Therapy Discontinuation after Myocardial Infarction
Abstract
1. Introduction
2. Tools and Methods
3. Results
3.1. The Proportion of Patients with Therapy Discontinuation
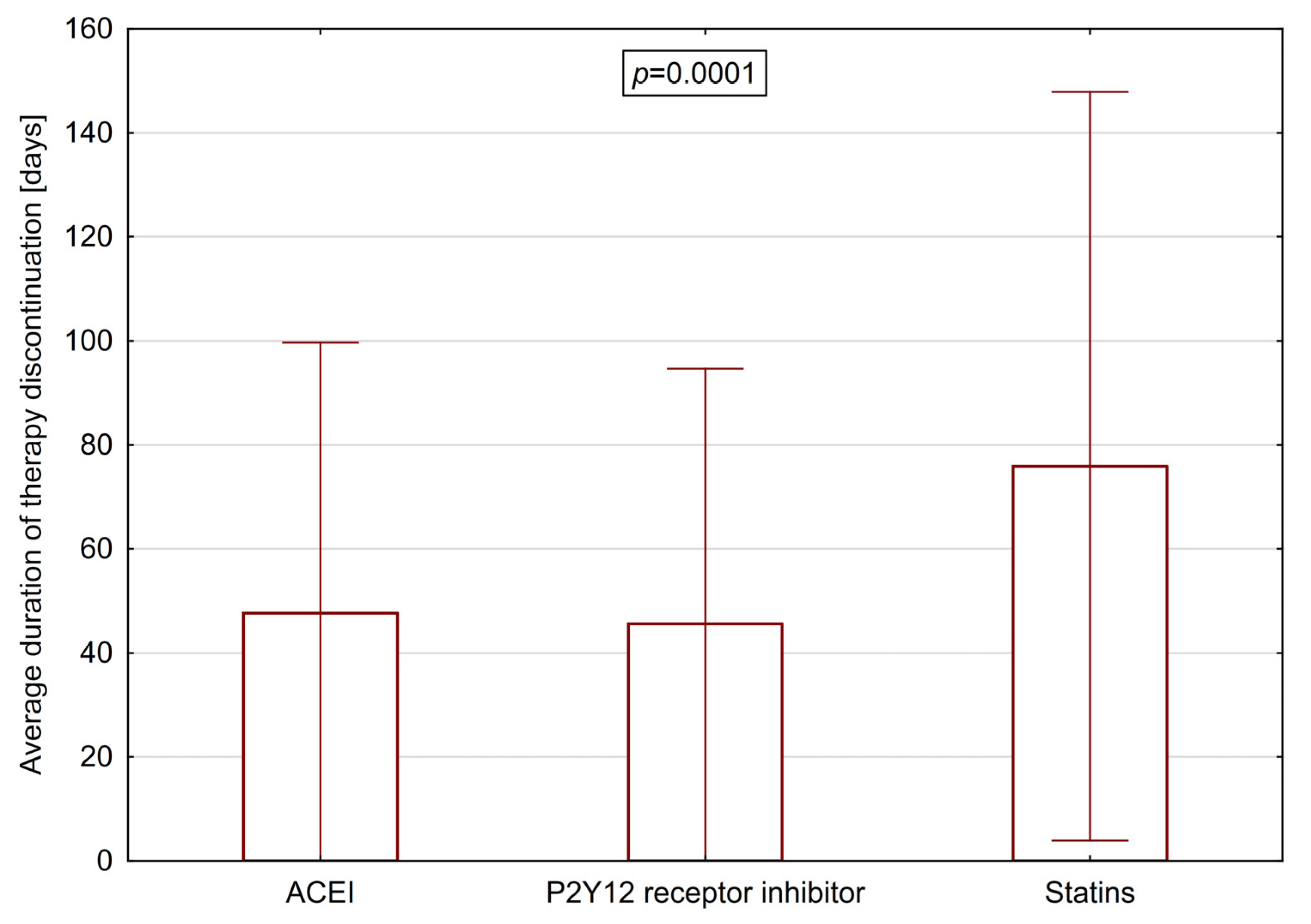
3.2. Average Duration of Therapy Discontinuation
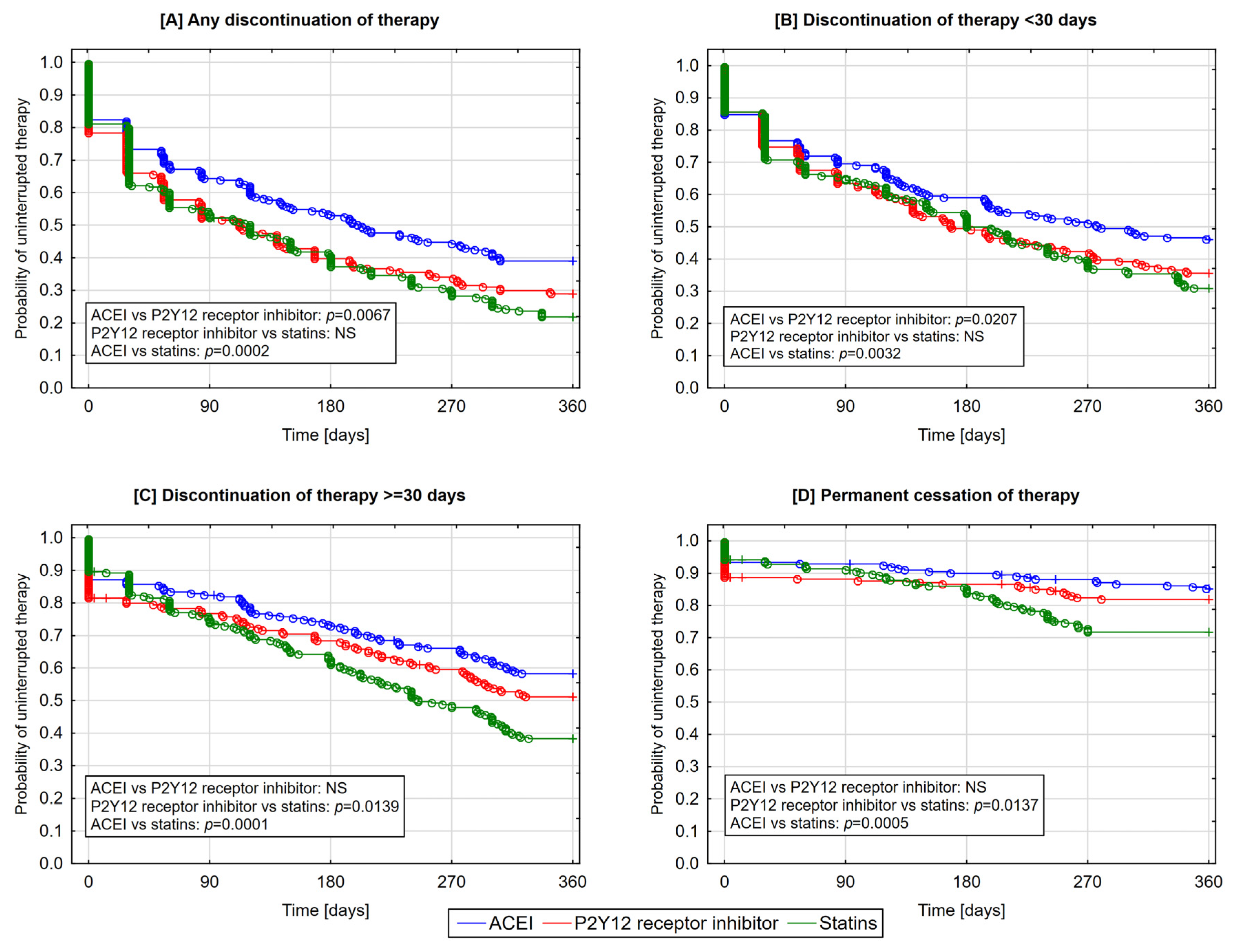
3.3. The Likelihood of Therapy Discontinuation according to Classes of Medications
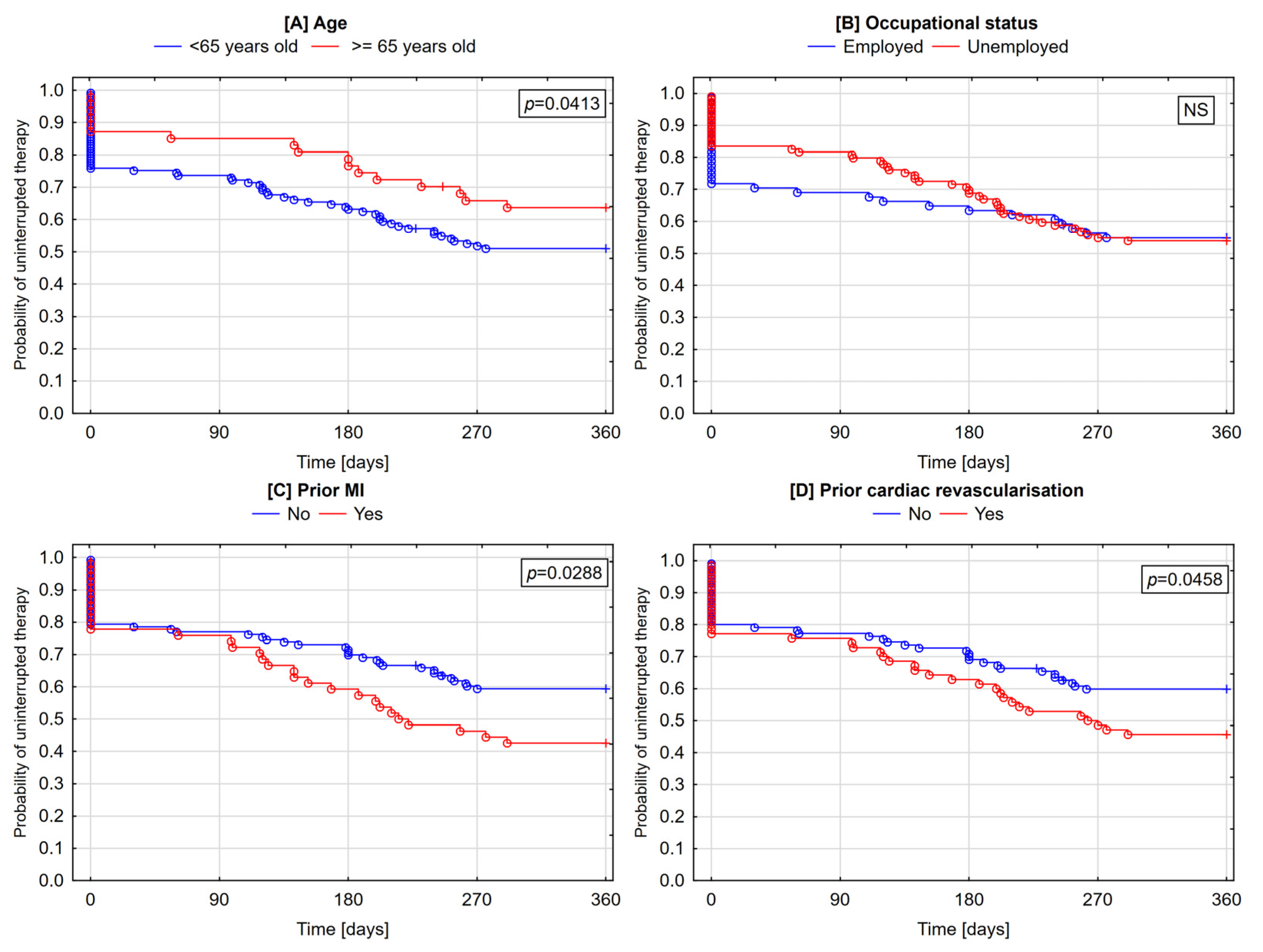
3.4. Determinants of Lack of Post-Discharge Therapy Initiation
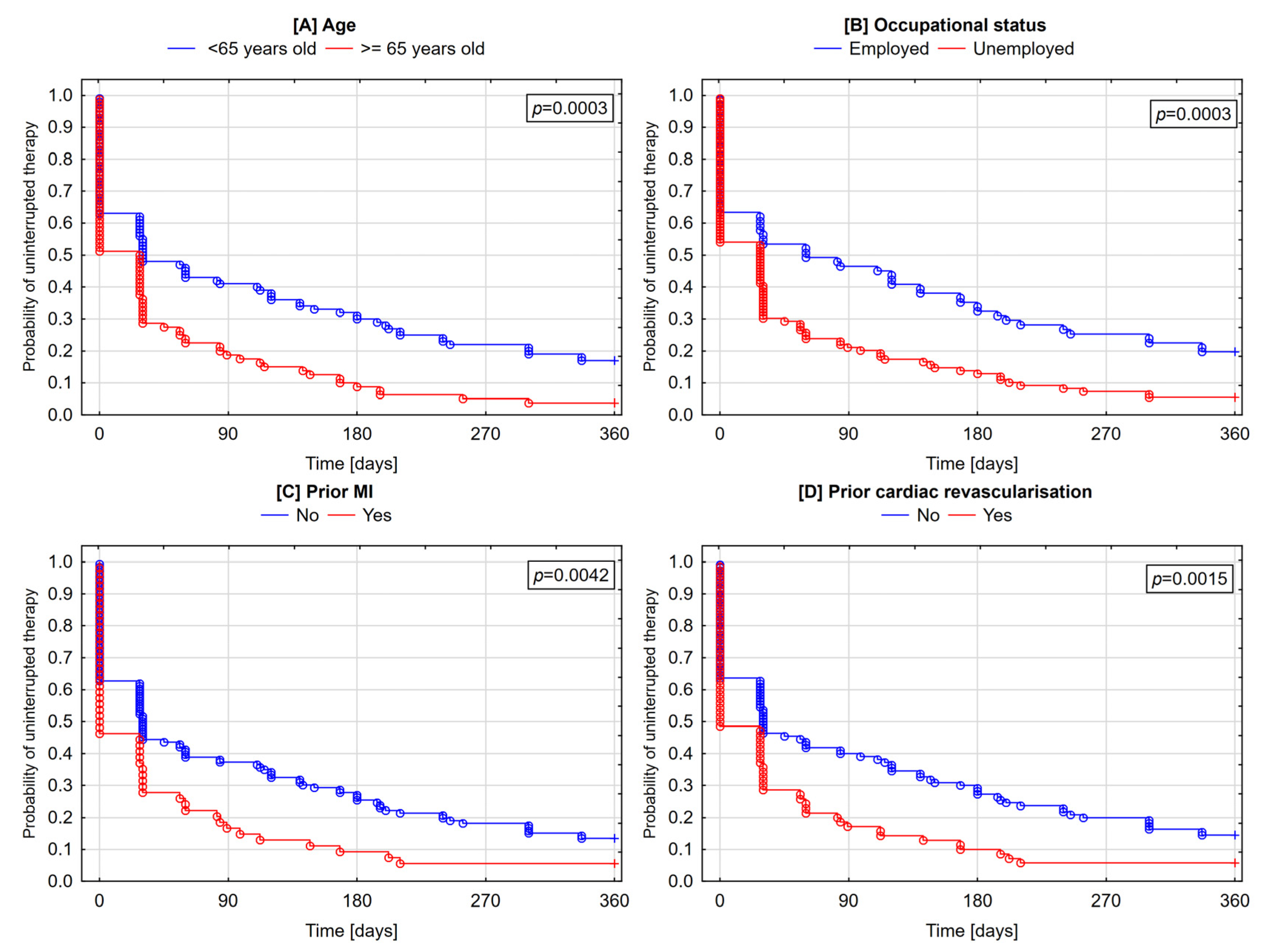
3.5. Determinants of Discontinuation and Cessation of Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.P.H.; Gaedke, M.Â.; Garcez, A.; Barcellos, N.T.; Paniz, V.M.V. Effect of characteristics of pharmacotherapy on non-adherence in chronic cardiovascular disease: A systematic review and meta-analysis of observational studies. Int. J. Clin. Pract. 2018, 72. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, E.; World Health Organization (Eds.) Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-154599-0. [Google Scholar]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. Am. J. Med. 2012, 125, 882–887.e1. [Google Scholar] [CrossRef] [PubMed]
- Kassab, Y.; Hassan, Y.; Abd Aziz, N.; Ismail, O.; AbdulRazzaq, H. Patients’ adherence to secondary prevention pharmacotherapy after acute coronary syndromes. Int. J. Clin. Pharm. 2013, 35, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Kosobucka, A.; Michalski, P.; Pietrzykowski, Ł.; Kasprzak, M.; Obońska, K.; Fabiszak, T.; Felsmann, M.; Kubica, A. Adherence to treatment assessed with the Adherence in Chronic Diseases Scale in patients after myocardial infarction. Patient Prefer. Adherence 2018, 12, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kubica, A.; Kosobucka, A.; Fabiszak, T.; Gorog, D.A.; Siller-Matula, J.M. Assessment