Scholar] [CrossRef]
- Cardi, V.; Albano, G.; Ambwani, S.; Cao, L.; Crosby, R.D.; Macdonald, P.; Treasure, J. A randomised clinical trial to evaluate the acceptability and efficacy of an early phase, online, guided augmentation of outpatient care for adults with anorexia nervosa. Psychol. Med. 2019, 16, 1–12. [Google Scholar] [CrossRef]
- Cardi, V.; Ambwani, S.; Robinson, E.; Albano, G.; MacDonald, P.; Aya, V.; Arcelus, J. Transition care in anorexia nervosa through guidance online from peer and carer expertise (TRIANGLE): Study protocol for a randomised controlled trial. Eur. Eat. Disord. Rev. 2017, 25, 512–523. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treasure, J.; Willmott, D.; Ambwani, S.; Cardi, V.; Clark Bryan, D.; Rowlands, K.; Schmidt, U. Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness. J. Clin. Med. 2020, 9, 630. https://doi.org/10.3390/jcm9030630
Treasure J, Willmott D, Ambwani S, Cardi V, Clark Bryan D, Rowlands K, Schmidt U. Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness. Journal of Clinical Medicine. 2020; 9(3):630. https://doi.org/10.3390/jcm9030630
Chicago/Turabian StyleTreasure, Janet, Daniel Willmott, Suman Ambwani, Valentina Cardi, Danielle Clark Bryan, Katie Rowlands, and Ulrike Schmidt. 2020. "Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness" Journal of Clinical Medicine 9, no. 3: 630. https://doi.org/10.3390/jcm9030630
APA StyleTreasure, J., Willmott, D., Ambwani, S., Cardi, V., Clark Bryan, D., Rowlands, K., & Schmidt, U. (2020). Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness. Journal of Clinical Medicine, 9(3), 630. https://doi.org/10.3390/jcm9030630





