A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia
Abstract
1. Introduction
1.1. How Can Cell Culture Be Used in PCD Diagnostics and Research?
1.2. A Revised Protocol with High Diagnostic Efficiency Is Being Used within the UK PCD Service
2. Methods
2.1. Nasal Epithelial Cell Culture Protocol (with Additional UHS COVID-19 Modifications)
2.2. Bio-Resource
2.3. Post-ALI Culture High-Speed Video Microscopy Analysis
2.4. Fast Fourier Transform Analysis of Cilia Coverage
2.5. Trans-Epithelial Electrical Resistance Measurements
2.6. Immunofluorescence Labelling of Ciliated Ali Cultures
2.7. Statistics
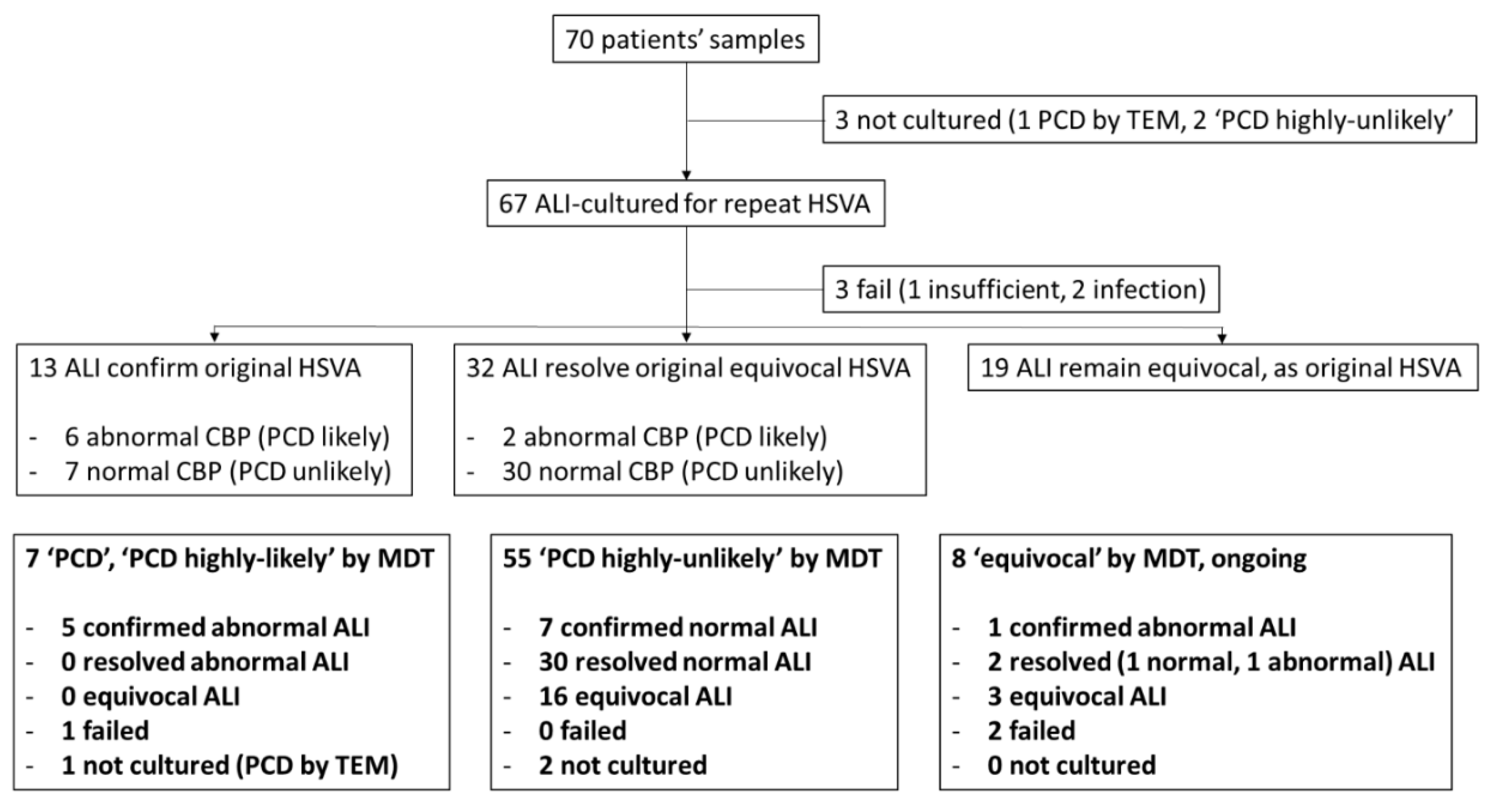
3. Results
3.1. How did the Nasal Epithelial Cell Culture Protocol Improve Diagnostic Accuracy at UHS
3.2. Was the Nasal Epithelial Cell Culture Protocol Reproducible across the UK PCD Service?
3.3. Can a Bio-Resource Extend Diagnostic Testing and Research?
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Prim. 2020, 6, 1–29. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Chetcuti, P.; Moya, E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch. Dis. Child. 2010, 95, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Davis, S.D.; Omran, H.; Shoemark, A. Primary ciliary dyskinesia in the genomics age. Lancet Respir. Med. 2020, 8, 202–216. [Google Scholar] [CrossRef]
- Behan, L.; Dimitrov, B.D.; Kuehni, C.E.; Hogg, C.; Carroll, M.; Evans, H.J.; Goutaki, M.; Harris, A.; Packham, S.; Walker, W.T.; et al. PICADAR: A diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Alanin, M.C.; Collins, S.; Harris, A.; Johansen, H.K.; Nielsen, K.G.; Papon, J.F.; Robinson, P.; Walker, W.T. Clinical care of children with primary ciliary dyskinesia. Expert Rev. Respir. Med. 2017, 11, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Leigh, M.W. Diagnosis of primary ciliary dyskinesia: Searching for a gold standard. Eur. Respir. J. 2014, 44, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef]
- Shoemark, A.; Dell, S.; Shapiro, A.; Lucas, J. ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: Similarities and differences in approach to diagnosis. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Jackson, C.L.; Behan, L.; Collins, S.A.; Goggin, P.M.; Adam, E.C.; Coles, J.L.; Evans, H.J.; Harris, A.; Lackie, P.M.; Packham, S.; et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 837–848. [Google Scholar] [CrossRef]