of adherence to medication in patients after myocardial infarction treated with percutaneous coronary intervention. Is there a place for newself-reported questionnaires? Curr. Med. Res. Opin. 2019, 35, 341–349. [Google Scholar] [CrossRef]
- Kubica, A.; Obońska, K.; Fabiszak, T.; Kubica, J. Adherence to antiplatelet treatment with P2Y12 receptor inhibitors. Is there anything we can do to improve it? A systematic review of randomized trials. Curr. Med. Res. Opin. 2016, 32, 1441–1451. [Google Scholar] [CrossRef]
- Ho, P.M.; Spertus, J.A.; Masoudi, F.A.; Reid, K.J.; Peterson, E.D.; Magid, D.J.; Krumholz, H.M.; Rumsfeld, J.S. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch. Intern. Med. 2006, 166, 1842–1847. [Google Scholar] [CrossRef]
- Iakovou, I. Incidence, Predictors, and Outcome of Thrombosis after Successful Implantation of Drug-Eluting Stents. JAMA 2005, 293, 2126. [Google Scholar] [CrossRef]
- Kuchulakanti, P.K.; Chu, W.W.; Torguson, R.; Ohlmann, P.; Rha, S.-W.; Clavijo, L.C.; Kim, S.-W.; Bui, A.; Gevorkian, N.; Xue, Z.; et al. Correlates and Long-Term Outcomes of Angiographically Proven Stent Thrombosis With Sirolimus- and Paclitaxel-Eluting Stents. Circulation 2006, 113, 1108–1113. [Google Scholar] [CrossRef]
- Spertus, J.A.; Kettelkamp, R.; Vance, C.; Decker, C.; Jones, P.G.; Rumsfeld, J.S.; Messenger, J.C.; Khanal, S.; Peterson, E.D.; Bach, R.G.; et al. Prevalence, Predictors, and Outcomes of Premature Discontinuation of Thienopyridine Therapy After Drug-Eluting Stent Placement: Results From the PREMIER Registry. Circulation 2006, 113, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Kubica, A.; Kasprzak, M.; Siller-Matula, J.; Koziński, M.; Pio Navarese, E.; Obońska, K.; Andruszkiewicz, A.; Sztuba, B.; Fabiszak, T.; Świątkiewicz, I.; et al. Time-related changes in determinants of antiplatelet effect of clopidogrel in patients after myocardial infarction. Eur. J. Pharmacol. 