Abstract
Randomized controlled clinical trials and real-life observations indicate that less than 50% of patients with Crohn’s disease (CD) or ulcerative colitis (UC) respond to vedolizumab, a humanized monoclonal antibody that blocks the α4β7 integrin. Since α4β7-expressing lymphocytes mainly infiltrate the left colon, we assessed whether localization of CD and UC influences vedolizumab-induced remission. One hundred and eighty-one patients (74 CD and 107 UC) receiving vedolizumab in 3 referral centers were retrospectively evaluated for clinical remission at week 14. Demographic and clinical characteristics were compared between remitters and non-responders, and multivariable multinomial analysis was performed to identify predictors of remission. Remission was achieved in 17 CD (23%) and 34 UC (32%) patients, respectively. In CD, localization of the lesions did not influence clinical remission. In UC, the remitters had more frequently a distal/left-sided colitis (21/34, 62%) as compared to the non-responders (9/47, 19%), and extensive colitis was more frequent in the non-responders (38/47, 81%) than in the remitters (13/34, 38%). The multivariable multinomial analysis showed that distal/left-sided colitis was associated with a higher probability of clinical remission while extensive colitis was inversely associated with induction of remission. Data indicate that UC patients with distal or left-sided colitis are more likely to achieve remission than patients with extensive colitis following vedolizumab treatment.
1. Introduction
In the last decades, a better understanding of the molecular mechanisms, which drive the pathological process in the gut of patients with Crohn’s disease (CD) and ulcerative colitis (UC), the major forms of inflammatory bowel diseases (IBD), has facilitated the development of drugs promoting resolution of mucosal inflammation and healing of the gut lesions [1,2,3,4,5].
One such a drug is vedolizumab, a gut-selective humanized monoclonal antibody that binds to the α4β7 integrin and selectively reduces intestinal immune cell trafficking, thereby providing a safe and effective treatment option for patients with IBD [6,7,8,9,10,11,12]. Vedolizumab is recommended for patients with active CD and active UC who have not responded to corticosteroids, immunosuppressants, or tumor necrosis factor (TNF) blockers. Almost half of the IBD patients respond to vedolizumab, but more than one-third of them relapse within 12–36 months from the induction phase [13,14,15,16,17,18,19,20,21,22,23]. These findings have boosted intensive research aimed at identifying predictors of response to the drug [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. It is now evident that prior exposure to anti-TNF α drugs associates with a reduced induction of clinical and endoscopic remission [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Both CD and UC patients with a severe clinical activity at baseline are less likely to respond to vedolizumab, as compared to patients with mild-to-moderate disease [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. In addition to prior anti-TNF α therapy, active smoking, penetrant behavior, and active perianal disease at baseline are independent predictors of nonresponse to the drug in CD patients [19].
More recent studies have shown that, at least in UC, patients achieving and maintaining clinical and endoscopic remission have significantly higher vedolizumab trough concentration during maintenance therapy than patients who do not respond [32,33,34,35,36,37,38,39]. Nevertheless, it remains plausible that further clinical and biological factors can influence the response to the drug. Indeed, it has been reported that accumulation of α4β7-expressing immune cells in the gut tissue can predict therapeutic success of vedolizumab [40]. Notably, in the healthy intestine, α4β7-expressing T cells accumulate preferentially in the left colon, raising the possibility that localization of the active lesions can further influence response to vedolizumab [41,42,43,44].
This study was aimed at examining whether disease location predicts clinical remission to vedolizumab.
2. Materials and Methods
2.1. Study Design and Aim
This was a retrospective study conducted on IBD patients treated with vedolizumab in three referral centers: Tor Vergata University Hospital (Rome, Italy), San Matteo Hospital of Pavia (Pavia, Italy), and Friedrich-Alexander-University Hospital of Erlangen (Erlangen, Germany). Patients’ data were retrospectively collected between April 2018 and October 2019 and, after a de-identification process, registered into an electronic database. The primary objective of the study was to assess whether the location of CD and UC influenced vedolizumab-induced remission at week 14. Moreover, we evaluated whether further clinical and demographic factors influenced both clinical response and remission at week 14. The study was approved by the local Ethics Committee (CEI Policlinico Tor Vergata, Rome).
