Intellectual Abilities of Children with Narcolepsy
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Procedure
2.2.1. General Data Collection
2.2.2. Diagnostic Procedure
2.2.3. Criteria for Idiopathic Narcolepsy
2.2.4. Anthropometric Measurements
2.2.5. Questionnaires
2.2.6. Neuropsychological Evaluation
2.2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| (BMI) | Body mass index |
| (CDI) | Child depression inventory |
| (GAI) | General aptitude index |
| (HP) | High potential |
| (ISI) | Insomnia severity index |
| (IQ) | Intelligence quotient |
| (MSLT) | Multiple sleep latency test |
| (OAHI) | Obstructive apnea hypopnea index |
| (OSA) | Obstructive sleep apnea |
| (PRI) | Perceptual reasoning index |
| (PSG) | Polysomnography |
| (PSI) | Processing speed index |
| (REM) | Rapid eye movement |
| (SVPD) | Significant verbal performance discrepancy |
| (VCI) | Verbal comprehension index |
| (WISC) | Wechsler Intelligence Scale for Children |
| (WMI) | Working memory index |
References
- Ohayon, M.M.; Priest, R.G.; Zulley, J.; Smirne, S.; Paiva, T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002, 58, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Peyron, C.; Tighe, D.K.; Pol, A.N.V.D.; De Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998, 18, 9996–10015. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Zeitzer, J.M.; Mignot, E. The Role of Hypocretins (Orexins) in Sleep Regulation and Narcolepsy. Annu. Rev. Neurosci. 2002, 25, 283–313. [Google Scholar] [CrossRef]
- Zamarian, L.; Högl, B.; Delazer, M.; Hingerl, K.; Gabelia, D.; Mitterling, T.; Brandauer, E.; Frauscher, B. Subjective deficits of attention, cognition and depression in patients with narcolepsy. Sleep Med. 2015, 16, 45–51. [Google Scholar] [CrossRef]
- Zald, D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003, 41, 88–123. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Bušková, J.; Kemlink, D.; Sonka, K.; Skibova, J. Does age at the onset of narcolepsy influence the course and severity of the disease? Sleep Med. 2009, 10, 967–972. [Google Scholar] [CrossRef]
- Zhang, M.; Inocente, C.O.; Villanueva, C.; Lecendreux, M.; Dauvilliers, Y.; Lin, J.S.; Arnulf, I.; Gustin, M.P.; Thieux, M.; Franco, P. Narcolepsy with cataplexy: Does age at diagnosis change the clinical picture? CNS Neurosci. Ther. 2020, 26, 1092–1102. [Google Scholar] [CrossRef]
- Inocente, C.O.; Gustin, M.-P.; Lavault, S.; Guignard-Perret, A.; Raoux, A.; Christol, N.; Gérard, D.; Dauvilliers, Y.; Reimão, R.; Bat-Pitault, F.; et al. Quality of Life in Children with Narcolepsy. CNS Neurosci. Ther. 2014, 20, 763–771. [Google Scholar] [CrossRef]
- Blackwell, J.E.; Alammar, H.A.; Weighall, A.R.; Kellar, I.; Nash, H.M. A systematic review of cognitive function and psychosocial well-being in school-age children with narcolepsy. Sleep Med. Rev. 2017, 34, 82–93. [Google Scholar] [CrossRef]
- Szakács, A.; Hallböök, T.; Tideman, P.; Darin, N.; Wentz, E. Psychiatric Comorbidity and Cognitive Profile in Children with Narcolepsy with or without Association to the H1N1 Influenza Vaccination. Sleep 2015, 38, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Bellebaum, C.; Daum, I. Cognitive deficits in narcolepsy. J. Sleep Res. 2006, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Broughton, R.; Stuss, D. Does memory impairment exist in Narcolepsy-Cataplexy? J. Clin. Exp. Neuropsychol. 1985, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-M.; Joo, E.Y.; Kim, J.Y.; Hwang, K.J.; Hong, S.B. Is High IQ Protective Against Cognitive Dysfunction in Narcoleptic Patients? J. Clin. Neurol. 2013, 9, 118–124. [Google Scholar] [CrossRef]
