Long-Term Influence of Platelet-Rich Plasma (PRP) on Dental Implants after Maxillary Augmentation: Implant Survival and Success Rates
Abstract
1. Introduction
- 3 mL PRP (drops) on 3 cm3 of cancellous bone;
- 0.5 mL calcium gluconate 10% (drops) on 3 cm3 of cancellous bone;
- 0.5mL of native wound blood (drops) on 3 cm3 of cancellous bone.
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Examiner Blinding and Calibration
2.3. Study Parameters
2.4. Statistical Analysis
2.5. Ethics and Privacy
3. Results
3.1. General Implants Evaluation
3.1.1. Patients Gender, Age, and Smoking Behavior
3.1.2. Implant Numbers and Survival Age
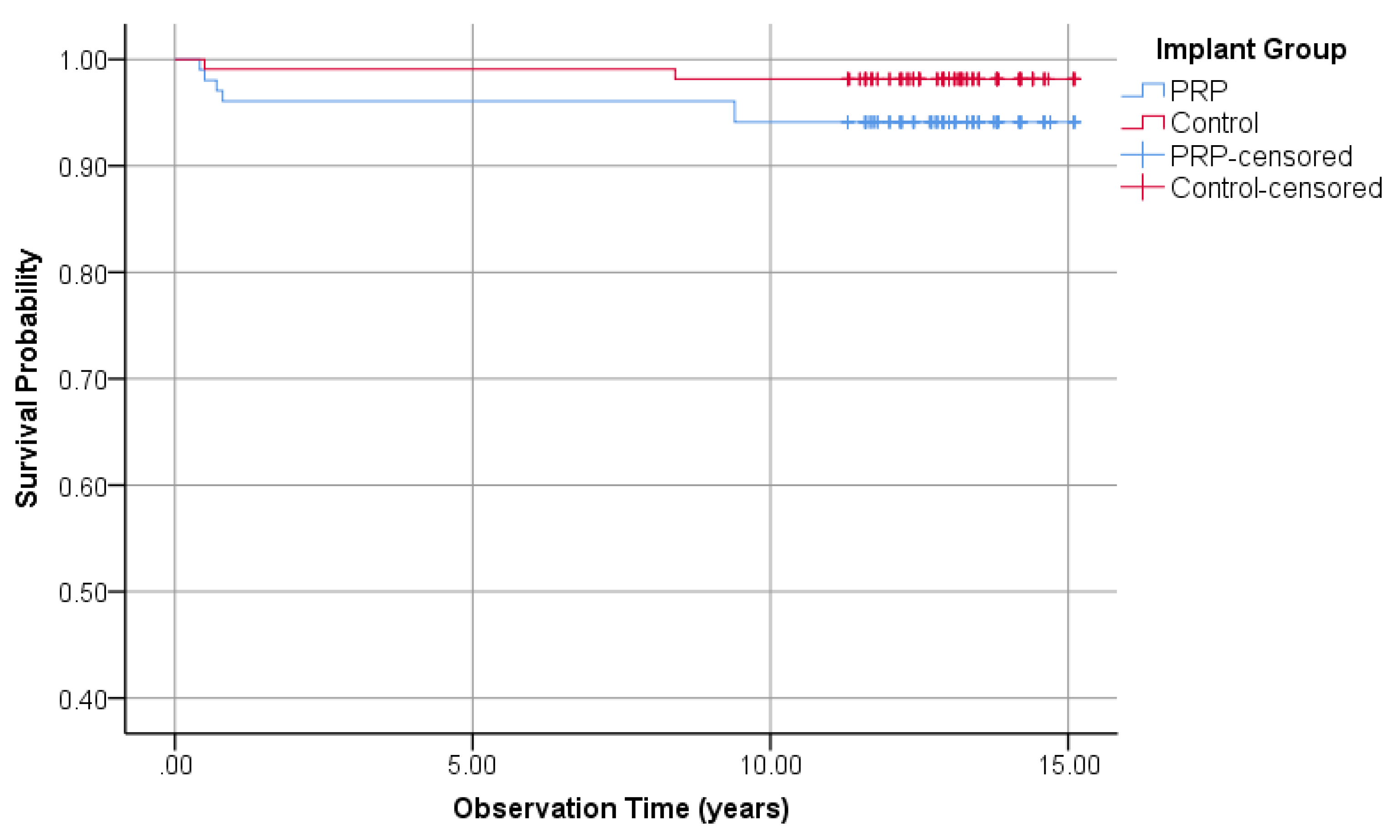
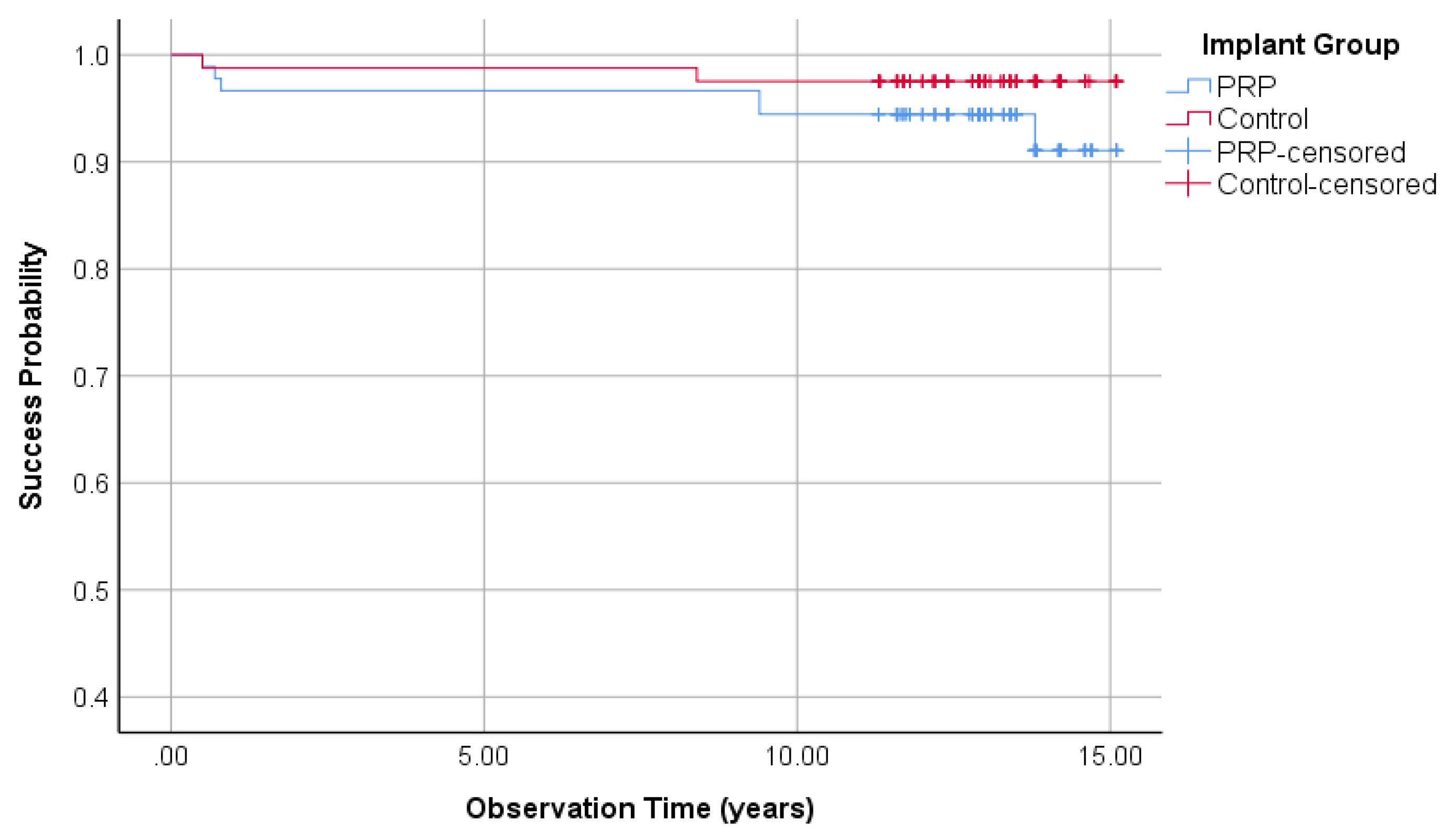
3.1.3. Survival Rate of Implants
3.1.4. Implant Success Rate Regarding Buser
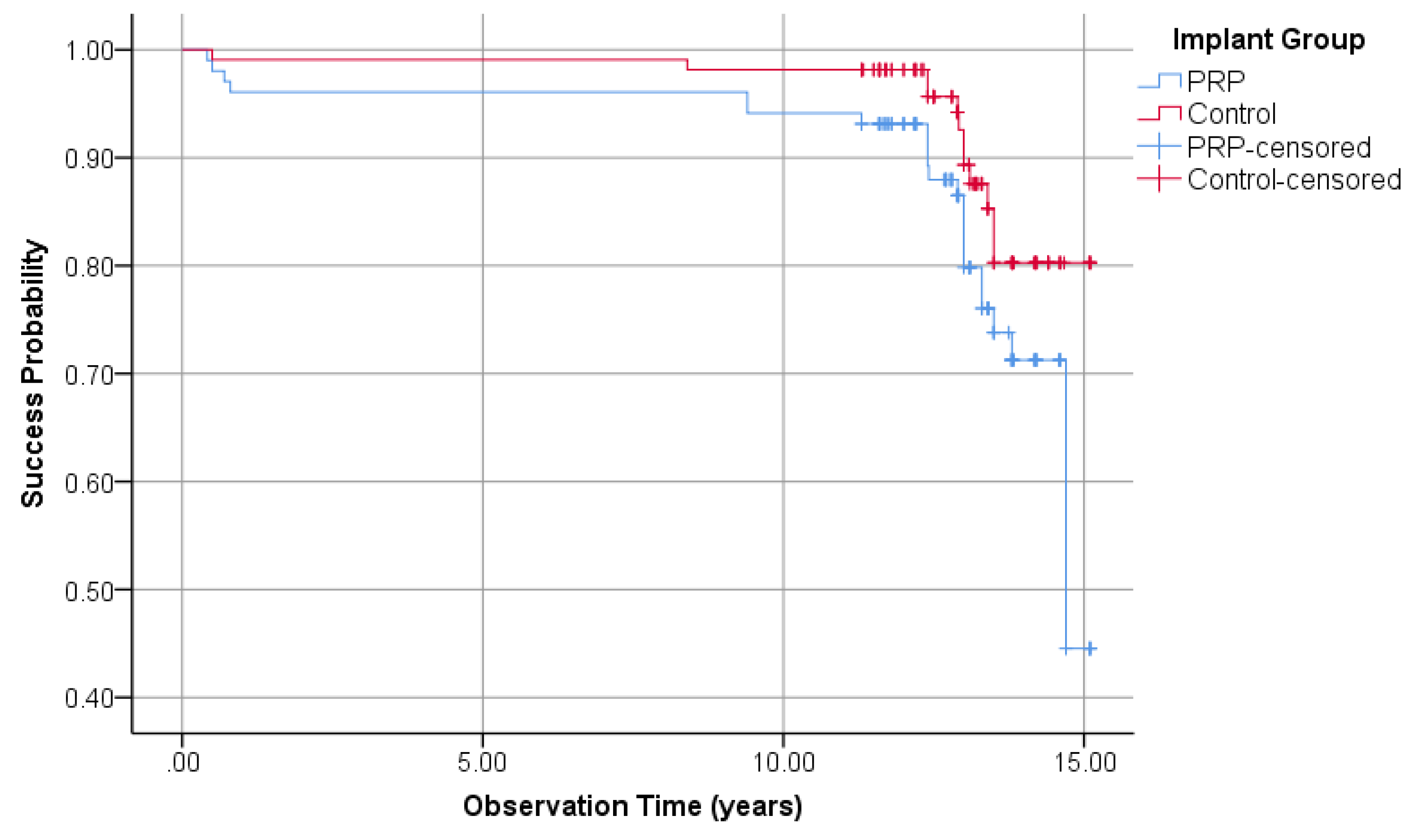
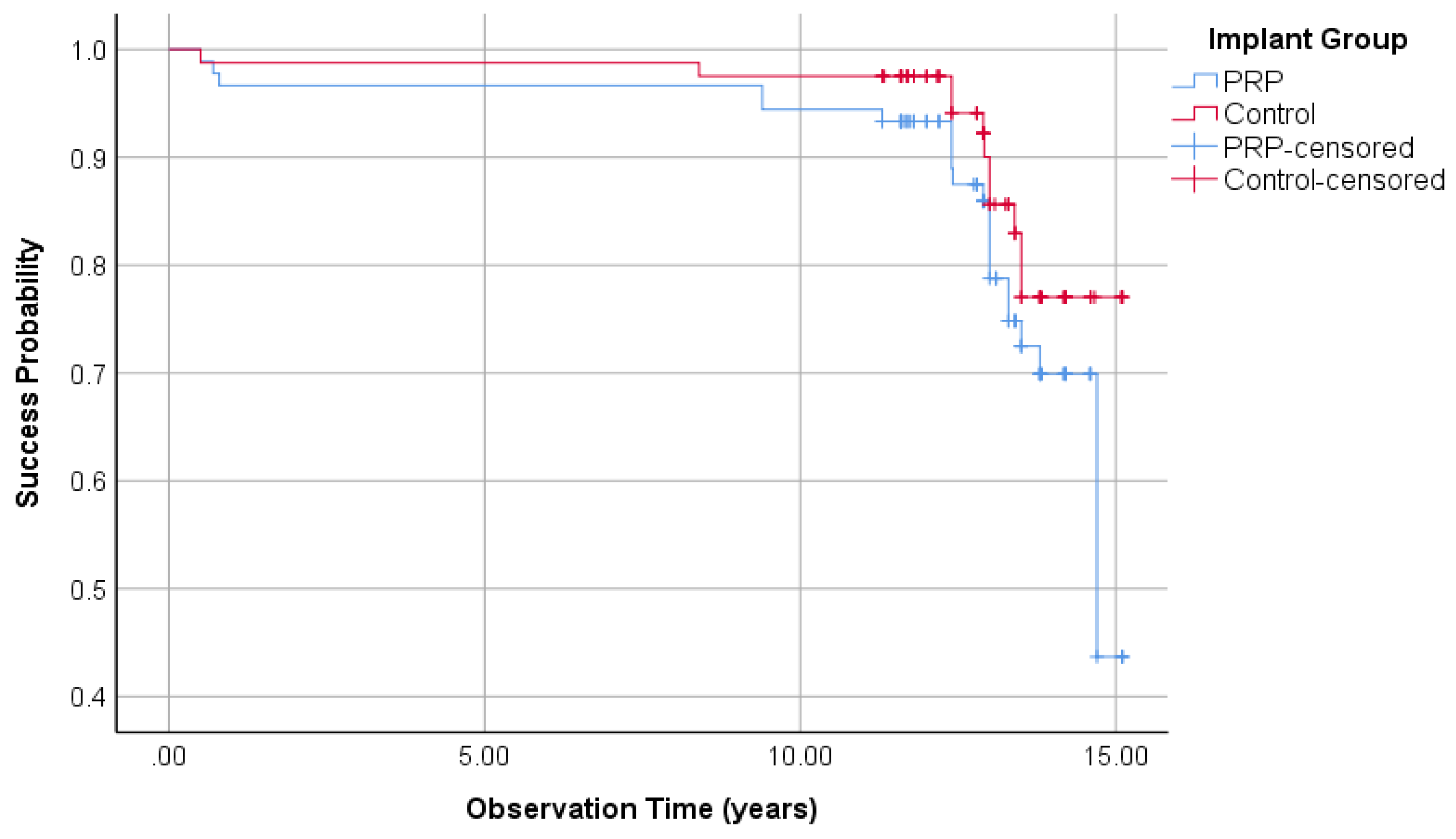
3.1.5. Implant Success Regarding Albrektsson
3.2. Split-Mouth Evaluation
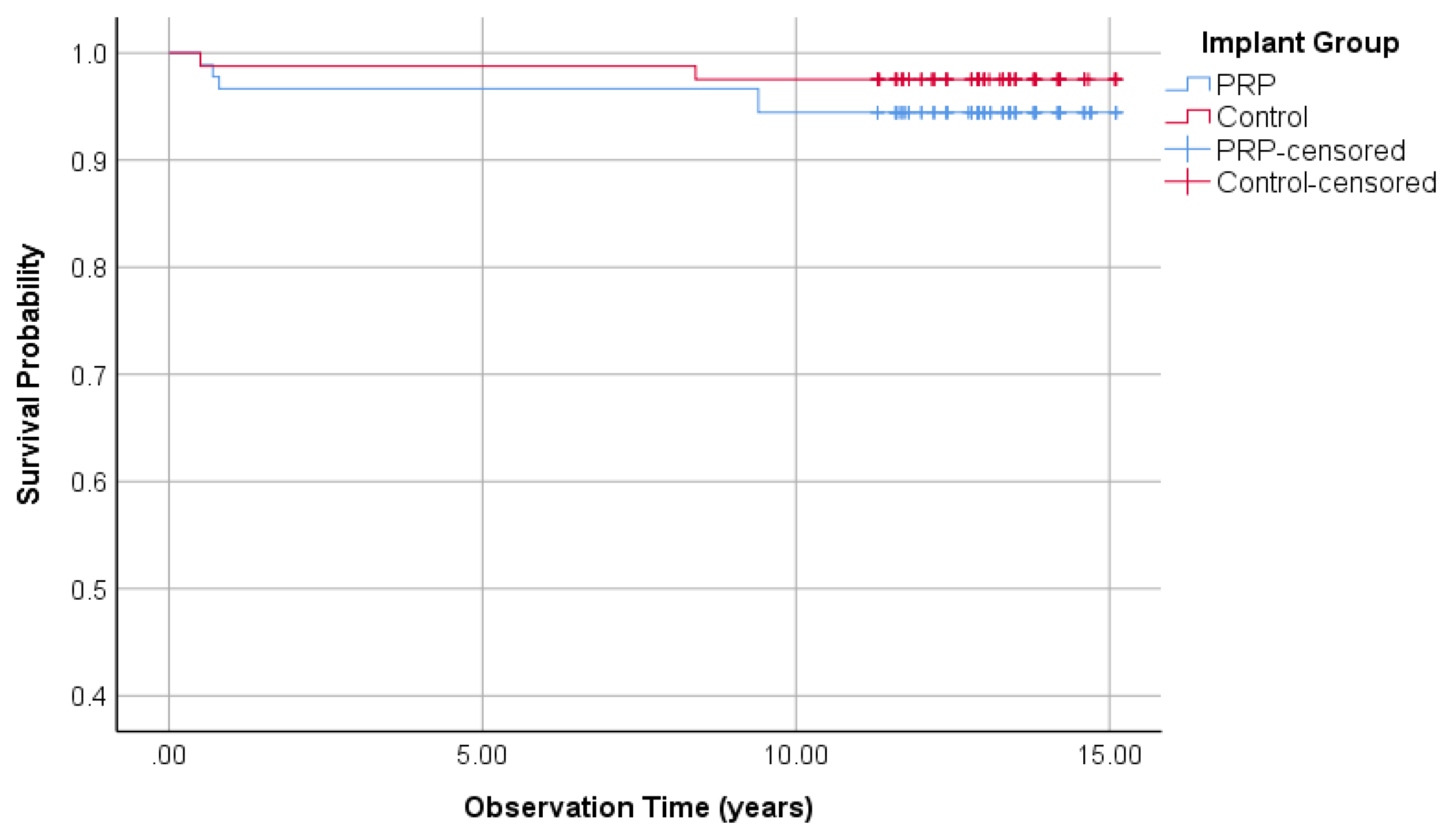
3.2.1. Survival Rate of the Implants (Split-Mouth)
3.2.2. Implant Success Rate Regarding Buser (Split-Mouth)
3.2.3. Implant Success Regarding Albrektsson (Split-Mouth)
3.3. Overall Evaluation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Helmi, M.F.; Huang, H.; Goodson, J.M.; Hasturk, H.; Tavares, M.; Natto, Z.S. Prevalence of periodontitis and alveolar bone loss in a patient population at Harvard School of Dental Medicine. BMC Oral Health 2019, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2009. [CrossRef]
