Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Literature Search
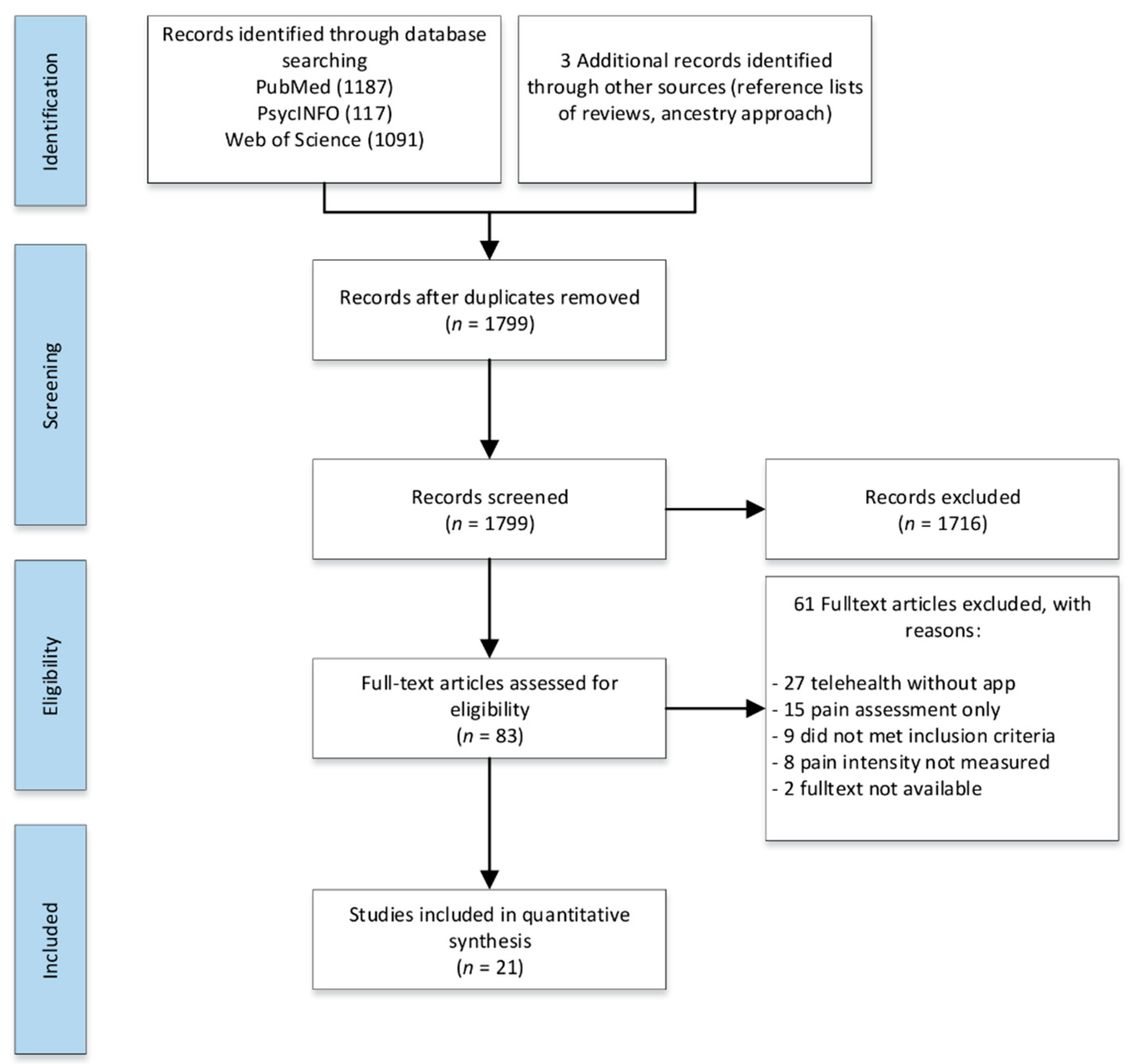
2.4. Study Selection
2.5. Data Extraction
2.6. Outcomes
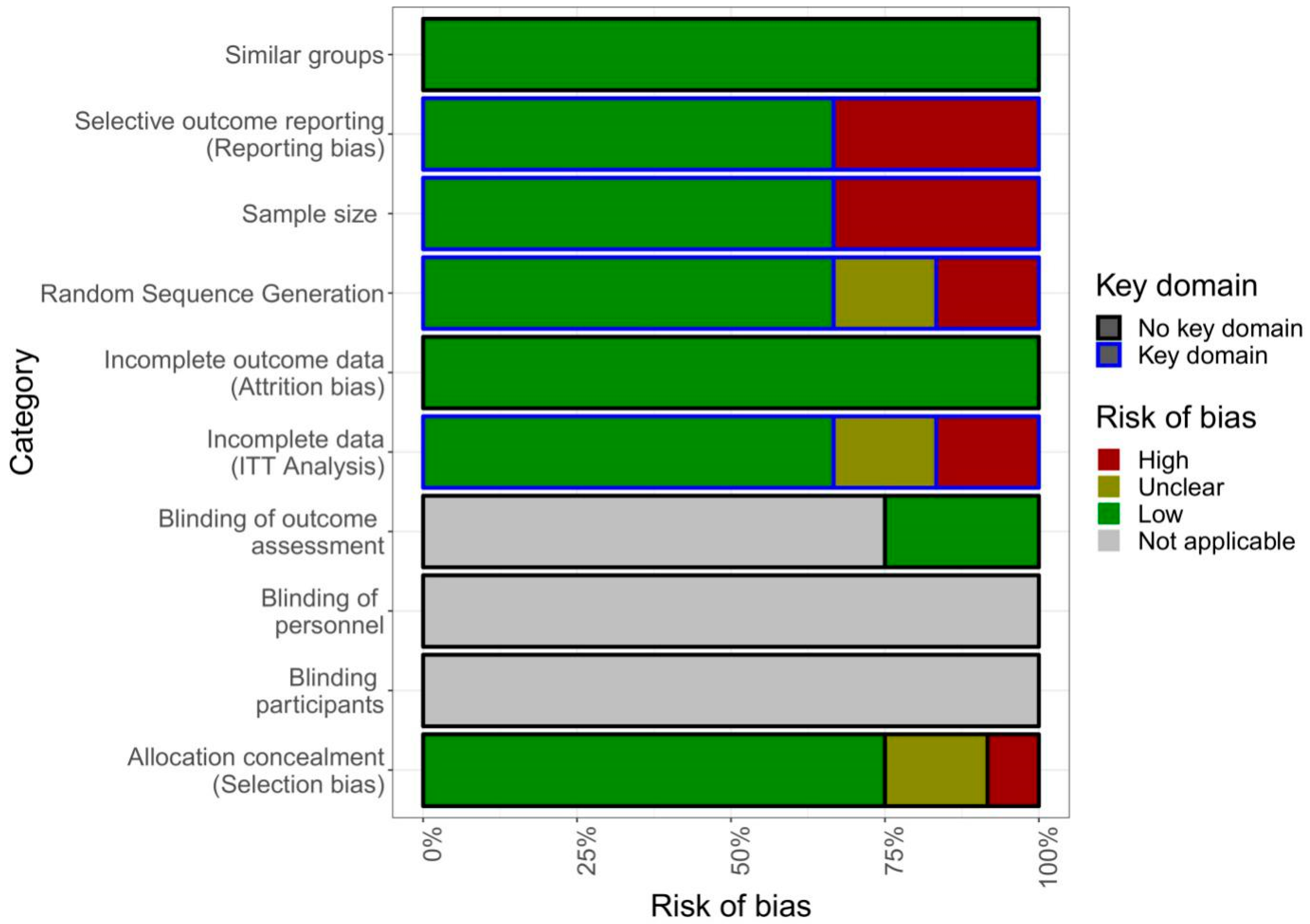
2.7. Quality of Studies
2.8. Strategy for Data Synthesis
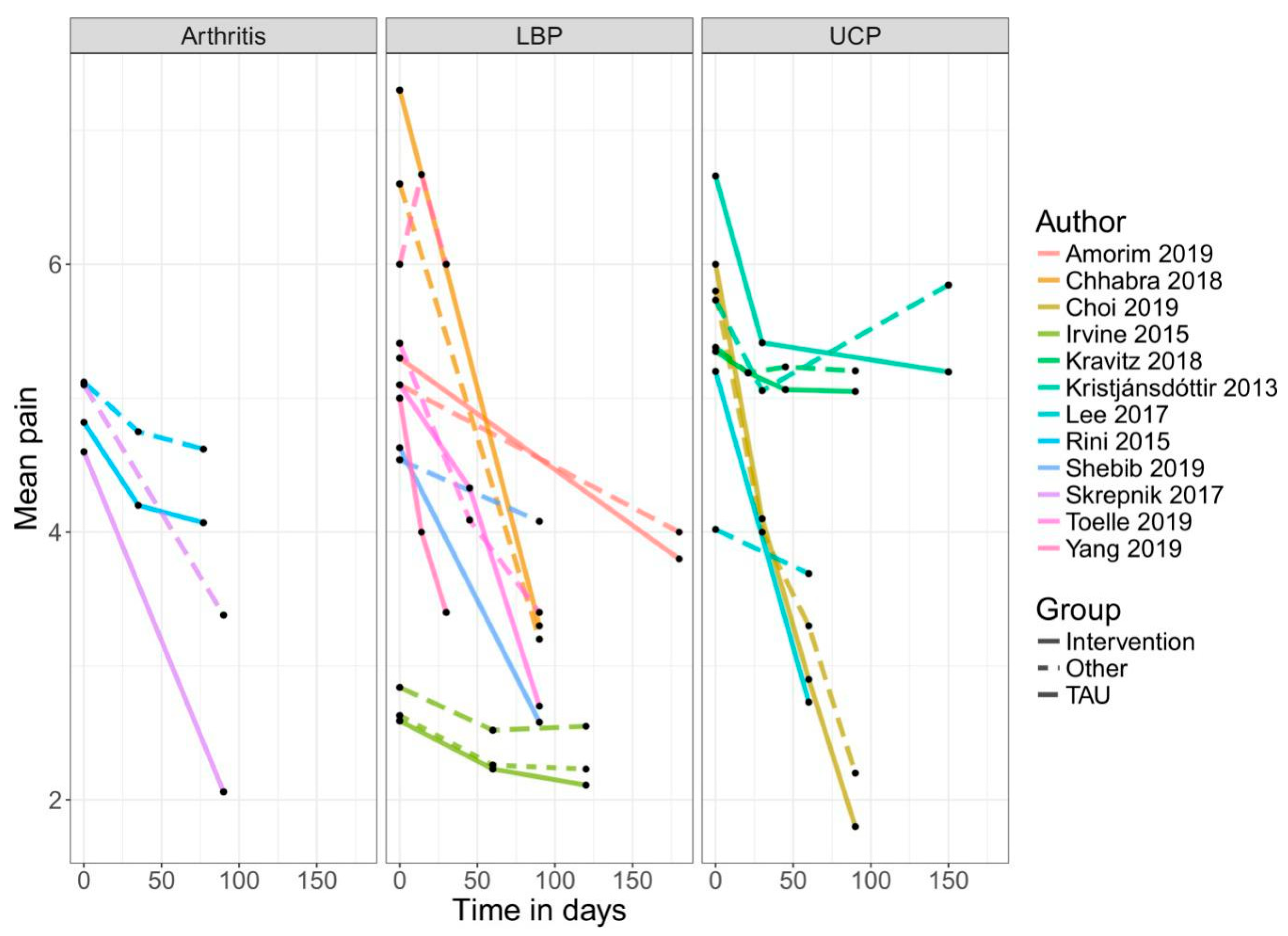
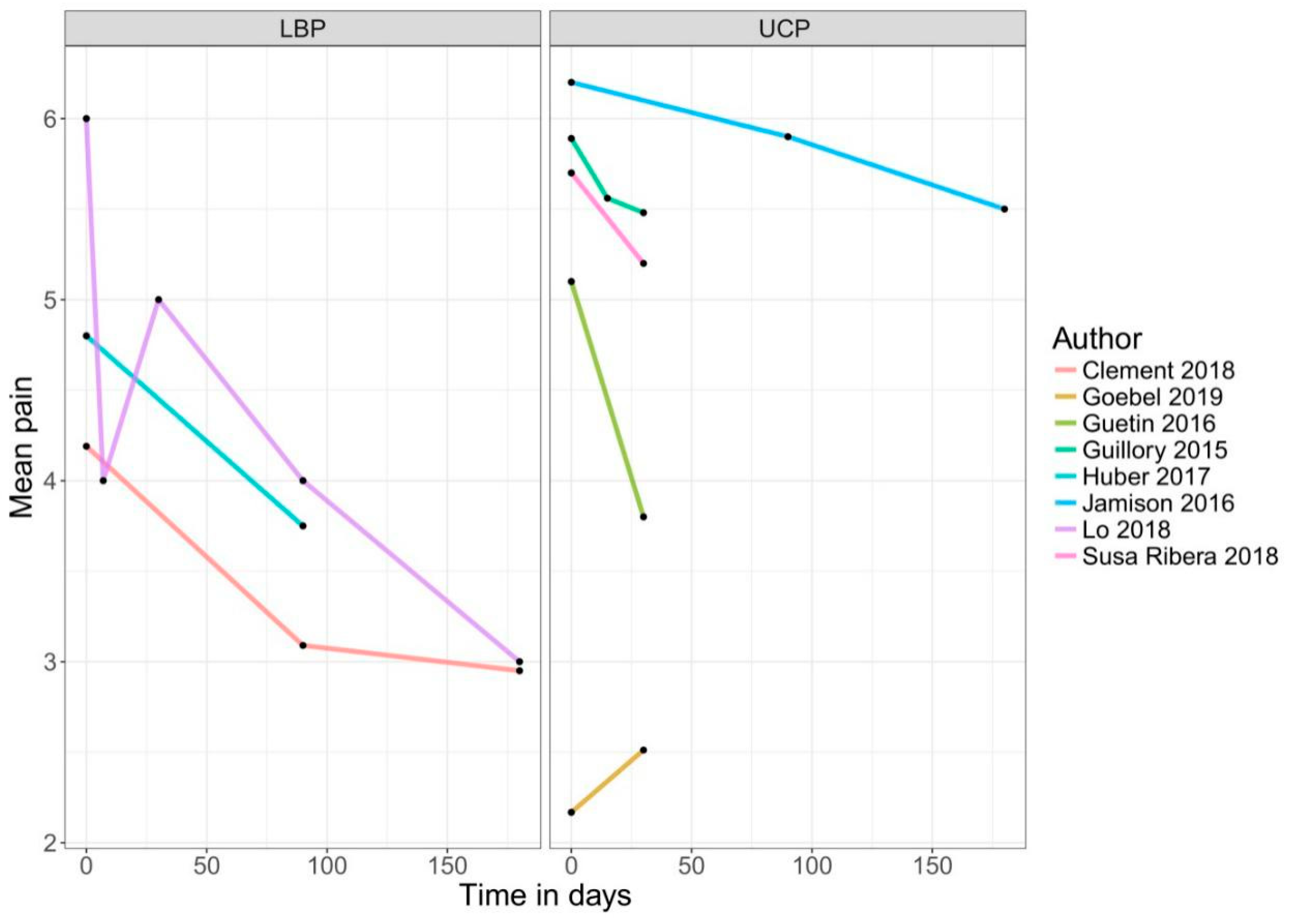
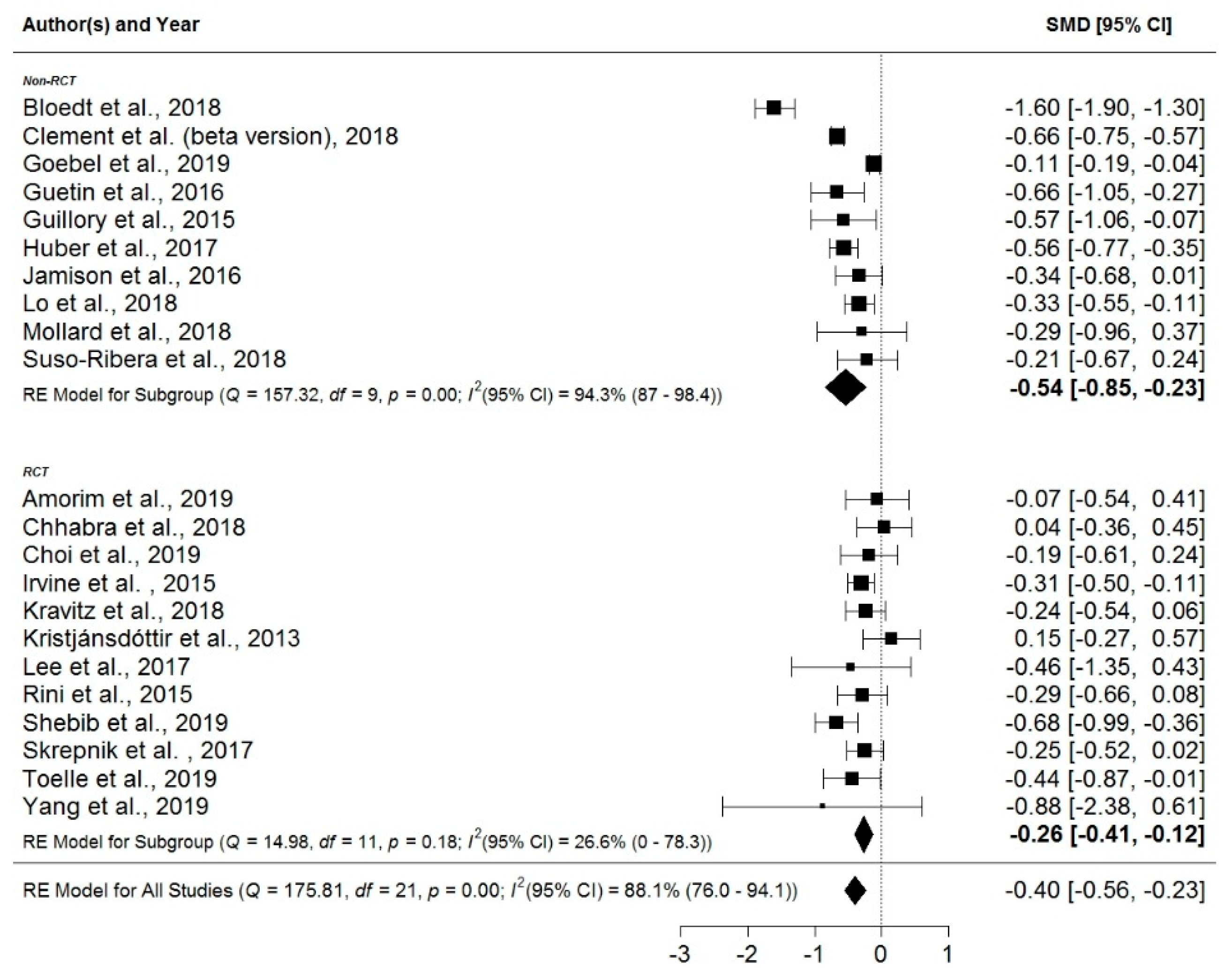
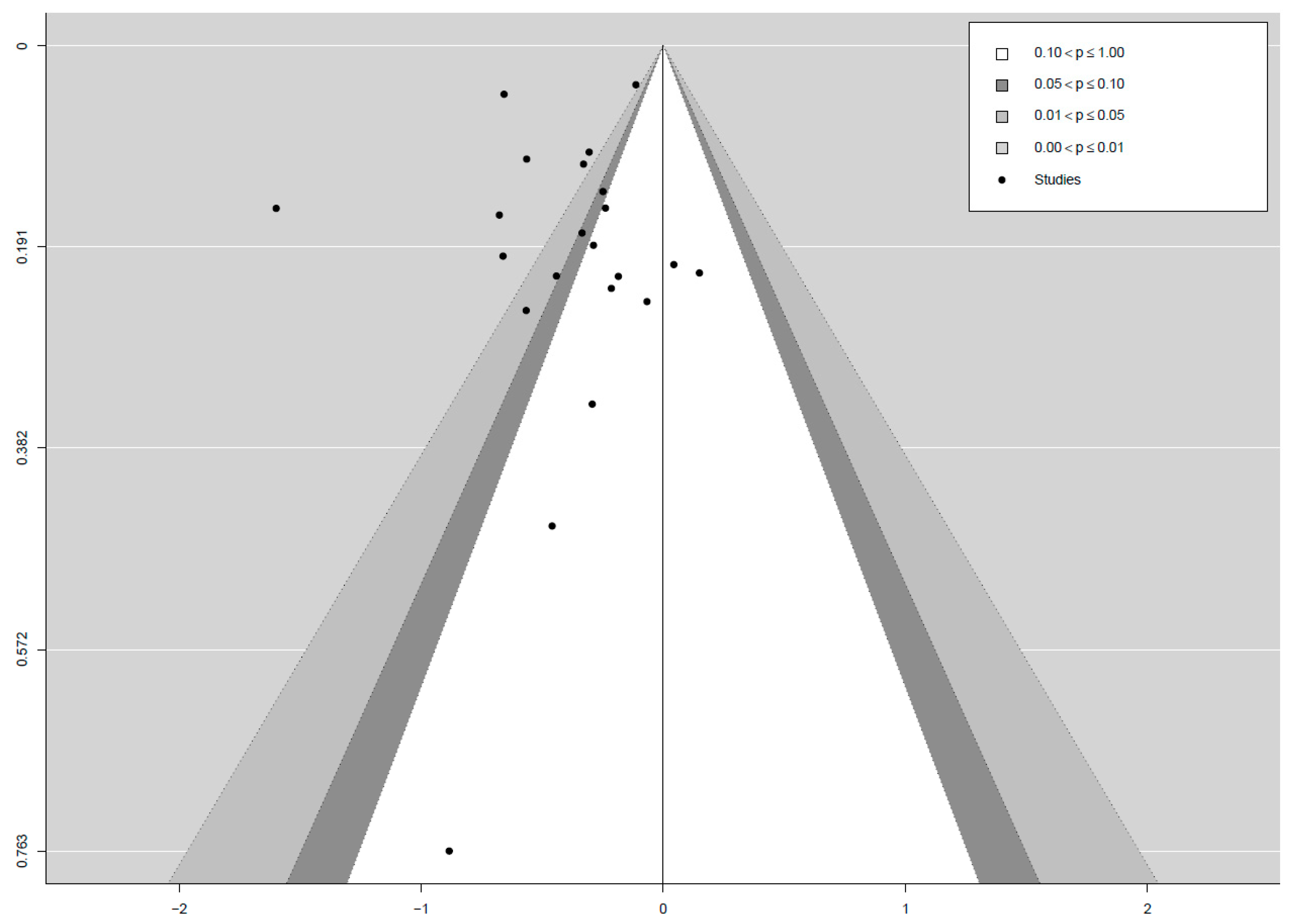
3. Results
Assessment of Quality of the Apps
4. Discussion
4.1. Efficacy of Mobile Application-Based Treatments
4.2. Quality of the Application-Based Treatments
4.3. Comparison with Existing Literature
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; IASP: Seattle, WA, USA, 1994. [Google Scholar]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003. [Google Scholar] [CrossRef]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef]
- Gureje, O.; Simon, G.E.; Von Korff, M. A cross-national study of the course of persistent pain in primary care. Pain 2001, 92, 195–200. [Google Scholar] [CrossRef]