2.2. Patients
Inclusion criteria included: a confirmed diagnosis of CD or UC [45,46]; a clinically active disease at baseline (regardless of the grade) requiring vedolizumab treatment; available data on clinical outcome at baseline and at week 14. Patients were excluded if they were in clinical remission at baseline, had unclassified/indeterminate colitis, or pouchitis and if the clinical data at the indicated time points were not available.
For each patient, several demographic and clinical variables were considered for the analysis (Table 1). Clinical disease activity for UC was evaluated by the partial Mayo (pMayo) score [47] and for CD by the Harvey Bradshaw index (HBI). [48] Clinical remission was defined as a pMayo score <2 (UC) and an HBI <5 (CD), while clinical response was defined as a reduction of minimum 3 points of pMayo score and HBI for UC and CD, respectively. Clinical evaluation of treatment response in the 3 participating centers took place at the same time using the same clinical scores above stated.

Table 1.
Clinical and demographic characteristics of the patients.
2.3. Statistical Analysis
Continuous variables were reported as median with interquartile range (IQR) and categorical variables were expressed as percentage. Distribution of the variables at baseline between the groups of comparison (remitters vs. non-responders and responders vs. non-responders) was evaluated with binomial analysis, using the χ2 or Fisher exact test. A multinomial logistic model for a constructed variable Y has been applied to assess the predictive factors of the clinical remission (Y1) and the clinical response (Y2) separately. The group of the non-responders was considered as the reference group for the binomial and multinomial logistic analysis. A p < 0.05 level was considered for statistical significance.
3. Results
3.1. Induction of Clinical Remission
One hundred and eighty-one IBD patients (74 CD and 107 UC) were enrolled. Twenty-two patients were excluded because their clinical data were not available. Patients had a median duration of disease longer than 10 years and most of them (85% of CD patients and 80% of UC patients) had been previously exposed to TNF α antagonists (Table 1). Most of the patients enrolled had a mild-to-moderate activity at baseline (Table 1). In CD, there was no statistical association between the clinical activity at baseline and disease location (Table S1). Similarly, no association was seen between the clinical activity and behavior except for the stricturing phenotype, which was significantly associated with a moderate activity (Table S2). In UC, the extent of the lesions was not associated with the clinical activity at baseline (Table S3).
At week 14, 17/74 (23%) CD patients and 34/107 (32%) UC patients were in clinical remission (Figure 1A). In CD, a mild clinical activity at baseline was significantly more frequent in the group of remitters (11/17, 65%) than in the group of the non-responders (7/40, 18%; p = 0.0004) (Table S4), while a moderate clinical activity was less frequent in patients with clinical remission (6/17, 35%) than in the non-responders (31/40, 77%; p = 0.002). There was no difference between remitters and non-responders for the remaining demographic and clinical variables, as well as for the prior or current use of drugs (Table S4).
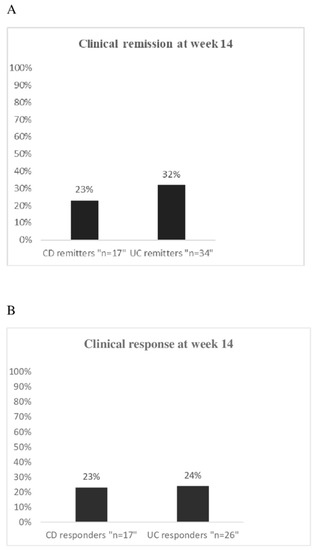
Figure 1.
(A) Percentage of clinical remission in 74 CD patients and 107 UC patients evaluated at week 14 upon vedolizumab treatment; (B) Percentage of clinical response in 74 CD patients and 107 UC patients evaluated at week 14 upon vedolizumab treatment.
In UC, severe clinical activity at baseline was documented in 9/ 47 (19%) non-responders and in no patient achieving clinical remission (p = 0.008) (Table S5). Moreover, the remitters had greater frequency of distal/left-sided colitis (21/34, 62%) as compared to the non-responders (9/47, 19%; p = 0.00008). In contrast, extensive colitis was more frequent in the non-responders (38/47, 81%) than in the remitters (13/34, 38%; p = 0.00008) (Table S5). No further association was observed between each of the two groups of remitters and non-responders and the remaining demographic and clinical variables or the prior or current use of drugs (Table S5).