- Zohrabian, V.M.; Sonick, M.; Hwang, D.; Abrahams, J.J. Dental Implants. Semin. Ultrasound CT MR 2015, 36, 415–426. [Google Scholar] [CrossRef]
- Watzek, G.; Fürhauser, R.; Mailath, G. Zahnärztliche Implantate. In Zahnärztliche Chirurgie, 4., vollst. überarb. und erw. Aufl. ed.; Schwenzer, N., Eckelt, U., Eds.; Thieme: Stuttgart, Germany, 2009; pp. 227–271. [Google Scholar]
- Pommer, B.; Frantal, S.; Willer, J.; Posch, M.; Watzek, G.; Tepper, G. Impact of dental implant length on early failure rates: A meta-analysis of observational studies. J. Clin. Periodontol. 2011, 38, 856–863. [Google Scholar] [CrossRef]
- Thor, A.; Franke-Stenport, V.; Johansson, C.B.; Rasmusson, L. Early bone formation in human bone grafts treated with platelet-rich plasma: Preliminary histomorphometric results. Int. J. Oral Maxillofac. Surg. 2007, 36, 1164–1171. [Google Scholar] [CrossRef]
- Ortolani, E.; Guerriero, M.; Coli, A.; Di Giannuario, A.; Minniti, G.; Polimeni, A. Effect of PDGF, IGF-1 and PRP on the implant osseointegration. An histological and immunohistochemical study in rabbits. Ann. Stomatol. 2014, 5, 66–68. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Nikolidakis, D.; Mulder, J.; Jansen, J.A.; Creugers, N.H.J. Effect of platelet-rich plasma on bone regeneration in dentistry: A systematic review. Clin. Oral Implants Res. 2008, 19, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, I.; Tsantis, S.; Georgakopoulos, P.; Korfiatis, P.; Fanti, E.; Martelli, M.; Costaridou, L.; Petsas, T.; Panayiotakis, G.; Martelli, F.S. The impact of Platelet Rich Plasma (PRP) in osseointegration of oral implants in dental panoramic radiography: Texture based evaluation. Clin. Cases Miner Bone Metab. 2014, 11, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wu, Y.-P.; Qian, S.-J.; Teng, C.; Chen, S.; Li, H. Research Progress in the Mechanism of Effect of PRP in Bone Deficiency Healing. Sci. World J. 2013, 2013, 134582. [Google Scholar] [CrossRef]
- Aimetti, M.; Romano, F.; Dellavia, C.; Paoli, S.D. Sinus grafting using autogenous bone and platelet-rich plasma: Histologic outcomes in humans. Int. J. Periodontics Restorative Dent. 2008, 28, 585–591. [Google Scholar]
- Consolo, U.; Zaffe, D.; Bertoldi, C.; Ceccherelli, G. Platelet-rich plasma activity on maxillary sinus floor augmentation by autologous bone. Clin. Oral Implants Res. 2007, 18, 252–262. [Google Scholar] [CrossRef]
- Khairy, N.M.; Shendy, E.E.; Askar, N.A.; El-Rouby, D.H. Effect of platelet rich plasma on bone regeneration in maxillary sinus augmentation (randomized clinical trial). Int. J. Oral Maxillofac. Surg. 2013, 42, 249–255. [Google Scholar] [CrossRef]