- Itz, C.J.; Geurts, J.; Van Kleef, M.; Nelemans, P. Clinical course of non-specific low back pain: A systematic review of prospective cohort studies set in primary care. Eur. J. Pain 2013, 17, 5–15. [Google Scholar] [CrossRef]
- Henningsen, P.; Zipfel, S.; Herzog, W. Management of functional somatic syndromes. Lancet 2007, 369, 946–955. [Google Scholar] [CrossRef]
- Mayou, R.; Farmer, A. Functional somatic symptoms and syndromes. BMJ 2002, 325, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Van der Feltz-Cornelis, C.M.; Hoedeman, R.; Keuter, E.J.; Swinkels, J.A. Presentation of the Multidisciplinary Guideline Medically Unexplained Physical Symptoms (MUPS) and somatoform disorder in the Netherlands: Disease management according to risk profiles. J. Sychosom. Res. 2012, 72, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.-C.; Penedo, J.M.G.; Ehrenthal, J.C.; Neubauer, E.; Amelung, D.; Schroeter, C.; Schiltenwolf, M. Impact of attachment behavior on the treatment process of chronic pain patients. J. Pain Res. 2018, 11, 2653. [Google Scholar] [CrossRef] [PubMed]
- Thurnheer, S.E.; Gravestock, I.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Benefits of mobile apps in pain management: Systematic review. JMIR mHealth uHealth 2018, 6, e11231. [Google Scholar] [CrossRef]
- Ehde, D.M.; Dillworth, T.M.; Turner, J.A. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am. Psychol. 2014, 69, 153. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.R.; Keefe, F.J.; Brigidi, B.; Naud, S.; Helzer, J.E. Therapeutic interactive voice response for chronic pain reduction and relapse prevention. Pain 2008, 134, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, F.; Lindenmeyer, A.; Powell, J.; Lowe, P.; Thorogood, M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J. Med Internet Res. 2006, 8, e10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Carrieri-Kohlman, V.; Rankin, S.H.; Slaughter, R.; Stulbarg, M.S. Internet-based patient education and support interventions: A review of evaluation studies and directions for future research. Comput. Biol. Med. 2004, 34, 95–112. [Google Scholar] [CrossRef]
- Insider, B. IQVIA Institute for Human Data Science Study: Impact of Digital Health Grows as Innovation, Evidence and Adoption of Mobile Health Apps Accelerate 2017. Available online: https://tinyurl.com/y7qamjat (accessed on 14 February 2019).