2014, 742, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kubica, J.; Adamski, P.; Niezgoda, P.; Alexopoulos, D.; Badarienė, J.; Budaj, A.; Buszko, K.; Dudek, D.; Fabiszak, T.; Gąsior, M.; et al. Prolonged antithrombotic therapy in patients after acute coronary syndrome: A critical appraisal of current European Society of Cardiology guidelines. Cardiol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen, K.; Alpert, J.S.; et al. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Greenberg, D.; Gilutz, H.; Arbelle, J.E.; Shiyovich, A. Mortality and healthcare resource utilization following acute myocardial infarction according to adherence to recommended medical therapy guidelines. Health Policy 2020, 124, 1200–1208. [Google Scholar] [CrossRef]
- Pietrzykowski, Ł.; Michalski, P.; Kosobucka, A.; Kasprzak, M.; Fabiszak, T.; Stolarek, W.; Siller-Matula, J.M.; Kubica, A. Medication adherence and its determinants in patients after myocardial infarction. Sci. Rep. 2020, 10, 12028. [Google Scholar] [CrossRef]
- Kubica, A.; Obońska, K.; Kasprzak, M.; Sztuba, B.; Navarese, E.P.; Koziński, M.; Świątkiewicz, I.; Kieszkowska, M.; Ostrowska, M.; Grześk, G.; et al. Prediction of high risk of non-adherence to antiplatelet treatment. Kardiol. Pol. 2016, 74, 61–67. [Google Scholar] [CrossRef]
- Kubica, A.; Kasprzak, M.; Obońska, K.; Fabiszak, T.; Laskowska, E.; Navarese, E.P.; Koziński, M.; Sztuba, B.; Świątkiewicz, I.; Grześk, G.; et al. Discrepancies in assessment of adherence to antiplatelet treatment after myocardial infarction. Pharmacology 2015, 95, 50–58. [Google Scholar] [CrossRef]