- Bayard, S.; Langenier, M.C.; De Cock, V.C.; Scholz, S.; Dauvilliers, Y. Executive Control of Attention in Narcolepsy. PLoS ONE 2012, 7, e33525. [Google Scholar] [CrossRef]
- Hood, B.; Bruck, D. Sleepiness and performance in narcolepsy. J. Sleep Res. 1996, 5, 128–134. [Google Scholar] [CrossRef]
- Ha, K.S.; Yoo, H.K.; Lyoo, I.K.; Jeong, D.U. Computerized assessment of cognitive impairment in narcoleptic patients. Acta Neurol. Scand. 2007, 116, 312–316. [Google Scholar] [CrossRef]
- Ramm, M.; Boentert, M.; Lojewsky, N.; Jafarpour, A.; Young, P.; Heidbreder, A. Disease-specific attention impairment in disorders of chronic excessive daytime sleepiness. Sleep Med. 2019, 53, 133–140. [Google Scholar] [CrossRef]
- Dorris, L.; Zuberi, S.M.; Scott, N.; Moffat, C.; McArthur, I. Psychosocial and intellectual functioning in childhood narcolepsy. Dev. Neurorehabilit. 2008, 11, 187–194. [Google Scholar] [CrossRef]
- Posar, A.; Pizza, F.; Parmeggiani, A.; Plazzi, G. Neuropsychological Findings in Childhood Narcolepsy. J. Child. Neurol. 2013, 29, 1370–1376. [Google Scholar] [CrossRef]
- Ryland, H.K.; Lundervold, A.J.; Elgen, I.; Hysing, M. Is there a protective effect of normal to high intellectual function on mental health in children with chronic illness? Child. Adolesc. Psychiatry Ment. Heal. 2010, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Iber, C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; Volume 1. [Google Scholar]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, CT, USA, 2014. [Google Scholar] [CrossRef]
- Pizza, F.; Barateau, L.; Jaussent, I.; Vandi, S.; Antelmi, E.; Mignot, E.; Dauvilliers, Y.; Plazzi, G.; for the MonBo Study Group. MonBo Study Group Validation of Multiple Sleep Latency Test for the diagnosis of pediatric narcolepsy type 1. Neurology 2019, 93, e1034–e1044. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Lammers, G.J.; Ripley, B.; Okun, M.; Nevsimalova, S.; Overeem, S.; Vankova, J.; Black, J.; Harsh, J.; Bassetti, C.; et al. The Role of Cerebrospinal Fluid Hypocretin Measurement in the Diagnosis of Narcolepsy and Other Hypersomnias. Arch. Neurol. 2002, 59, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Gozal, E.; Malhotra, A.; Tiosano, D.; Perlman, R.; Vega, C.; Shahar, E.; Gozal, D.; Hochberg, Z.; Pillar, G. Severe Hypersomnolence After Pituitary/Hypothalamic Surgery in Adolescents: Clinical Characteristics and Potential Mechanisms. Pediatrics 2002, 110, e74. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kovacs, M. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar]
- Goyette, C.H.; Conners, C.K.; Ulrich, R.F. Normative data on Revised Conners Parent and Teacher Rating Scales. J. Abnorm. Child. Psychol. 1978, 6, 221–236. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Newman, T.M. Assessment of Giftedness in School-Age Children Using Measures of Intelligence or Cognitive Abilities. In Handbook of Giftedness in Children; Springer: Boston, MA, USA, 2008; pp. 161–176. [Google Scholar]
- Guenole, F.; Louis, J.; Creveuil, C.; Baleyte, J.-M.; Montlahuc, C.; Fourneret, P.; Revol, O. Behavioral Profiles of Clinically Referred Children with Intellectual Giftedness. BioMed Res. Int. 2013, 2013, 540153. [Google Scholar] [CrossRef]
- Team 2009. R: A Language and Environment for Statistical Computing; Vienna University of Economics and Business: Vienna, Austria, 2012; Available online: http//www.R-project.org2012 (accessed on 6 June 2020).