- Reynoldson, C.; Stones, C.; Allsop, M.; Gardner, P.; Bennett, M.I.; Closs, S.J.; Jones, R.; Knapp, P. Assessing the quality and usability of smartphone apps for pain self-management. Pain Med. 2014, 15, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, C.; Jibb, L.A.; Rivera, J.; Agarwal, A.; Stinson, J.N. There’sa Pain App for that. Clin. J. Pain 2015, 31, 557–563. [Google Scholar] [CrossRef]
- Machado, G.C.; Pinheiro, M.B.; Lee, H.; Ahmed, O.H.; Hendrick, P.; Williams, C.; Kamper, S.J. Smartphone apps for the self-management of low back pain: A systematic review. Best Pract. Res. Clin. Rheumatol. 2016, 30, 1098–1109. [Google Scholar] [CrossRef]
- Bhattarai, P.; Newton-John, T.R.; Phillips, J.L. Quality and usability of arthritic pain self-management apps for older adults: A systematic review. Pain Med. 2018, 19, 471–484. [Google Scholar] [CrossRef]
- Portelli, P.; Eldred, C. A quality review of smartphone applications for the management of pain. Br. J. Pain 2016, 10, 135–140. [Google Scholar] [CrossRef]
- Chiew, T.K. A systematic literature review of the design approach and usability evaluation of the pain management mobile applications. Symmetry 2019, 11, 400. [Google Scholar]
- Pfeifer, A.-N.; Holl, F.; Swoboda, W.; Schröder-Pfeifer, P.; Schiltenwolf, M.; Uddin, R. Mobile application-based interventions for chronic non-cancer pain patients: A systematic review and meta-analysis of effectiveness and app quality. PROSPERO 2019, CRD42019139262.22. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019139262 (accessed on 4 November 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Logan, D.E.; Claar, R.L.; Scharff, L. Social desirability response bias and self-report of psychological distress in pediatric chronic pain patients. Pain 2008, 136, 366–372. [Google Scholar] [CrossRef]
- Lipsey, M.W.; Wilson, D.B. Practical Meta-Analysis; Sage Publications Inc.: Washington, DC, USA, 2001; Volume 49. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer. Available online: https://apps.automeris.io/WebPlotDigitizer (accessed on 31 October 2020).