3.2. Predictive Factors of Remission
The multinomial analysis revealed that, in CD, induction of clinical remission was significantly associated with male gender and a stricturing disease behavior, while there was an inverse association between the clinical activity of the disease at baseline and induction of remission (Table 2). No association between the other demographic and clinical variables and induction of remission was found (Table 2). Similarly, the prior use of TNF α antagonists, the prior or current use of immunosuppressors, and the concomitant use of steroids, as well as the baseline level of serum CRP did not associate with induction of remission (Table 2).

Table 2.
Predictive factors of clinical remission at week 14 in Crohn’s disease and ulcerative colitis patients.
In the UC group, distal/left-sided colitis was significantly associated with induction of remission (p = 0.0003) (Table 2). In contrast, extensive colitis and clinical activity at baseline were inversely associated with remission (p = 0.0003 and p = 0.037, respectively) (Table 2). There was no further association between induction of remission and the remaining variables considered in the study (Table 2).
3.3. Clinical Response and Predictors
Clinical response was achieved in 17/74 (23%) CD patients and in 26/107 (24%) UC patients (Figure 1B). For both CD and UC, all the considered variables at baseline were equally distributed between responders and non-responders (Tables S6 and S7).
In CD, the multivariable multinomial analysis showed an association between the concomitant use of immunosuppressors during the induction phase with vedolizumab and clinical response while an inverse association was seen between the penetrating behavior of the disease and clinical response (Table 3). No association between clinical response and the remaining variables considered in this study was seen (Table 3). In UC, there was no association between clinical response and any of the variables analyzed (Table 3). Mild activity of the disease at baseline was less frequent in the group of responders (4/26; 15%) than in the group of remitters (13/34; 38%, p = 0.05), while moderate-to-severe disease was more common in the group of responders (22/26; 85%) than in the group of remitters 21/34; 62%, p = 0.05).

Table 3.
Predictive factors of clinical response at week 14 in Crohn’s disease and ulcerative colitis patients.
4. Discussion
This study aimed to identify clinical predictors of response to vedolizumab. Through a retrospective analysis of clinical data of patients receiving the drug in 3 referral centers, we initially showed that induction of remission at week 14 was achieved in more than one fifth of CD patients and nearly one third of UC. Additionally, clinical response at the same time point was seen in another one fifth of CD patients and UC patients, thus confirming previous real-life studies showing that nearly half of the IBD patients can benefit from vedolizumab treatment [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Altogether, these findings indicate the need to identify predictive factors of response to the drug in order to optimize the therapeutic strategy.
In addition to the classical demographic and clinical variables considered in previous studies, we herein included the impact of intestinal location of disease on the induction of remission, as response to the treatment is strictly dependent on the number of α4β7-positive cells in inflamed gut and it is known that such cells are more frequent in the distal parts of the colon [43]. Our findings indicate that, in CD, induction of remission occurred more frequently in male, in line with data emerging from the GEMINI 2 study [11]. Surprisingly, stricturing behavior was associated with a greater probability of achieving clinical remission. The reasons why patients with strictures should benefit from vedolizumab treatment more than patients with other phenotypes remain unknown. A possibility is that such patients had a mild inflammatory process overlying the strictured area, which could be dampened by vedolizumab treatment. This hypothesis would be consistent with the demonstration that patients with a mild active disease respond better than patients with a more severe disease [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. This later finding emerges also from our analysis showing an inverse association between induction of remission and clinical activity of the disease at baseline. Another possibility is that vedolizumab can interfere with signaling pathways involved in the fibrogenetic process. In this context, it is noteworthy that α4β7 integrin, the molecular target of vedolizumab, could also bind to fibronectin, a component of the extracellular matrix abundantly produced in intestinal strictures and supposed to enhance collagen secretion [49,50]. Further work is warranted to address these issues.