- Kubica, A.; Kosobucka, A.; Michalski, P.; Fabiszak, T.; Felsmann, M. Self-reported questionnaires for assessment adherence to treatment in patients with cardiovascular diseases. Med. Res. J. 2018, 2, 115–122. [Google Scholar] [CrossRef]
- Kosobucka, A.; Michalski, P.; Pietrzykowski, Ł.; Kasprzak, M.; Fabiszak, T.; Felsmann, M.; Kubica, A. The impact of readiness to discharge from hospital on adherence to treatment in patients after myocardial infarction. Cardiol. J. 2020. [Google Scholar] [CrossRef]
- Suzuki, T.; Shiga, T.; Omori, H.; Tatsumi, F.; Nishimura, K.; Hagiwara, N. Adherence to medication and characteristics of Japanese patients with non-valvular atrial fibrillation. J. Cardiol. 2017, 70, 238–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winter, M.-P.; von Lewinski, D.; Wallner, M.; Prüller, F.; Kolesnik, E.; Hengstenberg, C.; Siller-Matula, J.M. Incidence, predictors, and prognosis of premature discontinuation or switch of prasugrel or ticagrelor: The ATLANTIS-SWITCH study. Sci. Rep. 2019, 9, 8194. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Chiu, M.; Guo, H.; Austin, P.C.; Marquis, J.-F.; Tu, J.V. Patterns of use of thienopyridine therapy after percutaneous coronary interventions with drug-eluting stents and bare-metal stents. Am. Heart J. 2009, 158, 592–598.e1. [Google Scholar] [CrossRef] [PubMed]
- Fosbøl, E.L.; Ju, C.; Anstrom, K.J.; Zettler, M.E.; Messenger, J.C.; Waksman, R.; Effron, M.B.; Baker, B.A.; Cohen, D.J.; Peterson, E.D.; et al. Early Cessation of Adenosine Diphosphate Receptor Inhibitors Among Acute Myocardial Infarction Patients Treated With Percutaneous Coronary Intervention: Insights From the TRANSLATE-ACS Study (Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome). Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef]