- Salazar, A.; de Sola, H.; Failde, I.; Moral-Munoz, J.A. Measuring the quality of mobile apps for the management of pain: Systematic search and evaluation using the mobile app rating scale. JMIR mHealth uHealth 2018, 6, e10718. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. Available online: https://www.ncbi.nlm.nih.gov/books/NBK99082/bin/appb-fm4.pdf (accessed on 31 October 2020).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C.J.B. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R.J.B. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Amorim, A.B.; Pappas, E.; Simic, M.; Ferreira, M.L.; Jennings, M.; Tiedemann, A.; Carvalho-e-Silva, A.P.; Caputo, E.; Kongsted, A.; Ferreira, P.H. Integrating mobile-health, health coaching, and physical activity to reduce the burden of chronic low back pain trial (IMPACT): A pilot randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20. [Google Scholar] [CrossRef]
- Chhabra, H.S.; Sharma, S.; Verma, S. Smartphone app in self-management of chronic low back pain: A randomized controlled trial. Eur. Spine J. 2018, 27, 2862–2874. [Google Scholar] [CrossRef]
- Choi, Y.; Nam, J.; Yang, D.; Jung, W.; Lee, H.-R.; Kim, S.H. Effect of smartphone application-supported self-rehabilitation for frozen shoulder: A prospective randomized control study. Clin. Rehabil. 2019, 33, 653–660. [Google Scholar] [CrossRef]
- Irvine, A.B.; Russell, H.; Manocchia, M.; Mino, D.E.; Glassen, T.C.; Morgan, R.; Gau, J.M.; Birney, A.J.; Ary, D.V. Mobile-web app to self-manage low back pain: Randomized controlled trial. J. Med Internet Res. 2015, 17. [Google Scholar] [CrossRef]
- Kravitz, R.L.; Schmid, C.H.; Marois, M.; Wilsey, B.; Ward, D.; Hays, R.D.; Duan, N.; Wang, Y.; MacDonald, S.; Jerant, A.; et al. Effect of mobile device—Supported single-patient multi-crossover trials on treatment of chronic musculoskeletal pain: A randomized clinical trial. JAMA Intern. Med. 2018, 178, 1368–1377. [Google Scholar] [CrossRef]
- Kristjansdottir, O.B.; Fors, E.A.; Eide, E.; Finset, A.; Stensrud, T.L.; van Dulmen, S.; Wigers, S.H.; Eide, H. A smartphone-based intervention with diaries and therapist feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain. Part 2: 11-month follow-up results of a randomized trial. J. Med Internet Res. 2013, 15, 152–170. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.; Lim, T.; Kim, T.; Kim, S.; Suh, D.; Lee, S.; Yoon, B. Effectiveness of an application-based neck exercise as a pain management tool for office workers with chronic neck pain and functional disability: A pilot randomized trial. Eur. J. Integr. Med. 2017, 12, 87–92. [Google Scholar] [CrossRef]
- Rini, C.; Porter, L.S.; Somers, T.J.; McKee, D.C.; DeVellis, R.F.; Smith, M.; Winkel, G.; Ahern, D.K.; Goldman, R.; Stiller, J.L.; et al. Automated, internet-based pain coping skills training to manage osteoarthritis pain: A randomized controlled trial. Pain 2015, 156, 837. [Google Scholar] [CrossRef]
- Shebib, R.; Bailey, J.F.; Smittenaar, P.; Perez, D.A.; Mecklenburg, G.; Hunter, S. Randomized controlled trial of a 12-week digital care program in improving low back pain. NPJ Digit. Med. 2019, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Skrepnik, N.; Spitzer, A.; Altman, R.; Hoekstra, J.; Stewart, J.; Toselli, R. Assessing the impact of a novel smartphone application compared with standard follow-up on mobility of patients with knee osteoarthritis following treatment with Hylan G-F 20: A randomized controlled trial. JMIR mHealth uHealth 2017, 5. [Google Scholar] [CrossRef]
- Toelle, T.R.; Utpadel-Fischler, D.A.; Haas, K.-K.; Priebe, J.A. App-based multidisciplinary back pain treatment versus combined physiotherapy plus online education: A randomized controlled trial. NPJ Digit. Med. 2019, 2, 34. [Google Scholar] [CrossRef]