- Bettega, G.; Brun, J.-P.; Boutonnat, J.; Cracowski, J.-L.; Quesada, J.-L.; Hegelhofer, H.; Drillat, P.; Richard, M.J. Autologous platelet concentrates for bone graft enhancement in sinus lift procedure. Transfusion 2009, 49, 779–785. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Schortinghuis, J.; Liem, R.S.B.; Ruben, J.L.; van der Wal, J.E.; Vissink, A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin. Oral Implants Res. 2005, 16, 349–356. [Google Scholar] [CrossRef]
- Arora, N.S.; Ramanayake, T.; Ren, Y.-F.; Romanos, G.E. Platelet-Rich Plasma in Sinus Augmentation Procedures: A Systematic Literature Review: Part II. Implant Dent. 2010, 19, 145–157. [Google Scholar] [CrossRef]
- Schaaf, H.; Streckbein, P.; Lendeckel, S.; Heidinger, K.; Görtz, B.; Bein, G.; Boedeker, R.-H.; Schlegel, K.A.; Howaldt, H.-P. Topical use of platelet-rich plasma to influence bone volume in maxillary augmentation: A prospective randomized trial. Vox Sang. 2008, 94, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, H.; Streckbein, P.; Lendeckel, S.; Heidinger, K.S.; Rehmann, P.; Boedeker, R.-H.; Howaldt, H.-P. Sinus lift augmentation using autogenous bone grafts and platelet-rich plasma: Radiographic results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Khouly, I.; Pardiñas López, S.; Aliaga, I.; Froum, S.J. Long-Term Implant Survival After 100 Maxillary Sinus Augmentations Using Plasma Rich in Growth Factors. Implant Dent. 2017, 26, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Arad, D.; Ofec, R.; Eliyahu, G.; Ruban, A.; Sterer, N. Long