- Latry, P.; Martin-Latry, K.; Lafitte, M.; Peter, C.; Couffinhal, T. Dual antiplatelet therapy after myocardial infarction and percutaneous coronary intervention: Analysis of patient adherence using a French health insurance reimbursement database. EuroIntervention 2012, 7, 1413–1419. [Google Scholar] [CrossRef]
- Choudhry, N.K.; Fischer, M.A.; Avorn, J.; Liberman, J.N.; Schneeweiss, S.; Pakes, J.; Brennan, T.A.; Shrank, W.H. The Implications of Therapeutic Complexity on Adherence to Cardiovascular Medications. Arch. Intern. Med. 2011, 171. [Google Scholar] [CrossRef]
- Kirchmayer, U.; Agabiti, N.; Belleudi, V.; Davoli, M.; Fusco, D.; Stafoggia, M.; Arcà, M.; Barone, A.P.; Perucci, C.A. Socio-demographic differences in adherence to evidence-based drug therapy after hospital discharge from acute myocardial infarction: A population-based cohort study in Rome, Italy: Socio-demographic differences in evidence-based drug therapy after infarction. J. Clin. Pharm. Ther. 2012, 37, 37–44. [Google Scholar] [CrossRef]
- Jánosi, A.; Ofner, P.; Kiss, Z.; Kiss, L.; Kiss, R.G.; Dinnyés, J.; Járai, Z.; Nagy, G.; Veress, G.; Ferenci, T. Szívinfarktust túlélt betegek terápiahűsége a másodlagos megelőzés szempontjából fontos gyógyszeres kezelésekhez. Orv. Hetil. 2017, 158, 1051–1057. [Google Scholar] [CrossRef]
- Dayoub, E.J.; Seigerman, M.; Tuteja, S.; Kobayashi, T.; Kolansky, D.M.; Giri, J.; Groeneveld, P.W. Trends in Platelet Adenosine Diphosphate P2Y 12 Receptor Inhibitor Use and Adherence Among Antiplatelet-Naive Patients After Percutaneous Coronary Intervention, 2008-2016. JAMA Intern. Med. 2018, 178, 943. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Saragoni, S.; Batacchi, P.; Benemei, S.; Geppetti, P.; Sturani, A.; Buda, S.; Degli Esposti, E. Adherence to statin treatment and health outcomes in an Italian cohort of newly treated patients: Results from an administrative database analysis. Clin. Ther. 2012, 34, 190–199. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, Z.; McCollam, P.; Anderson, J.; Bae, J.P.; Fu, H.; Zettler, M.; LeNarz, L. Factors associated with clopidogrel use, adherence, and persistence in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Curr. Med. Res. Opin. 2011, 27, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.D.; Tsang, P.P.M.; Li, W.T.L.; Wang, H.H.X.; Liu, K.Q.L.; Griffiths, S.M.; Wong, M.C.S. Determinants of medication adherence and blood pressure control among hypertensive patients in Hong Kong: A cross-sectional study. Int. J. Cardiol. 2015, 182, 250–257. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Grosso, A.; Abu-Assi, E.; Kinnaird, T.; Ariza-Solé, A.; Manzano-Fernández, S.; Templin, C.; Velicki, L.; Xanthopoulou, I.; Cerrato, E.; et al. Incidence and predictors of bleeding in ACS patients treated with PCI and prasugrel or ticagrelor: An analysis from the RENAMI registry. Int. J. Cardiol. 2018, 273, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Jiang, J.Y.; Griffiths, S.M. Antihypertensive Drug Adherence among 6408 Chinese Patients on Angiotensin-Converting Enzyme Inhibitors in Hong Kong: A Cohort Study. J. Clin. Pharmacol. 2010, 50, 598–605. [Google Scholar] [CrossRef]
- Crowley, M.J.; Zullig, L.L.; Shah, B.R.; Shaw, R.J.; Lindquist, J.H.; Peterson, E.D.; Bosworth, H.B. Medication non-adherence after myocardial infarction: An exploration of modifying factors. J. Gen. Intern. Med. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Tuppin, P.; Neumann, A.; Danchin, N.; de Peretti, C.; Weill, A.; Ricordeau, P.; Allemand, H. Evidence-based pharmacotherapy after myocardial infarction in France: Adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch. Cardiovasc. Dis. 2010, 103, 363–375. [Google Scholar] [CrossRef]