No association was found between induction of remission and the remaining variables analyzed in the study. In particular, our data did not confirm previous studies reporting an inverse association between the prior use of TNF α blockers and response to vedolizumab [19,20]. The reason for such a discrepancy remains unclear, but it is conceivable that our data could be somehow biased by the fact that only a minority of the patients (15%) was naïve to TNF α antagonists. Similarly, smoking, active perianal disease, location of the disease, and abnormal CRP at baseline, which appeared to influence response to the treatment in some studies, were not associated with induction of remission in our study [15,19,20,51].
Our data indicate that, in the CD cohort, there was a positive association between concomitant immunosuppressive therapy during the induction phase and a better clinical outcome at week 14, probably reflecting the ability of vedolizumab and thiopurines to suppress distinct inflammatory pathways, which sustain the pathologic process in CD.
Notably, a different scenario emerged when UC-related data were analyzed, as extent of mucosal lesions largely influenced induction of remission. Indeed, the remitters had more frequently a distal/left-sided colitis as compared to the non-responders. The multivariable multinomial analysis showed that distal/left-sided colitis was associated with a higher probability of clinical remission while extensive colitis was inversely associated with induction of remission. This association was independent of the baseline disease activity. These data are in line with those of a recent study showing that distal/left-sided colitis was associated with a higher percentage of clinical remission at week 14 as compared to extensive colitis following vedolizumab treatment [14]. However, other studies failed to document an association between extent of UC and response to vedolizumab [22,23,52]. Although, it remains unclear why such studies have generated divergent results, it is conceivable that discrepancies can rely on differences in the selection of the objectives, study population, and statistical analysis adopted.
Despite patients with distal/left-sided colitis were more likely to achieve remission following vedolizumab treatment, no association was seen between the induction of clinical response and extent of colitis. This could in part rely on the different grade of activity of the disease at baseline in the groups of remitters and responders. Indeed, remitters had a higher frequency of mild disease as compared to responders while moderate-to-severe disease was more frequent in the group of responders.
This study has important strengths, such as the multicentric origin of the data and the novelty of the primary objective. However, the retrospective nature of the data and the lack of information on endoscopic/histological response to the treatment represent major limits. As mentioned above, the majority of the patients was already exposed to anti-TNF therapy, as vedolizumab at the time-point of investigation was mainly used in patients who were unresponsive or intolerant to TNF blockers in the examined centers. Therefore, we are aware that the present data deserve further confirmation prior to be generalized to the whole IBD population.
5. Conclusions
In conclusion, we here show that the extent of mucosal inflammation in UC is a major determinant of clinical remission induced by vedolizumab. However, prospective studies on large populations investigating clinical and endoscopic/histological remission are needed to confirm such data.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/2/385/s1, Table S1: Distribution of the clinical activity of disease at baseline in Crohn’s disease patients with ileal disease (L1), colonic disease (L2), and ileocolonic disease (L3); Table S2: Distribution of the clinical activity of disease at baseline in Crohn’s disease patients with inflammatory behavior (B1), stricturing behaviour (B2) and penetrating behavior (B3). Table S3: Distribution of the clinical activity of disease at baseline in patients with distal/left-sided colitis or extensive ulcerative colitis. Table S4: Distribution of baseline characteristics in Crohn’s disease patients with clinical remission (remitters) as compared to patients without clinical response (non-responders) at week 14; Table S5: Distribution of baseline characteristics in ulcerative colitis patients with clinical remission (remitters) as compared to patients without clinical response (non-responders) at week 14; Table S6: Distribution of baseline characteristics in Crohn’s disease patients with and without clinical response at week 14; Table S7: Distribution of baseline characteristics in ulcerative colitis patients with and without clinical response at week 14.
Author Contributions
P.S., acquisition of data, analysis and interpretation of data, drafting of the manuscript; I.M., F.L., E.T., H.S., M.V.L., S.C., E.D.C., S.S., and L.F., acquisition of data; I.R., statistical analysis; A.D.S., R.A., M.F.N., E.C., critical revision of the manuscript for important intellectual content; G.M.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and study supervision. All authors approved the final version of the article, including the authorship list. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors have no potential conflicts (financial, professional, or personal) that are relevant to the manuscript.