- Kempeneers, C.; Seaton, C.; Chilvers, M.A. Variation of Ciliary Beat Pattern in Three Different Beating Planes in Healthy Subjects. Chest 2017, 151, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, B.; Shoemark, A.; Jackson, C.L.; Hirst, R.; Thompson, J.; Hayes, J.; Frost, E.; Copeland, F.; Hogg, C.; O’Callaghan, C.; et al. Accuracy of High-Speed Video Analysis to Diagnose Primary Ciliary Dyskinesia. Chest 2019, 155, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, A.; Dixon, M.; Corrin, B.; Dewar, A. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J. Clin. Pathol. 2011, 65, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, A.; Boon, M.; Brochhausen, C.; Bukowy-Bieryllo, Z.; De Santi, M.M.; Goggin, P.; Griffin, P.; Hegele, R.G.; Hirst, R.A.; Leigh, M.W.; et al. International consensus guideline for reporting transmission electron microscopy results in the diagnosis of Primary Ciliary Dyskinesia (BEAT PCD TEM Criteria). Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Shoemark, A.; Frost, E.; Dixon, M.; Ollosson, S.; Kilpin, K.; Patel, M.; Scully, J.; Rogers, A.V.; Mitchison, H.M.; Bush, A.; et al. Accuracy of Immunofluorescence in the Diagnosis of Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2017, 196, 94–101. [Google Scholar] [CrossRef]
- Liu, Z.; Nguyen, Q.P.H.; Guan, Q.; Albulescu, A.; Erdman, L.; Mahdaviyeh, Y.; Kang, J.; Ouyang, H.; Hegele, R.G.; Moraes, T.J.; et al. A quantitative super-resolution imaging toolbox for diagnosis of motile ciliopathies. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Dougherty, G.W.; Loges, N.T.; Klinkenbusch, J.A.; Olbrich, H.; Pennekamp, P.; Menchen, T.; Raidt, J.; Wallmeier, J.; Werner, C.; Westermann, C.; et al. DNAH11 Localization in the Proximal Region of Respiratory Cilia Defines Distinct Outer Dynein Arm Complexes. Am. J. Respir. Cell Mol. Biol. 2016, 55, 213–224. [Google Scholar] [CrossRef]
- Loges, N.T.; Antony, D.; Maver, A.; Deardorff, M.; Güleç, E.Y.; Gezdirici, A.; Nöthe-Menchen, T.; Höben, I.M.; Jelten, L.; Frank, D.; et al. Recessive DNAH9 Loss-of-Function Mutations Cause Laterality Defects and Subtle Respiratory Ciliary-Beating Defects. Am. J. Hum. Genet. 2018, 103, 995–1008. [Google Scholar] [CrossRef]
- Cindrić, S.; Dougherty, G.W.; Olbrich, H.; Hjeij, R.; Loges, N.T.; Amirav, I.; Philipsen, M.C.; Marthin, J.K.; Nielsen, K.G.; Sutharsan, S.; et al. SPEF2- and HYDIN-Mutant Cilia Lack the Central Pair–associated Protein SPEF2, Aiding Primary Ciliary Dyskinesia Diagnostics. Am. J. Respir. Cell Mol. Biol. 2020, 62, 382–396. [Google Scholar] [CrossRef]
- Marshall, C.R.; Scherer, S.W.; Zariwala, M.A.; Lau, L.; Paton, T.A.; Stockley, T.L.; Jobling, R.K.; Ray, P.N.; Knowles, M.R.; Hall, D.A.; et al. Whole-Exome Sequencing and Targeted Copy Number Analysis in Primary Ciliary Dyskinesia. G3 Genes Genomes Genet. 2015, 5, 1775–1781. [Google Scholar] [CrossRef]