- Guignard-Perret, A.; Thieux, M.; Guyon, A.; Mazza, S.; Zhang, M.; Revol, O.; Plancoulaine, S.; Franco, P. Sleep of Children with High Potentialities: A Polysomnographic Study. J. Clin. Med. 2020, 9, 3182. [Google Scholar] [CrossRef]
- Corral, M.; Rodriguez, M.; Amenedo, E.; Sanchez, J.L.; Díaz, F. Cognitive Reserve, Age, and Neuropsychological Performance in Healthy Participants. Dev. Neuropsychol. 2006, 29, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.H.; Salmond, C.H.; Jones, P.B.; Sahakian, B.J. Cognitive reserve in neuropsychiatry. Psychol. Med. 2006, 36, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.; Simonoff, E.; Stevenson, J. The Impact of Child IQ, Parent IQ and Sibling IQ on Child Behavioural Deviance Scores. J. Child. Psychol. Psychiatry 1995, 36, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.C.; Koot, H.M. DSM-IV Disorders in Children with Borderline to Moderate Intellectual Disability. I: Prevalence and Impact. J. Am. Acad. Child. Adolesc. Psychiatry 2003, 42, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Stormacq, C.; Broucke, S.V.D.; Wosinski, J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot. Int. 2019, 34, e1–e17. [Google Scholar] [CrossRef]
- Von Stumm, S.; Plomin, R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence 2015, 48, 30–36. [Google Scholar] [CrossRef]
- Raggi, A.; Plazzi, G.; Pennisi, G.; Tasca, D.; Ferri, R. Cognitive evoked potentials in narcolepsy: A review of the literature. Neurosci. Biobehav. Rev. 2011, 35, 1144–1153. [Google Scholar] [CrossRef]
- Kayaalp, D.; Yaman, M.; Karakaya, F.; Aydin, T.; Mayda, H.; Güzel, H.I. Evaluation of the Effect of Modafinil on Cognitive Functions in Patients with Idiopathic Hypersomnia with P300. Med. Sci. Monit. 2015, 21, 1850–1855. [Google Scholar] [CrossRef]
- Leonard, B.E.; McCartan, D.; White, J.; King, D.J. Methylphenidate: A review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum. Psychopharmacol. Clin. Exp. 2004, 19, 151–180. [Google Scholar] [CrossRef]