- Yang, J.; Wei, Q.; Ge, Y.; Meng, L.; Zhao, M. Smartphone-based remote self-management of chronic low back pain: A preliminary study. J. Healthcare Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Blodt, S.; Pach, D.; von Eisenhart-Rothe, S.; Lotz, F.; Roll, S.; Icke, K.; Witt, C.M. Effectiveness of app-based self-acupressure for women with menstrual pain compared to usual care: A randomized pragmatic trial. Am. J. Obstet. Gynecol. 2018, 218. [Google Scholar] [CrossRef]
- Clement, I.; Lorenz, A.; Ulm, B.; Plidschun, A.; Huber, S. Implementing systematically collected user feedback to increase user retention in a mobile app for self-management of low back pain: Retrospective cohort study. JMIR mHealth uHealth 2018, 6, e10422. [Google Scholar] [CrossRef]
- Göbel, H.; Frank, B.; Heinze, A.; Zimmermann, W.; Göbel, C.; Göbel, A.; Brunkhorst, J.; Rupp, K.J.D.S. Gesundheitsverhalten von Migräne-und Kopfschmerzpatienten bei digitaler Therapiebegleitung mit der Migräne-App. Schmerz 2019, 33, 147–155. [Google Scholar] [CrossRef]
- Guétin, S.; de Diego, E.; Mohy, F.; Adolphe, C.; Hoareau, G.; Touchon, J.; Thayer, J.F.; Koenig, J. A patient-controlled, smartphone-based music intervention to reduce pain—A multi-center observational study of patients with chronic pain. Eur. J. Integr. Med. 2016, 8, 182–187. [Google Scholar] [CrossRef]
- Guillory, J.; Chang, P.; Henderson, C.R., Jr.; Shengelia, R.; Lama, S.; Warmington, M.; Jowza, M.; Gay, G. Piloting a text message-based social support intervention for patients with chronic pain: Establishing feasibility and preliminary efficacy. Clin. J. Pain 2015, 31, 548. [Google Scholar] [CrossRef]
- Huber, S.; Priebe, J.A.; Baumann, K.M.; Plidschun, A.; Schiessl, C.; Tolle, T.R. Treatment of low back pain with a digital multidisciplinary pain treatment app: Short-term results. JMIR Rehabil. Assist. Technol. 2017, 4, e11. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Lei, D.; Li, L.; Huang, D.F.; Tong, K.F. The perceived benefits of an artificial intelligence-embedded mobile app implementing evidence-based guidelines for the self-management of chronic neck and back pain: Observational study. JMIR mHealth uHealth 2018, 6. [Google Scholar] [CrossRef]
- Mollard, E.; Michaud, K. A mobile app with optical imaging for the self-management of hand rheumatoid arthritis: Pilot study. JMIR mHealth uHealth 2018, 6. [Google Scholar] [CrossRef]
- Suso-Ribera, C.; Castilla, D.; Zaragoza, I.; Ribera-Canudas, M.V.; Botella, C.; Garcia-Palacios, A. Validity, reliability, feasibility, and usefulness of pain monitor: A multidimensional smartphone app for daily monitoring of adults with heterogenous chronic pain. Clin. J. Pain 2018, 34, 900–908. [Google Scholar] [CrossRef]
- Jamison, R.N.; Mei, A.; Ross, E.L. Longitudinal trial of a smartphone pain application for chronic pain patients: Predictors of compliance and satisfaction. J. Telemed. Telecare 2016, 24, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jamison, R.N.; Jurcik, D.C.; Edwards, R.R.; Huang, C.-C.; Ross, E.L. A pilot comparison of a smartphone app with or without 2-way messaging among chronic pain patients: Who benefits from a pain app? Clin. J. Pain 2017, 33, 676. [Google Scholar] [CrossRef]
- Blodt, S.; Witt, C.M.; Holmberg, C. Women’s reasons for participation in a clinical trial for menstrual pain: A qualitative study. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Cuijpers, P. Internet-based and other computerized psychological treatments for adult depression: A meta-analysis. Cogn. Behav. Ther. 2009, 38, 196–205. [Google Scholar] [CrossRef]
- Spek, V.; Cuijpers, P.; Nyklíček, I.; Riper, H.; Keyzer, J.; Pop, V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: A meta-analysis. Psychol. Med. 2007, 37, 319–328. [Google Scholar] [CrossRef]
- Von Korff, M.; Dworkin, S.F.; Le Resche, L. Graded chronic pain status: An epidemiologic evaluation. Pain 1990, 40, 279–291. [Google Scholar] [CrossRef]