Term Follow-Up of Dental Implants Placed in Autologous Onlay Bone Graft. Clin. Implant Dent. Relat. Res. 2016, 18, 449–461. [Google Scholar] [CrossRef]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Marginal bone-level alterations at implants installed in block versus particulate onlay bone grafts mixed with platelet-rich plasma in atrophic maxilla. a prospective 5-year follow-up study of 15 patients. Clin. Implant Dent. Relat. Res. 2013, 15, 7–14. [Google Scholar] [CrossRef]
- Robiony, M.; Zorzan, E.; Polini, F.; Sembronio, S.; Toro, C.; Politi, M. Osteogenesis distraction and platelet-rich plasma: Combined use in restoration of severe atrophic mandible. Long-term results. Clin. Oral Implants Res. 2008, 19, 1202–1210. [Google Scholar] [CrossRef]
- Velich, N.; Németh, Z.; Tóth, C.; Szabó, G. Long-term results with different bone substitutes used for sinus floor elevation. J. Craniofac. Surg. 2004, 15, 38–41. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Buser, D.; Weber, H.P.; Lang, N.P. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implants Res. 1990, 1, 33–40. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Moraschini, V.; Poubel, L.A.D.C.; Ferreira, V.F.; Barboza, E.D.S.P. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Schaaf, H.; El Khassawna, T.; Malhan, D.; Mausbach, K.; Howaldt, H.