- Ingravallo, F.; Gnucci, V.; Pizza, F.; Vignatelli, L.; Govi, A.; Dormi, A.; Pelotti, S.; Cicognani, A.; Dauvilliers, Y.; Plazzi, G. The burden of narcolepsy with cataplexy: How disease history and clinical features influence socio-economic outcomes. Sleep Med. 2012, 13, 1293–1300. [Google Scholar] [CrossRef]
- Broughton, R.; Ghanem, Q.; Hishikawa, Y.; Sugita, Y.; Nevsimalova, S.; Roth, B. Life Effects of Narcolepsy in 180 Patients from North America, Asia and Europe Compared to Matched Controls. Can. J. Neurol. Sci. J. Can. Des. Sci. Neurol. 1981, 8, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Lewin, D.S.; Rosen, R.C.; England, S.J.; Dahl, R.E. Preliminary evidence of behavioral and cognitive sequelae of obstructive sleep apnea in children. Sleep Med. 2002, 3, 5–13. [Google Scholar] [CrossRef]
- Ponziani, V.; Gennari, M.; Pizza, F.; Balsamo, A.; Bernardi, F.; Plazzi, G. Growing Up with Type 1 Narcolepsy: Its Anthropometric and Endocrine Features. J. Clin. Sleep Med. 2016, 12, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.-H.; Clément, O.; Sapin, E.; Gervasoni, D.; Peyron, C.; Léger, L.; Salvert, D.; Fort, P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med. Rev. 2011, 15, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Born, J. About Sleep’s Role in Memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, L.; Yang, G.; Gan, W.-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Lacaux, C.; Izabelle, C.; Santantonio, G.; De Villèle, L.; Frain, J.; Lubart, T.; Pizza, F.; Plazzi, G.; Arnulf, I.; Oudiette, D. Increased creative thinking in narcolepsy. Brain 2019, 142, 1988–1999. [Google Scholar] [CrossRef]
- Rinaldi, L.; Karmiloff-Smith, A. Intelligence as a Developing Function: A Neuroconstructivist Approach. J. Intell. 2017, 5, 18. [Google Scholar] [CrossRef]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; Gogtay, N.; Evans, A.; Rapoport, J.; Giedd, J. Intellectual ability and cortical development in children and adolescents. Nat. Cell Biol. 2006, 440, 676–679. [Google Scholar] [CrossRef]
- Brant, A.M.; Munakata, Y.; Boomsma, D.I.; DeFries, J.C.; Haworth, C.M.A.; Keller, M.C.; Martin, N.G.; McGue, M.; Petrill, S.A.; Plomin, R.; et al. The Nature and Nurture of High IQ. Psychol. Sci. 2013, 24, 1487–1495. [Google Scholar] [CrossRef]