References
- Duijvestein, M.; Battat, R.; Vande, C.N.; D’Haens, G.R.; Sandborn, W.J.; Khanna, R.; Jairath, V.; Feagan, B.G. Novel Therapies and Treatment Strategies for Patients with Inflammatory Bowel Disease. Curr. Treat. Options Gastroenterol. 2018, 16, 129–146. [Google Scholar] [CrossRef]
- Argollo, M.; Fiorino, G.; Hindryckx, P.; Peyrin-Biroulet, L.; Danese, S. Novel therapeutic targets for inflammatory bowel disease. J. Autoimmun. 2017, 85, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Papadakis, K.A. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver 2017, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chudy-Onwugaje, K.O.; Christian, K.E.; Farraye, F.A.; Cross, R.K. A State-of-the-Art Review of New Emerging Therapies for the Treatment of, I.B.D. Inflamm. Bowel Dis. 2019, 25, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Soler, D.; Chapman, T.; Yang, L.L.; Wyant, T.; Egan, R.; Fedyk, E.R. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J. Pharm. Exp. 2009, 330, 864–875. [Google Scholar] [CrossRef]
- Erle, D.J.; Briskin, M.J.; Butcher, E.C.; Garcia-Pardo, A.; Lazarovits, A.I.; Tidswell, M. Expression and function of the MAdCAM−1 receptor, integrin alpha 4 beta 7, on human leukocytes. J. Immunol. 1994, 153, 517–528. [Google Scholar]
- Fedyk, E.R.; Wyant, T.; Yang, L.-L.; Csizmadia, V.; Burke, K.; Yang, H.; Kadambi, V.J. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm. Bowel Dis. 2012, 18, 2107–2119. [Google Scholar] [CrossRef]
- Feagan, B.G.; Greenberg, G.R.; Wild, G.; Fedorak, R.N.; Paré, P.; McDonald, J.W.; Dubé, R.; Cohen, A.; Steinhart, A.H.; Landau, S.; et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N. Engl. J. Med. 2005, 352, 2499–2507. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment had failed. Gastroenterology 2014, 147, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Ungar, B.; Yung, D.E.; Ben-Horin, S.; Eliakim, R.; Kopylov, U. Vedolizumab in IBD-lessons from real-world experience; a systematic review and pooled analysis. J. Crohns Colitis 2018, 12, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Bokemeyer, B.; Drabik, A.; Stallmach, A.; Schreiber, S.; Vedolizumab, G.C. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment Pharm. 2016, 43, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Marsal, J.; Bergemalm, D.; Vigren, L.; Björk, J.; Eberhardson, M.; Karling, P.; Söderman, C.; SWIBREGVedolizumab Study Group; Myrelid, P.; et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: A national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand. J. Gastroenterol. 2017, 52, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Grimaud, J.C.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1593–1601. [Google Scholar] [CrossRef]
- Kopylov, U.; Ron, Y.; Avni-Biron, I.; Koslowsky, B.; Waterman, M.; Daher, S.; Ungar, B.; Yanai, H.; Maharshak, N.; Ben-Bassat, O.; et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm. Bowel Dis. 2017, 23, 404–408. [Google Scholar] [CrossRef]
- Vivio, E.E.; Kanuri, N.; Gilbertsen, J.J.; Monroe, K.; Dey, N.; Chen, C.H.; Gutierrez, A.M.; Ciorba, M.A. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J. Crohns Colitis 2016, 10, 402–409. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Jiang, X.; Peerani, F.; Narula, N.; Chaudrey, K.; Whitehead, D.; Hudesman, D.; Lukin, D.; Swaminath, A.; et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: Results from the US VICTORY consortium. Am. J. Gastroenterol. 2016, 111, 1147–1155. [Google Scholar] [CrossRef]
- Narula, N.; Peerani, F.; Meserve, J.; Kochhar, G.; Chaudrey, K.; Hartke, J.; Chilukuri, P.; Koliani-Pace, J.; Winters, A.; Katta, L.; et al. Vedolizumab for ulcerative colitis: Treatment outcomes from the VICTORY consortium. Am. J. Gastroenterol. 2018, 113, 1345–1354. [Google Scholar] [CrossRef]