- Olbrich, H.; Cremers, C.; Loges, N.T.; Werner, C.; Nielsen, K.G.; Marthin, J.K.; Philipsen, M.; Wallmeier, J.; Pennekamp, P.; Menchen, T.; et al. Loss-of-Function GAS8 Mutations Cause Primary Ciliary Dyskinesia and Disrupt the Nexin-Dynein Regulatory Complex. Am. J. Hum. Genet. 2015, 97, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, J.R.; Becker-Heck, A.; Castleman, V.H.; Al-Mutairi, D.A.; Liu, Y.; Loges, N.T.; Pathak, N.; Austin-Tse, C.; Sheridan, E.; Schmidts, M.; et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat. Genet. 2012, 44, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, A.; Moya, E.; Hirst, R.A.; Patel, M.P.; Robson, E.A.; Hayward, J.; Scully, J.; Fassad, M.R.; Lamb, W.; Schmidts, M.; et al. High prevalence of CCDC103 p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations. Thorax 2018, 73, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.; Wallmeier, J.; Ma, L.; Loges, N.T.; Jaspers, M.; Olbrich, H.; Dougherty, G.W.; Raidt, J.; Werner, C.; Amirav, I.; et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014, 5, 4418. [Google Scholar] [CrossRef]
- Wallmeier, J.; Al-Mutairi, D.A.; Chen, C.-T.; Loges, N.T.; Pennekamp, P.; Menchen, T.; Ma, L.; Shamseldin, H.; Olbrich, H.; Dougherty, G.W.; et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014, 46, 646–651. [Google Scholar] [CrossRef]
- Wallmeier, J.; Frank, D.; Shoemark, A.; Nöthe-Menchen, T.; Cindric, S.; Olbrich, H.; Loges, N.T.; Aprea, I.; Dougherty, G.W.; Pennekamp, P.; et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am. J. Hum. Genet. 2019, 105, 1030–1039. [Google Scholar] [CrossRef]
- Hirst, R.A.; Jackson, C.L.; Coles, J.L.; Williams, G.; Rutman, A.; Goggin, P.M.; Adam, E.C.; Page, A.; Evans, H.J.; Lackie, P.M.; et al. Culture of Primary Ciliary Dyskinesia Epithelial Cells at Air-Liquid Interface Can Alter Ciliary Phenotype but Remains a Robust and Informative Diagnostic Aid. PLoS ONE 2014, 9, e89675. [Google Scholar] [CrossRef]
- Dixon, M.; Shoemark, A. Secondary defects detected by transmission electron microscopy in primary ciliary dyskinesia diagnostics. Ultrastruct. Pathol. 2017, 41, 390–398. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Rutman, A.; Williams, G.; Kulkarni, N.; Hayes, J.; Hirst, R.A. Ciliated conical epithelial cell protrusions point towards a diagnosis of primary ciliary dyskinesia. Respir. Res. 2018, 19, 125. [Google Scholar] [CrossRef]
- Toskala, E.; Haataja, J.; Shirasaki, H.; Rautiainen, M. Culture of cells harvested with nasal brushing: A method for evaluating ciliary function. Rhinology 2005, 43, 121–124. [Google Scholar]