- Latorre, D.; Kallweit, U.; Armentani, E.; Foglierini, M.; Mele, F.; Cassotta, A.; Jovic, S.; Jarrossay, D.; Mathis, J.; Zellini, F.; et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nat. Cell Biol. 2018, 562, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Payton, A.; Boogerd, E.V.D.; Davidson, Y.; Gibbons, L.; Ollier, W.; Rabbitt, P.; Worthington, J.; Horan, M.; Pendleton, N. Influence and interactions of cathepsin D, HLA-DRB1 and APOE on cognitive abilities in an older non-demented population. Genes Brain Behav. 2006, 5, 23–31. [Google Scholar] [CrossRef]
- Megarbane, A.; Noguier, F.; Stora, S.; Manchon, L.; Mircher, C.; Bruno, R.; Dorison, N.; Pierrat, F.; Réthoré, M.-O.; Trentin, B.; et al. The intellectual disability of trisomy 21: Differences in gene expression in a case series of patients with lower and higher IQ. Eur. J. Hum. Genet. 2013, 21, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Miyagawa, T.; Toyoda, H.; Tokunaga, K.; Honda, M. Epigenome-wide association study of DNA methylation in narcolepsy: An integrated genetic and epigenetic approach. Sleep 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.K.; Ferris, M.J.; Locke, J.L.; Brodnik, Z.D.; Jones, S.R.; España, R.A. Hypocretin/orexin knock-out mice display disrupted behavioral and dopamine responses to cocaine. Addict. Biol. 2016, 22, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Huber, R.; Kurth, S.; Ringli, M.; Jenni, O.G.; Achermann, P. The Sleep EEG as a Marker of Intellectual Ability in School Age Children. Sleep 2011, 34, 181–189. [Google Scholar] [CrossRef] [PubMed]
| Global | n | HP | n | No-HP | n | p | |
|---|---|---|---|---|---|---|---|
| 74 | 28 | 46 | |||||
| Age, years | 11.5 (5.5–17.4) | 74 | 11.3 (6.6–17.1) | 28 | 11.7 (5.5–17.4) | 46 | 0.87 |
| Sex, male % (n) | 58 (43) | 74 | 46 (13) | 28 | 65 (30) | 46 | 0.61 |
| BMI z-score | 2.8 (−1.8–9) | 73 | 2.2 (−0.5–7.6) | 28 | 3.3 (−1.8–9) | 45 | 0.18 |
| BMI z- score classification | 73 | 28 | 45 | 0.28 | |||
| Normal, % (n) | 26 (19) | 43 (12) | 16 (7) | ||||
| Obesity, % (n) | 64 (47) | 50 (14) | 73 (33) | ||||