- Vermeire, S.; Loftus, E.V.; Colombel, J.F.; Feagan, B.G.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.R.; Kaser, A.; Panaccione, R.; et al. Long-term effectiveness and safety of vedolizumab in patients with Crohn’s disease: 5-year cumulative exposure of GEMINI 2 completers rolling into the GEMINI open-label extension study. Gastroenterology 2017, 152, S601. [Google Scholar]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multicentre cohort study. Aliment Pharm. 2017, 46, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Serrero, M.; Peyrin-Biroulet, L.; Filippi, J.; Pariente, B.; Roblin, X.; Buisson, A.; Stefanescu, C.; Trang-Poisson, C.; Altwegg, R.; et al. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: A prospective multi-centre cohort study. Alim. Pharm. Ther. 2019, 50, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rubin, D.T.; Danese, S.; Vermeire, S.; Abhyankar, B.; Sankoh, S.; James, A.; Smyth, M. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin. Gastroenterol. Hepatol. 2017, 15, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Van Assche, G.; Lukas, M.; Xu, J.; James, A.; Abhyankar, B.; Lasch, K. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm. Bowel Dis. 2017, 23, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Langbein, C.; Atreya, R.; Bruns, T.; Dignass, A.; Ende, K.; Hampe, J.; Hartmann, F.; Neurath, M.F.; Maul, J.; et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease—A prospective multicenter observational study. Aliment. Pharm. 2016, 44, 1199–1212. [Google Scholar] [CrossRef]
- Danese, S.; Sandborn, W.J.; Colombel, J.F.; Vermeire, S.; Glover, S.C.; Rimola, J.; Siegelman, J.; Jones, S.; Bornstein, J.D.; Feagan, B.G. Endoscopic; radiologic; and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019, 157, 1007–1018. [Google Scholar] [CrossRef]
- Shelton, E.; Allegretti, J.R.; Stevens, B.; Lucci, M.; Khalili, H.; Nguyen, D.D.; Sauk, J.; Giallourakis, C.; Garber, J.; Hamilton, M.J.; et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: A multicenter cohort. Inflamm. Bowel Dis. 2015, 21, 2879–2885. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Barnes, E.L.; Stevens, B.; Storm, M.; Ananthakrishnan, A.; Yajnik, V.; Korzenik, J. Predictors of clinical response and remission at 1 year among a multicenter cohort of patients with inflammatory bowel disease treated with vedolizumab. Dig. Dis. Sci. 2017, 62, 1590–1596. [Google Scholar] [CrossRef]
- Lenti, M.V.; Levison, S.; Eliadou, E. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: The Cross Pennine study. Dig. Liver Dis. 2018, 50, 1299–1304. [Google Scholar] [CrossRef]
- Digby-Bell, J.L.; Atreya, R.; Monteleone, G.; Powell, N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; Dirks, N.L.; Gastonguay, M.R.; Fasanmade, A.A.; Wyant, T.; Parikh, A.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Fox, I. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharm. 2015, 42, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; French, J.L.; Dirks, N.L.; Sankoh, S.; Parikh, A.; Yang, H.; Danese, S.; Colombel, J.F.; Smyth, M.; Sandborn, W.J.; et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J. Crohn’s Colitis 2017, 11, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Williet, N.; Boschetti, G.; Fovet, M.; Di Bernado, T.; Claudez, P.; Del Tedesco, E.; Jarlot, C.; Rinaldi, L.; Berger, A.; Phelip, J.M.; et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin. Gastroenterol. Hepatol. 2016, 15, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Pouillon, L.; Rousseau, H.; Busby-Venner, H.; De Carvalho Bittencourt, M.; Choukour, M.; Gauchotte, G.; Zallot, C.; Danese, S.; Baumann, C.; Peyrin-Biroulet, L. Vedolizumab Trough Levels and Histological Healing During Maintenance Therapy in Ulcerative Colitis. J. Crohns Colitis 2019, 13, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Liefferinckx, C.; Minsart, C.; Cremer, A.; Amininejad, L.; Tafciu, V.; Quertinmont, E.; Tops, S.; Devière, J.; Gils, A.; van Gossum, A.; et al. Early vedolizumab trough levels at induction in inflammatory bowel disease patients with treatment failure during maintenance. Eur. J. Gastroenterol. Hepatol. 