-P.; Streckbein, P. Oral Rehabilitation of Hypodontia Patients Using an Endosseous Dental Implant: Functional and Aesthetic Results. J. Clin. Med. 2019, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Schaper, E.; Schaaf, H.; Pons-Kühnemann, J.; Schlenz, M.A.; Streckbein, P.; Böttger, S.; Howaldt, H.-P.; Wilbrand, J.-F. Evaluation of Implant Success in Patients with Dental Aplasia. BioMed Res. Int. 2019, 2019, 1680158. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Wu, Y.; Wang, X.D.; Huang, W.; Zhang, Z.; Zhang, Z. A retrospective 3- to 5-year study of the reconstruction of oral function using implant-supported prostheses in patients with hypohidrotic ectodermal dysplasia. J. Oral Implantol. 2014, 40, 571–580. [Google Scholar] [CrossRef]
- Stang, A. Randomized controlled trials—An indispensible part of clinical research. Deutsches Ärzteblatt Int. 2011, 108, 661. [Google Scholar]
- Buch, R.S.; Weibrich, G.; Wagner, W. Criteria of success in implantology. Mund Kiefer Gesichtschir 2003, 7, 42–46. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 307–318. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Del Fabbro, M. Long-term outcomes for the treatment of atrophic posterior maxilla: A systematic review of literature. Clin. Implant Dent. Relat. Res. 2015, 17, 120–132. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Marrelli, M.; Paduano, F.; Tatullo, M. Human periapical cyst–mesenchymal stem cells differentiate into neuronal cells. J. Dent. Res. 2015, 94, 843–852. [Google Scholar] [CrossRef]