| Overweight, % (n) | 10 (7) | 7 (2) | 11 (5) | ||||
| SEL, % (n) | 66 | 26 | 40 | 0.64 | |||
| Farmers | 3 (2) | 4 (1) | 3 (1) | ||||
| Artisans, shopkeepers, CEOs | 5 (3) | 4 (1) | 5 (2) | ||||
| Executive and intellectual professions | 20 (13) | 31 (8) | 12 (5) | ||||
| Intermediate professions | 24 (16) | 27 (7) | 22 (9) | ||||
| Employees | 27 (18) | 27 (7) | 28 (11) | ||||
| Workers | 6 (4) | 0 (0) | 10 (4) | ||||
| Students and unemployed | 15 (10) | 7 (2) | 20 (8) | ||||
| School difficulties, % (n) | 48 (34) | 71 | 21 (6) | 28 | 65 (28) | 43 | 0.01 |
| Global | n | HP | n | No-HP | n | p | |
|---|---|---|---|---|---|---|---|
| 74 | 28 | 46 | |||||
| Age at diagnosis, years | 11.7 (5.5–17.6) | 74 | 11 (6.7–7.5) | 28 | 12 (5.5–17.4) | 46 | 0.86 |
| Age at onset, years | 9.6 (4.2–15.8) | 71 | 10 (5.9–15.6) | 28 | 8.5 (4.2–15.8) | 43 | 0.54 |
| H1N1 vaccine, % (n) | 23 (13) | 56 | 33 (7) | 21 | 17 (6) | 35 | 0.61 |
| Cataplexies, % (n) | 91 (67) | 74 | 96 (27) | 28 | 87 (40) | 46 | 0.61 |
| Sleep paralysis, % (n) | 18 (13) | 74 | 18 (5) | 28 | 17 (8) | 46 | 1.00 |
| Hypnagogic hallucinations, % (n) | 39 (29) | 74 | 39 (11) | 28 | 39 (18) | 46 | 1.00 |
| HLA +, % (n) | 100 (71) | 71 | 100 (28) | 28 | 100 (43) | 43 | 1.00 |
| Hypocretin, pg/mL | 21 (0–178) | 40 | 20 (0–178) | 11 | 22 (1–90) | 29 | 0.71 |
| Global | n | HP | n | No-HP | n | p | |
|---|---|---|---|---|---|---|---|
| PSG | 28 | 46 | |||||
| Total sleep time, min | 478 (270–615) | 72 | 463 (324–561) | 27 | 485 (270–615) | 45 | 0.45 |
| Sleep efficiency, % | 84.1 (52.5–95.2) | 71 | 83.7 (56.6–91.4) | 27 | 84.1 (52.5–95.2) | 44 | 0.54 |
| Sleep latency, min | 4.8 (0–78) | 72 | 6 (0–78) | 27 | 4 (0–77) | 45 | 0.64 |
| REM latency, min | 5.8 (0–225) | 72 | 10.3 (0–225) | 27 | 4.5 (0–215.5) | 45 | 0.86 |
| Stage 1, % | 15.3 (0.2–32) | 72 | 16 (0.2–28.3) | 27 | 15.3 (2.8–32) | 45 | 0.90 |
| Stage 1, min | 67 (1–173) | 72 | 64 (1–137) | 27 | 68 (11–173) | 45 | 0.64 |
| Stage 2, % | 41.4 (17.4–64.7) | 72 | 39 (26.3–64.7) | 27 | 42.4 (17.4–56.7) | 45 | 0.64 |
| Stage 2, min | 191 (23.5–293) | 72 | 178 (23.5–293) | 27 | 201 (72–291) | 45 | 0.35 |