- Willems, T.; Jorissen, M. Sequential monolayer-suspension culture of human airway epithelial cells. J. Cyst. Fibros. 2004, 3 (Suppl. 2), 53–54. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, M.; Montemurro, F.; Cangiotti, A.M.; Ragazzo, V.; Di Cicco, M.; Vinci, B.; Vozzi, G.; Macchia, P.; Boner, A.L. Simplified cell culture method for the diagnosis of atypical primary ciliary dyskinesia. Thorax 2009, 64, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, M.; Bush, A.; Montemurro, F.; Pioggia, G.; Piras, M.; Tartarisco, G.; Di Cicco, M.E.; Chinellato, I.; Cangiotti, A.M.; Boner, A.L. Rapid diagnosis of primary ciliary dyskinesia: Cell culture and soft computing analysis. Eur. Respir. J. 2013, 41, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Marthin, J.K.; Stevens, E.; Larsen, L.A.; Christensen, S.T.; Nielsen, K.G. Patient-specific three-dimensional explant spheroids derived from human nasal airway epithelium: A simple methodological approach for ex vivo studies of primary ciliary dyskinesia. Cilia 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Rutman, A.; Williams, G.; O’Callaghan, C. Ciliated Air-Liquid Cultures as an Aid to Diagnostic Testing of Primary Ciliary Dyskinesia. Chest 2010, 138, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.X.; Jackson, C.L.; Cole, J.L.; Lackie, P.M.; Traini, D.; Young, P.M.; Lucas, J.; Conway, J. Primary Air–Liquid Interface Culture of Nasal Epithelium for Nasal Drug Delivery. Mol. Pharm. 2016, 13, 2242–2252. [Google Scholar] [CrossRef]
- Collins, S.A.; Kelso, M.J.; Rineh, A.; Yepuri, N.R.; Coles, J.; Jackson, C.L.; Halladay, G.D.; Walker, W.T.; Webb, J.S.; Hall-Stoodley, L.; et al. Cephalosporin-3′-Diazeniumdiolate NO Donor Prodrug PYRRO-C3D Enhances Azithromycin Susceptibility of Nontypeable Haemophilus influenzae Biofilms. Antimicrob. Agents Chemother. 2017, 61, 02086-16. [Google Scholar] [CrossRef]
- Walker, W.T.; Jackson, C.L.; Allan, R.N.; Collins, S.A.; Kelso, M.J.; Rineh, A.; Yepuri, N.R.; Nicholas, B.; Lau, L.; Johnston, D.; et al. Primary ciliary dyskinesia ciliated airway cells show increased susceptibility to Haemophilus influenzae biofilm formation. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Walker, W.T.; Jackson, C.L.; Coles, J.; Lackie, P.M.; Faust, S.N.; Hall-Stoodley, L.; Lucas, J. Ciliated cultures from patients with primary ciliary dyskinesia produce nitric oxide in response to Haemophilus influenzae infection and proinflammatory cytokines. Chest 2014, 145, 668–669. [Google Scholar] [CrossRef]