- Ray, S.; Thormann, U.; Eichelroth, M.; Budak, M.; Biehl, C.; Rupp, M.; Sommer, U.; El Khassawna, T.; Alagboso, F.I.; Kampschulte, M.; et al. Strontium and bisphosphonate coated iron foam scaffolds for osteoporotic fracture defect healing. Biomaterials 2018, 157, 1–16. [Google Scholar] [CrossRef] [PubMed]

| Parameter | PRP-Group | Control-Group | |||
|---|---|---|---|---|---|
| Value | Rating | Value | Rating | ||
| General-Evaluation | Survival rate | 94.1% | - | 98.1% | + |
| Cumulative survival rate | 94.1% | - | 98.1% | + | |
| Success according to Buser | 93.1% | - | 98.1% | + | |
| Cumulative success according to Buser | 90.9% | - | 98.6% | + | |
| Success according to Albrektsson | 77.5% | - | 88.9% | + | |
| Cumulative success according to Albrektsson | 44.5% | - | 80.3% | + | |
| Mean years of Evaluation | 12.53 | 12.90 | |||
| Split-Mouth-Evaluation | Survival rate | 94.4% | - | 97.5% | + |
| Cumulative survival rate | 94.4% | - | 97.5% | + | |
| Success according to Buser | 93.3% | - | 97.5% | + | |
| Cumulative success according to Buser | 91.1% | - | 97.5% | + | |
| Success according to Albrektsson | 75.6% | - | 86.4% | + | |
| Cumulative success according to Albrektsson | 43.7% | - | 77% | + | |