| Stage 3, % | 19.6 (0.2–59.5) | 72 | 19.8 (9.1–34.6) | 27 | 19.4 (0.2–59.5) | 45 | 0.55 |
| Stage 3, min | 94 (1–246) | 72 | 93 (28.2–145.5) | 27 | 95 (1–246) | 45 | 0.94 |
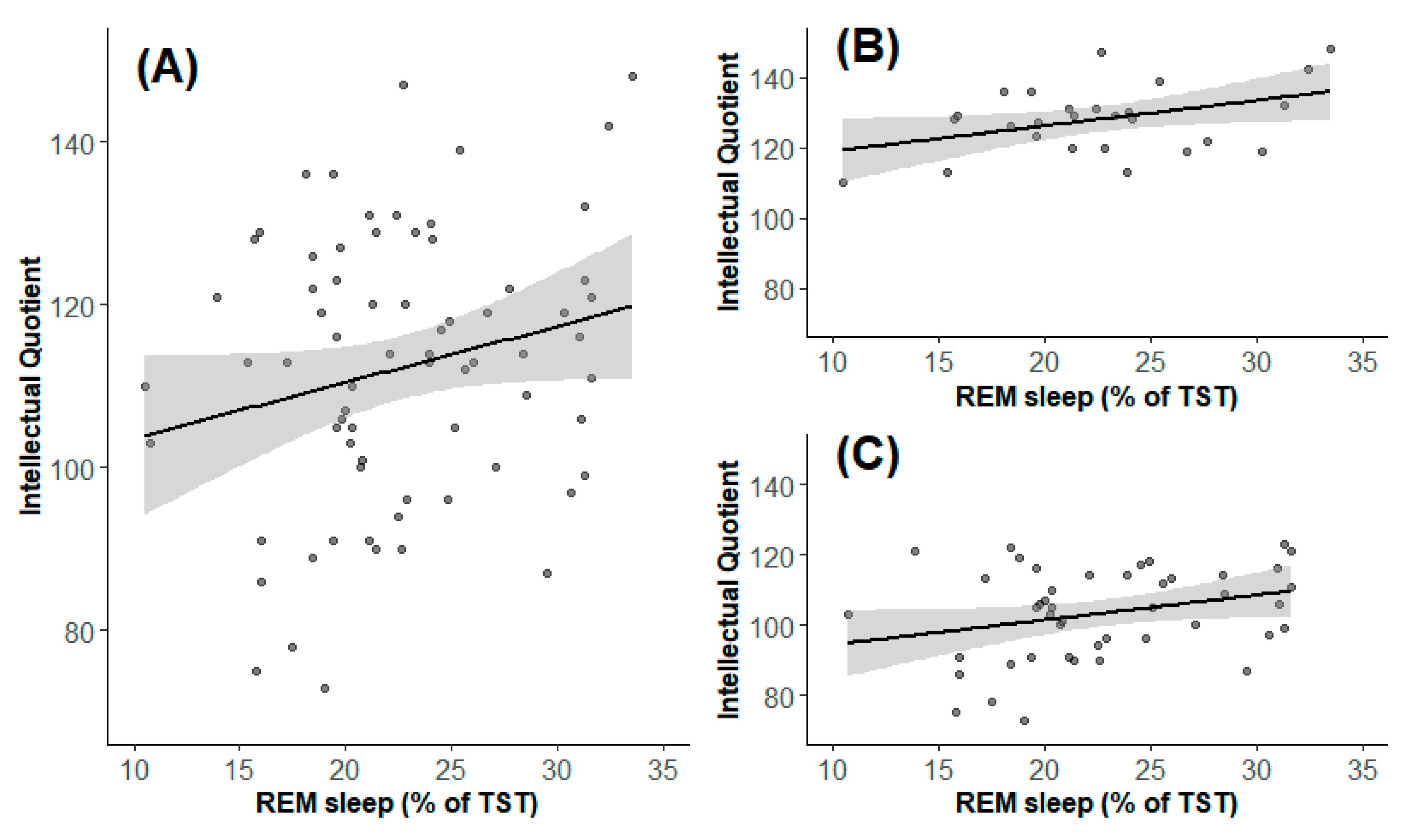
| REM sleep, % | 22.3 (10.5–39.3) | 72 | 22.7 (10.5-39.3) | 27 | 21.4 (10.7–31.6) | 45 | 0.90 |
| REM sleep, min | 110 (17.9–190) | 72 | 102 (17.9-190) | 27 | 112.5 (29–178) | 45 | 0.54 |
| Total arousal index, % | 11.8 (0–66.6) | 69 | 12.3 (6.2-66.6) | 26 | 11.5 (0–37.4) | 43 | 0.90 |
| Desaturation ≥3% index, % | 0.05 (0–19.3) | 68 | 0 (0–1.5) | 25 | 0.4 (0–19.3) | 43 | 0.01 |
| Minimal saturation, % | 93 (30–98) | 70 | 92.8 (69.3–97) | 25 | 93 (30–98) | 45 | 0.88 |
| OAHI | 0.5 (0–7.5) | 68 | 0.40 (0-3.6) | 26 | 0.55 (0–7.5) | 42 | 0.54 |
| MSLT | |||||||
| MSLT, n | 4 (4,5) | 74 | 4 (4,5) | 28 | 4 (4,5) | 46 | 0.59 |
| SOREM, % | 100 (25–100) | 74 | 100 (25–100) | 28 | 100 (25–100) | 46 | 0.97 |
| Sleep latency, min | 2.5 (0–10) | 74 | 2 (0–10) | 28 | 3.3 (0.4–8) | 46 | 0.54 |
| Global | n | HP | n | No-HP | n | p | |
|---|---|---|---|---|---|---|---|
| Epworth total score | 17 (9–23) | 71 | 16 (9–23) | 27 | 18 (9–23) | 44 | 0.63 |
| Epworth pathologic score, % (n) | 96 (68) | 71 | 96 (26) | 27 | 96 (42) | 44 | 1.00 |
| ISI total score | 12 (2–22) | 62 | 12 (4–22) | 23 | 12 (2–21) | 39 | 0.86 |
| ISI pathologic score, % (n) | 71 (44) | 62 | 70 (16) | 23 | 72 (28) | 39 | 1.00 |
| CDI total score | 10 (0–38) | 62 | 9 (0–38) | 25 | 10 (2–30) | 37 | 0.63 |
| CDI pathologic score, % (n) | 21 (13) | 62 | 20 (5) | 25 | 22 (8) | 37 | 1.00 |
| Conners Total Score | 18 (1–58) | 54 | 14.5 (1–58) | 20 | 21 (1–44) | 34 | 0.24 |
| Conners pathologic score, % (n) | 0 (0) | 54 | 0 (0) | 20 | 0 (0) | 34 | 1.00 |
| Conduct disorders | 49 (9–92) | 54 | 43.5 (39–92) | 20 | 50 (9–78) | 34 | 0.20 |
| Learning disorders | 57 (36–107) | 54 | 45 (36–98) | 20 | 57 (38–107) | 34 | 0.18 |
| Psychosomatization | 53 (42–106) | 54 | 45 (42–106) | 20 | 53 (42–83) | 34 | 0.62 |
| Impulsivity | 46 (35–75) | 54 | 43 (35–68) | 20 | 47 (35–75) | 34 | 0.24 |
| Anxiety | 50 (39–78) | 54 | 51 (40–78) | 20 | 49 (39–68) | 34 | 0.63 |