- Laba, T.-L.; Bleasel, J.; Brien, J.-A.; Cass, A.; Howard, K.; Peiris, D.; Redfern, J.; Salam, A.; Usherwood, T.; Jan, S. Strategies to improve adherence to medications for cardiovascular diseases in socioeconomically disadvantaged populations: A systematic review. Int. J. Cardiol. 2013, 167, 2430–2440. [Google Scholar] [CrossRef]
| Parameter | Variable | Total Sample | |
|---|---|---|---|
| n | % | ||
| Gender | Female | 60 | 26.7 |
| Male | 165 | 73.3 | |
| Age | <65 | 129 | 57.3 |
| ≥65 | 96 | 42.7 | |
| Employment status | Employed | 93 | 41.3 |
| Unemployed | 132 | 58.7 | |
| Education | Primary | 30 | 13.3 |
| Vocational | 83 | 36.9 | |
| Secondary | 82 | 36.4 | |
| Higher | 30 | 13.3 | |
| Self-reported economic status | Very good | 14 | 6.2 |
| Satisfactory | 199 | 88.4 | |
| Bad | 12 | 5.3 | |
| Very bad | 0 | 0 | |
| Marital status | Unmarried | 25 | 11.1 |
| Widowed | 33 | 14.7 | |
| Married | 167 | 74.2 | |
| Place of residence * | City | 117 | 52.0 |
| Town | 44 | 19.6 | |
| The country | 64 | 28.4 | |
| History of CAD | Yes | 102 | 45.3 |
| No | 123 | 54.7 | |
| Prior MI | Yes | 64 | 28.4 |
| No | 161 | 71.6 | |
| Prior cardiac revascularization | Yes | 85 | 37.8 |
| No | 140 | 62.2 | |
| Hyperlipidemia | Yes | 151 | 67.6 |
| No | 73 | 32.4 | |
| Diabetes | Yes | 63 | 28.0 |
| No | 157 | 71.0 | |
| Hypertension | Yes | 165 | 73.3 |
| No | 60 | 26.7 | |
| Smoking status (current) | Yes | 85 | 37.8 |
| No | 140 | 62.2 | |