| Mean years of Evaluation | 13.1 | 13.1 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, S.; Narberhaus, C.; Schaaf, H.; Streckbein, P.; Pons-Kühnemann, J.; Schmitt, C.; Neukam, F.W.; Howaldt, H.-P.; Böttger, S. Long-Term Influence of Platelet-Rich Plasma (PRP) on Dental Implants after Maxillary Augmentation: Implant Survival and Success Rates. J. Clin. Med. 2020, 9, 391. https://doi.org/10.3390/jcm9020391
Attia S, Narberhaus C, Schaaf H, Streckbein P, Pons-Kühnemann J, Schmitt C, Neukam FW, Howaldt H-P, Böttger S. Long-Term Influence of Platelet-Rich Plasma (PRP) on Dental Implants after Maxillary Augmentation: Implant Survival and Success Rates. Journal of Clinical Medicine. 2020; 9(2):391. https://doi.org/10.3390/jcm9020391
Chicago/Turabian StyleAttia, Sameh, Clara Narberhaus, Heidrun Schaaf, Philipp Streckbein, Jörn Pons-Kühnemann, Christian Schmitt, Friedrich Wilhelm Neukam, Hans-Peter Howaldt, and Sebastian Böttger. 2020. "Long-Term Influence of Platelet-Rich Plasma (PRP) on Dental Implants after Maxillary Augmentation: Implant Survival and Success Rates" Journal of Clinical Medicine 9, no. 2: 391. https://doi.org/10.3390/jcm9020391
APA StyleAttia, S., Narberhaus, C., Schaaf, H., Streckbein, P., Pons-Kühnemann, J., Schmitt, C., Neukam, F. W., Howaldt, H.-P., & Böttger, S. (2020). Long-Term Influence of Platelet-Rich Plasma (PRP) on Dental Implants after Maxillary Augmentation: Implant Survival and Success Rates. Journal of Clinical Medicine, 9(2), 391. https://doi.org/10.3390/jcm9020391