| Hyperactivity | 52 (33–88) | 54 | 48.5 (35–88) | 20 | 54 (33–76) | 34 | 0.41 |
| Global | n | HP | n | No-HP | n | p | |
|---|---|---|---|---|---|---|---|
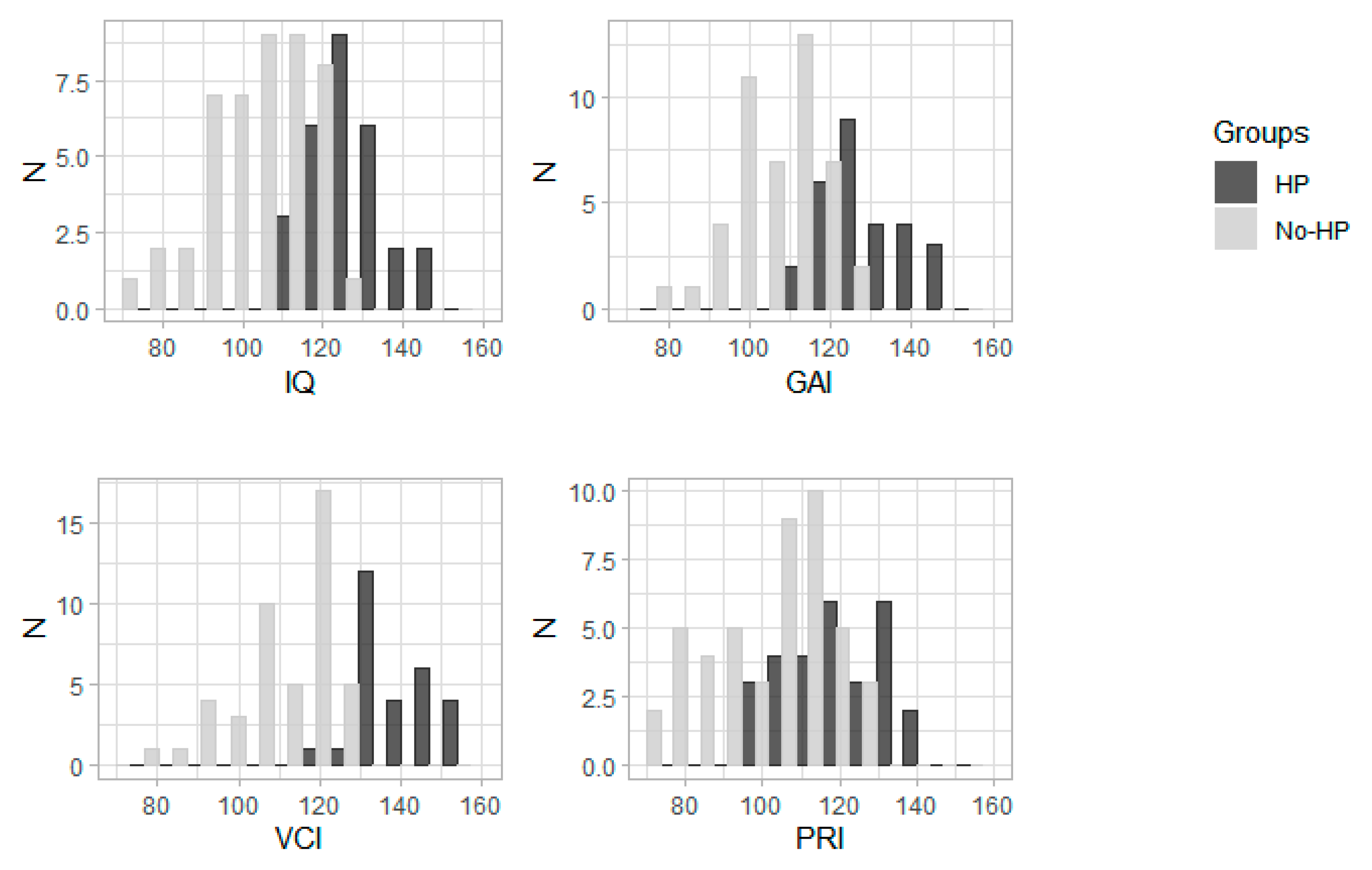
| VCI | 118 (76–155) | 74 | 136.5 (118–155) | 28 | 113 (76–128) | 46 | <0.0001 |
| PRI | 109 (72–142) | 74 | 116 (99–142) | 28 | 103 (72–128) | 46 | <0.0001 |
| WMI | 103 (58–130) | 65 | 109 (76–130) | 25 | 95.5 (58–118) | 40 | <0.0001 |
| PSI | 100 (64–151) | 72 | 112 (76–151) | 27 | 96 (64–124) | 45 | 0.006 |
| GAI | 115 (80–148) | 74 | 128 (115–148) | 28 | 107 (80–123) | 46 | <0.0001 |
| IQ | 114 (73–148) | 74 | 128.5 (110–148) | 28 | 105 (73–123) | 46 | <0.0001 |
| SVPD, % (n) | 18 (13) | 74 | 25 (7) | 28 | 13 (6) | 46 | 0.61 |
| Treatment, % (n) | 58 (43) | 74 | 57 (16) | 28 | 59 (27) | 46 | 1.00 |
| Therapy type | 74 | 28 | 46 | 1.00 | |||
| None | 42 (31) | 43 (12) | 41 (19) | ||||
| Monotherapy | 41 (30) | 39 (11) | 41 (19) | ||||
| Bi-therapy | 17 (13) | 18 (5) | 17 (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thieux, M.; Zhang, M.; Marcastel, A.; Herbillon, V.; Guignard-Perret, A.; Seugnet, L.; Lin, J.-S.; Guyon, A.; Plancoulaine, S.; Franco, P. Intellectual Abilities of Children with Narcolepsy. J. Clin. Med. 2020, 9, 4075. https://doi.org/10.3390/jcm9124075
Thieux M, Zhang M, Marcastel A, Herbillon V, Guignard-Perret A, Seugnet L, Lin J-S, Guyon A, Plancoulaine S, Franco P. Intellectual Abilities of Children with Narcolepsy. Journal of Clinical Medicine. 2020; 9(12):4075. https://doi.org/10.3390/jcm9124075
Chicago/Turabian StyleThieux, Marine, Min Zhang, Agathe Marcastel, Vania Herbillon, Anne Guignard-Perret, Laurent Seugnet, Jian-Sheng Lin, Aurore Guyon, Sabine Plancoulaine, and Patricia Franco. 2020. "Intellectual Abilities of Children with Narcolepsy" Journal of Clinical Medicine 9, no. 12: 4075. https://doi.org/10.3390/jcm9124075
APA StyleThieux, M., Zhang, M., Marcastel, A., Herbillon, V., Guignard-Perret, A., Seugnet, L., Lin, J.-S., Guyon, A., Plancoulaine, S., & Franco, P. (2020). Intellectual Abilities of Children with Narcolepsy. Journal of Clinical Medicine, 9(12), 4075. https://doi.org/10.3390/jcm9124075