2019, 31, 478–485. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Ramos, G.P.; Willrich, M.A.V.; Jenkins, S.M.; Park, S.H.; Aniwan, S.; Schoenoff, S.A.; Bruining, D.H.; Papadakis, K.A.; Raffals, L.; et al. Vedolizumab Drug Level Correlation with Clinical Remission, Biomarker Normalization, and Mucosal Healing in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 580–586. [Google Scholar] [CrossRef]
- Dreesen, E.; Verstockt, B.; Bian, S.; de Bruyn, M.; Compernolle, G.; Tops, S.; Noman, M.; Van Assche, G.; Ferrante, M.; Gils, A.; et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1937–1946. [Google Scholar] [CrossRef]
- Pouillon, L.; Vermeire, S.; Bossuyt, P. Vedolizumab trough level monitoring in inflammatory bowel disease: A state-of-the-art overview. Bmc. Med. 2019, 17, 89. [Google Scholar] [CrossRef]
- Rath, T.; Billmeier, U.; Ferrazzi, F.; Vieth, M.; Ekici, A.; Neurath, M.F.; Atreya, R. Effects of Anti-Integrin Treatment with Vedolizumab on Immune Pathways and Cytokines in Inflammatory Bowel Diseases. Front Immunol. 2018, 31, 1700. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Vande Casteele, N.; Boland, B.S.; Rivera-Nieves, J.; Ernst, P.B.; Eckmann, L.; Barrett, K.E.; Chang, J.T.; Sandborn, W.J. Should We Divide Crohn’s Disease into Ileum-Dominant and Isolated Colonic Diseases? Clin. Gastroenterol. Hepatol. 2019, 19, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, R.; Lamb, C.A.; Eastham-Anderson, J.; Scherl, A.; Raffals, L.; Faubion, W.A.; Bennett, M.R.; Long, A.K.; Mansfield, J.C.; Kirby, J.A.; et al. AlphaE Integrin Expression Is Increased in the Ileum Relative to the Colon and Unaffected by Inflammation. J. Crohns Coliti. 2018, 12, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Elewaut, D.; Van Damme, N.; De Keyser, F.; Scherl, A.; Raffals, L.; Faubion, W.A.; Bennett, M.R.; Long, A.K.; Mansfield, J.C.; Kirby, J.A.; et al. Altered expression of alpha E beta 7 integrin on intra-epithelial and lamina propria lymphocytes in patients with Crohn’s disease. Acta. Gastroenterol. Belg. 1998, 61, 288–294. [Google Scholar] [PubMed]
- Zundler, S.; Fischer, A.; Schillinger, D.; Binder, M.T.; Atreya, R.; Rath, T.; Lopez-Pósadas, R.; Voskens, C.J.; Watson, A.; Atreya, I.; et al. The α4β1 Homing Pathway Is Essential for Ileal Homing of Crohn’s Disease Effector T Cells In Vivo. Inflamm. Bowel Dis. 2017, 23, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s Disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Zundler, S.; Becker, E.; Schulze, L.L.; Neurath, M.F. Immune cell trafficking and retention in inflammatory bowel disease: Mechanistic insights and therapeutic advances. Gut 2019, 68, 1688–1700. [Google Scholar] [CrossRef]
- De Bruyn, J.R.; Becker, M.A.; Steenkamer, J.; Wildenberg, M.E.; Meijer, S.L.; Buskens, C.J.; Bemelman, W.A.; Löwenberg, M.; Ponsioen, C.Y.; van den Brink, G.R.; et al. Intestinal fibrosis is associated with lack of response to Infliximab therapy in Crohn’s disease. PLoS ONE 2018, 13, e0190999. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of primary response to biologic treatment (anti-TNF, vedolizumab and ustekinumab) in patients with inflammatory bowel disease: From basic science to clinical practice. J. Crohns Colitis 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Randall, C.; Betancourt, R.; Keene, S.; Lilly, A.; Fowler, M.; Dellon, E.S.; Herfarth, H.H. Mucosal Eosinophilia Is an Independent Predictor of Vedolizumab Efficacy in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).