- Smith, C.M.; Kulkarni, H.; Radhakrishnan, P.; Rutman, A.; Bankart, M.J.; Williams, G.; Hirst, R.A.; Easton, A.J.; Andrew, P.W.; O’Callaghan, C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 2014, 43, 485–496. [Google Scholar] [CrossRef]
- Smith, C.M.; Sandrini, S.; Datta, S.; Freestone, P.; Shafeeq, S.; Radhakrishnan, P.; Williams, G.; Glenn, S.M.; Kuipers, O.P.; Hirst, R.A.; et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am. J. Respir. Crit. Care Med. 2014, 190, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Swindle, E.J.; Dennison, P.; Jayasekera, N.P.; Dudley, S.; Monk, P.; Behrendt, H.; Schmidt-Weber, C.; Holgate, S.T.; Howarth, P.H.; et al. Barrier responses of human bronchial epithelial cells to grass pollen exposure. Eur. Respir. J. 2012, 42, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.A.; Conforti, F.; Perotin-Collard, J.M.; Frank, M.; Crispin, M.; Coles, J.; et al. A novel isoform of ACE2 is expressed in human nasal and bronchial respiratory epithelia and is upregulated in response to RNA respiratory virus infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Kuehni, C.E.; Lucas, J.S. Diagnosis of primary ciliary dyskinesia: Summary of the ERS Task Force report. Breathe 2017, 13, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Evans, H.J.; Haarman, E.G.; Hirst, R.A.; Hogg, C.; Jackson, C.L.; Nielsen, K.G.; Omran, H.; Papon, J.-F.; Robinson, P.; et al. Exploring the Art of Ciliary Beating. Chest 2017, 152, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Serafini, S.M.; Michaelson, E.D. Length and distribution of cilia in human and canine airways. Bull. Eur. Physiopathol. Respir. 1977, 13, 551–559. [Google Scholar]
- Lucas, J.S.; Burgess, A.; Mitchison, H.M.; Moya, E.; Williamson, M.; Hogg, C.; National PCD Service, UK. Diagnosis and management of primary ciliary dyskinesia. Arch. Dis. Child. 2014, 99, 850–856. [Google Scholar] [CrossRef]
- Lucas, J.; Chetcuti, P.; Copeland, F.; Hogg, C.; Kenny, T.; Moya, E.; O’Callaghan, C.; Walker, W.T. Overcoming challenges in the management of primary ciliary dyskinesia: The UK model. Paediatr. Respir. Rev. 2014, 15, 142–145. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-Differentiated Human Airway Epithelial Cell Cultures. Methods Mol. Med. 2005, 107, 183–206. [Google Scholar] [CrossRef]
- Lai, M.; Pifferi, M.; Bush, A.; Piras, M.; Michelucci, A.; Di Cicco, M.; Del Grosso, A.; Quaranta, P.; Cursi, C.; Tantillo, E.; et al. Gene editing ofDNAH11restores normal cilia motility in primary ciliary dyskinesia. J. Med. Genet. 2016, 53, 242–249. [Google Scholar] [CrossRef]


| PCD Positive/PCD Highly-Likely (n = 7) | PCD Highly-Unlikely (n = 55) | Equivocal/Ongoing (n = 8) | |
|---|---|---|---|