| Form of Therapy Discontinuation | ACEI N = 210 [%] | P2Y12 Receptor Inhibitors N = 194 [%] | Statins N = 222 [%] | p * | ACEI + P2Y12 Receptor Inhibitors + Statins (Discontinuation of Any Medication) N = 180 [%] | ACEI + P2Y12 Receptor Inhibitors + Statins (Simultaneous Discontinuation of All Medications) N = 180 [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | ||
| Any discontinuation of therapy | 39.1 | 60.9 | 28.9 | 71.1 | 22.1 | 77.9 | 0.0006 | 12.2 | 87.8 | 92.8 | 7.2 |
| Short-term discontinuation of therapy (<30 days) | 56.7 | 43.3 | 53.1 | 46.9 | 50.5 | 49.5 | 0.4310 | 15.6 | 84.4 | 96.7 | 3.3 |
| Long-term discontinuation of therapy (≥30 days) | 56.6 | 41.4 | 51.5 | 48.5 | 37.7 | 61.3 | 0.0002 | 16.6 | 83.9 | 96.2 | 3.8 |
| Permanent cessation of therapy | 85.2 | 14.8 | 82.0 | 12.8 | 72.1 | 27.9 | 0.0001 | 55.0 | 45.0 | 97.8 | 2.2 |
| Lack of post-discharge therapy initiation | 93.3 | 6.7 | 88.7 | 11.3 | 94.1 | 5.9 | 0.0860 | 75.1 | 24.1 | 98.3 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzykowski, Ł.; Kasprzak, M.; Michalski, P.; Kosobucka, A.; Fabiszak, T.; Kubica, A. Therapy Discontinuation after Myocardial Infarction. J. Clin. Med. 2020, 9, 4109. https://doi.org/10.3390/jcm9124109
Pietrzykowski Ł, Kasprzak M, Michalski P, Kosobucka A, Fabiszak T, Kubica A. Therapy Discontinuation after Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(12):4109. https://doi.org/10.3390/jcm9124109
Chicago/Turabian StylePietrzykowski, Łukasz, Michał Kasprzak, Piotr Michalski, Agata Kosobucka, Tomasz Fabiszak, and Aldona Kubica. 2020. "Therapy Discontinuation after Myocardial Infarction" Journal of Clinical Medicine 9, no. 12: 4109. https://doi.org/10.3390/jcm9124109
APA StylePietrzykowski, Ł., Kasprzak, M., Michalski, P., Kosobucka, A., Fabiszak, T., & Kubica, A. (2020). Therapy Discontinuation after Myocardial Infarction. Journal of Clinical Medicine, 9(12), 4109. https://doi.org/10.3390/jcm9124109