| Median age (min, max) | 11.15 (0.1, 32.2) | 4.5 (0.1, 70.4) | 13.5 (4.0, 63.7) |
| Female n (%) | n = 5 (71.4%) | n = 26 (47.3%) | n = 5 (62.5%) |
| Chronic wet cough % | 100 | 63.6 | 87.5 |
| Rhinosinusitis % | 85.7 | 80 | 75 |
| Situs abnormality % | 71.4 | 12.7 | 0 |
| Median nNO nl/min | 24.4 (Q1 18.8, Q3 47.5, min 18, max 67, n = 6) | 380 (Q1 240, Q3 596, min 1.2, max 1280, n = 49) | 280 (Q1 93, Q 3 548.5, min 5, max 762, n = 8) |
| TEM | n = 3 normal n = 2 outer arm defects n = 2 outer arm defects with possible inner arm defect | n = 49 normal n = 6 no data | n = 7 normal n = 1 no data |
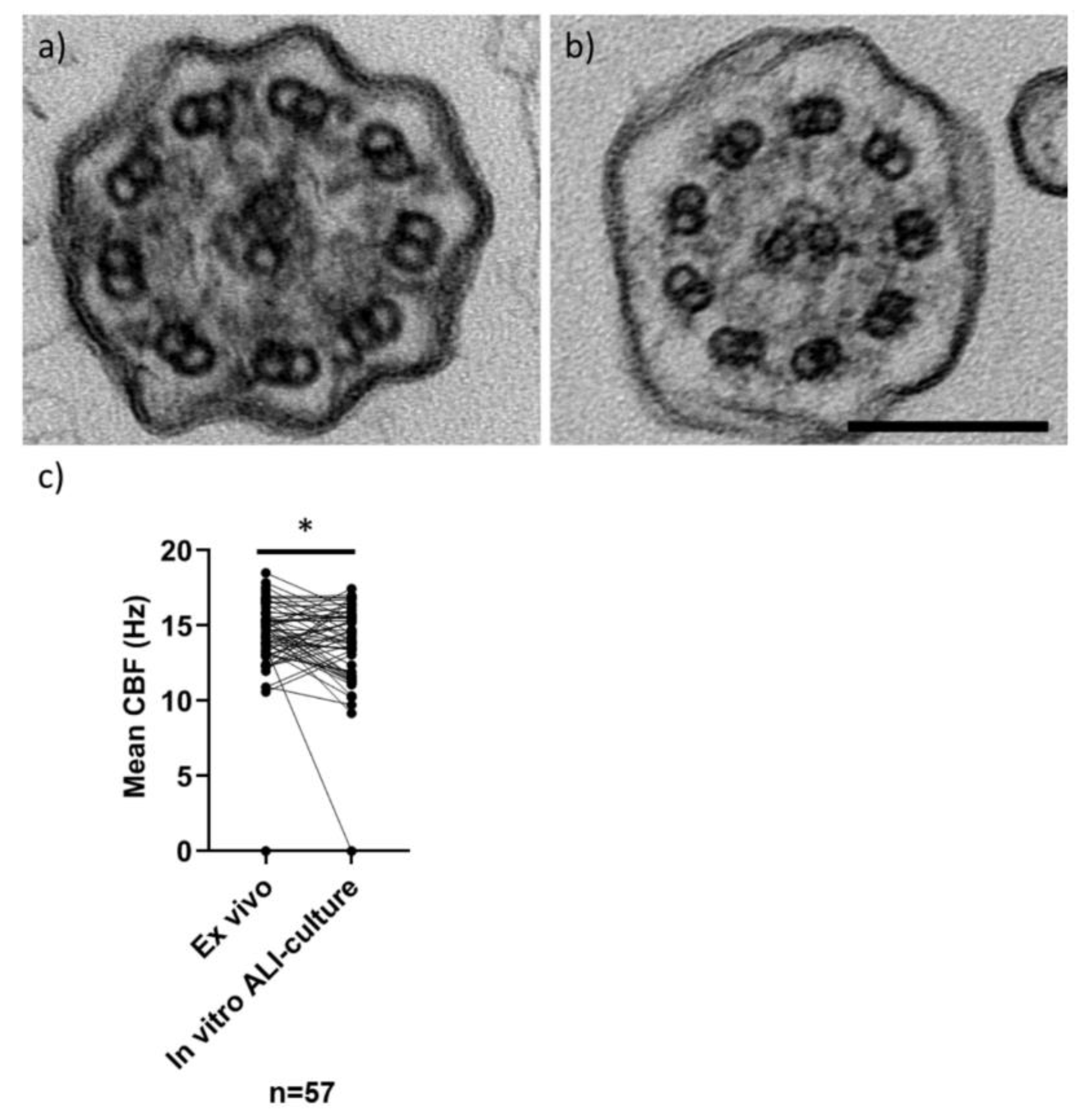
| Mean ex vivo sample CBF or CBP | n = 5 static, 0 Hz n = 2 variable dyskinesia | 14.8 Hz, SD ± 1.8 (n = 52) n = 2 variable dyskinesia n = 1 insufficient | 13.9 Hz, SD ± 1.2 (n = 6) n = 2 variable dyskinesia |
| Mean in vitro ALI-culture CBF or CBP | n = 5 static, 0 Hz n = 2 no data (1 failed due to bacteria, 1 sample frozen) | 13.9 Hz, SD ± 2.1 (n = 52) n = 3 not done | 14.4 Hz, SD ± 2.4 (n = 4) n = 2 became static, 0 Hz n = 2 no data (failed due to bacteria) |
| Causative genes | RSPH4 homozygous CCDC151 homozygous DNAAF3 homozygous DNAAF5 heterozygous DNAH11 homozygous n = 2 no data | n = 13 no mutation found n = 42 no data | n = 4 no mutation found n = 1 CCDC164 single heterozygous mutation n = 3 no data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coles, J.L.; Thompson, J.; Horton, K.L.; Hirst, R.A.; Griffin, P.; Williams, G.M.; Goggin, P.; Doherty, R.; Lackie, P.M.; Harris, A.; et al. A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia. J. Clin. Med. 2020, 9, 3753. https://doi.org/10.3390/jcm9113753
Coles JL, Thompson J, Horton KL, Hirst RA, Griffin P, Williams GM, Goggin P, Doherty R, Lackie PM, Harris A, et al. A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia. Journal of Clinical Medicine. 2020; 9(11):3753. https://doi.org/10.3390/jcm9113753
Chicago/Turabian StyleColes, Janice L., James Thompson, Katie L. Horton, Robert A. Hirst, Paul Griffin, Gwyneth M. Williams, Patricia Goggin, Regan Doherty, Peter M. Lackie, Amanda Harris, and et al. 2020. "A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia" Journal of Clinical Medicine 9, no. 11: 3753. https://doi.org/10.3390/jcm9113753
APA StyleColes, J. L., Thompson, J., Horton, K. L., Hirst, R. A., Griffin, P., Williams, G. M., Goggin, P., Doherty, R., Lackie, P. M., Harris, A., Walker, W. T., O’Callaghan, C., Hogg, C., Lucas, J. S., Blume, C., & Jackson, C. L. (2020). A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia. Journal of Clinical Medicine, 9(11), 3753. https://doi.org/10.3390/jcm9113753