- Rosser, B.A.; Eccleston, C. Smartphone applications for pain management. J. Telemed. Telecare 2011, 17, 308–312. [Google Scholar] [CrossRef]
- Firth, J.; Torous, J.; Nicholas, J.; Carney, R.; Pratap, A.; Rosenbaum, S.; Sarris, J. The efficacy of smartphone-based mental health interventions for depressive symptoms: A meta-analysis of randomized controlled trials. World Psychiatry 2017, 16, 287–298. [Google Scholar] [CrossRef]
- Firth, J.; Torous, J.; Nicholas, J.; Carney, R.; Rosenbaum, S.; Sarris, J. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J. Affect. Disord. 2017, 218, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Farzandipour, M.; Nabovati, E.; Sharif, R.; Arani, M.H.; Anvari, S. Patient self-management of asthma using mobile health applications: A systematic review of the functionalities and effects. Appl. Clin. Inform. 2017, 8, 1068–1081. [Google Scholar] [CrossRef]
- Steinert, A.; Haesner, M.; Steinhagen-Thiessen, E. App-basiertes selbstmonitoring bei Typ-2-diabetes. Z. Gerontol. Geriatr. 2017, 50, 516–523. [Google Scholar] [CrossRef]
- Andersson, G.; Cuijpers, P. Pros and cons of online cognitive–behavioural therapy. Br. J. Psychiatry 2008, 193, 270–271. [Google Scholar] [CrossRef]
- Younger, J.; McCue, R.; Mackey, S. Pain outcomes: A brief review of instruments and techniques. Curr. Pain Headache Rep. 2009, 13, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Deloach, J.; Porucznik, C.A.; Powell, A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J. Shoulder Elbow Surg. 2009, 18, 927–932. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Type of Study | Target Population | % Female | Recruitment | Inclusion | N | Intervention | Additional Support | Intervention Duration | Primary Endpoint | Outcome Measure | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amorim 2019 | RCT | Adults (18–65 years) | 50% | via clinic | Chronic low back pain: - mechanical LBP for over 12 weeks | 68 | 1. Intervention group: Mobile web app; 2. Control group: Information booklet and staying active | YES: After the first face-to-face coaching session, the health coach contacted participants fortnightly and information booklet + Fitbit tracker | not specified | 6 months | Pain NRS | Australia |
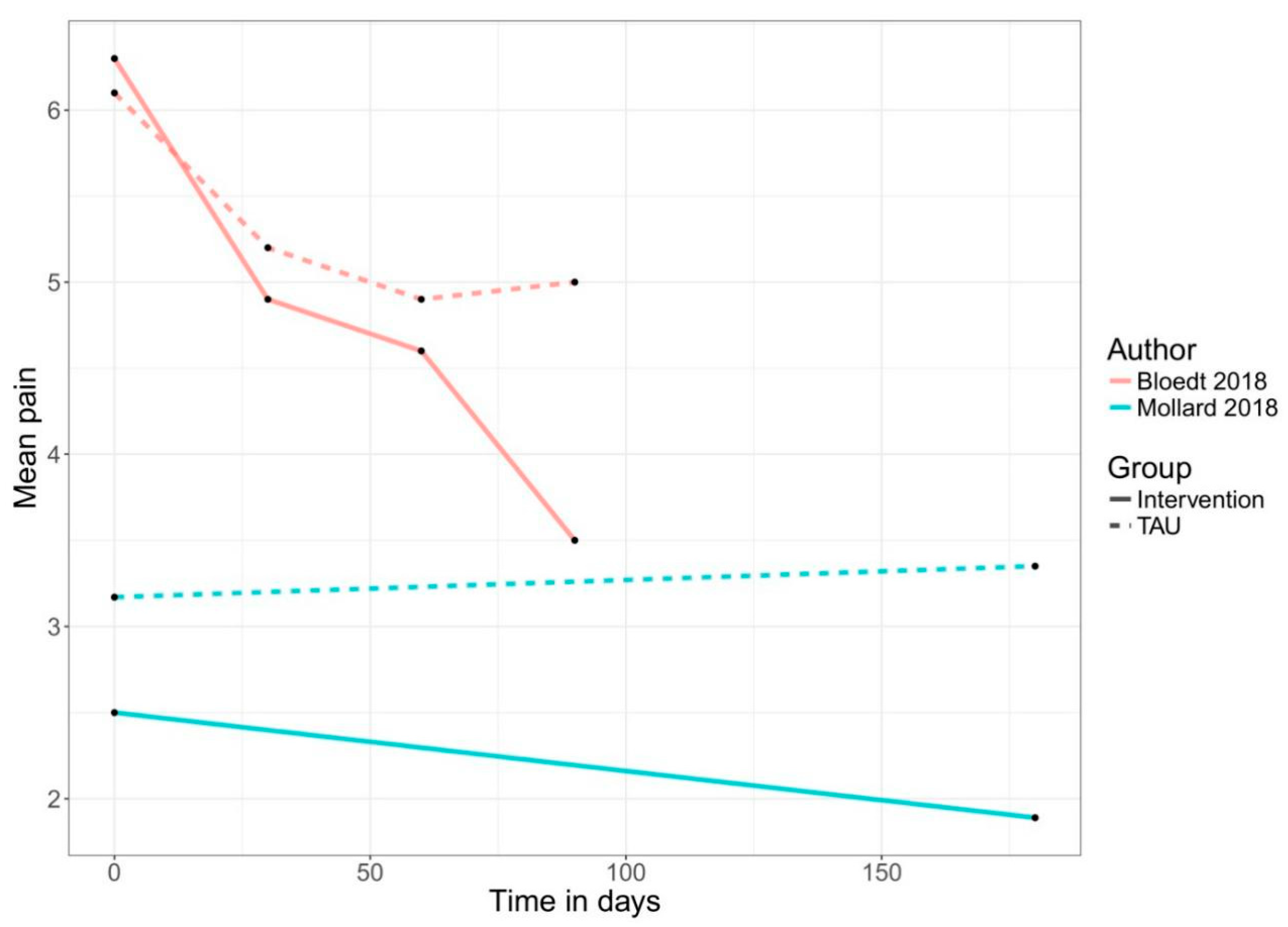
| Bloedt 2018 | Randomized pragmatic trial (observational study) | Women (18–34 years) | 100% | via research institution | Menstrual pain (cramping): - being diagnosed with dysmenorrhea | 221 | 1. Intervention group: AKUD App with acupressure features | No: Usual care | not specified | 6 months (6 menstrual cycles) | Pain NRS | Germany |
| Chhabra 2018 | RCT | Adults (>18 years) | n/a | via clinic | Chronic low back pain: - mechanical LBP >12 weeks with or without radicular symptoms | 93 | 1. Intervention group: Snapcare App; 2. Control group: Usual care with written prescription of medication and physical activity | No: Usual care (Written prescription of medication and physical activity) | 12 weeks | 12 weeks | Pain NRS | India |
| Choi 2019 | RCT | Adults (>20 years) | 68% | via clinic | Frozen shoulder: - shoulder pain for at least one month | 84 | 1. Intervention group: Exercise app, including feedback, motivation, reminder; 2. Control group: Self-exercise group | YES: both groups were prescribed nonsteroidal anti-inflammatory drugs (celecoxib) for two months, and educated and encouraged to perform self-exercise | not specified | 12 weeks | Pain VAS | Korea |
| Clement 2018 | retrospective analysis of the user database | Adults (>18 years) | 49% | via online channels (Facebook, Google Ads, company home page) | Low back pain: - declaration of medical treatment of back pain | 1055 | 1. Intervention group: Updated 1.4 version of the Kaia App featuring physiotherapy, mindfulness, and education | No | not specified | 24 weeks | Pain NRS | Germany |
| Goebel 2019 | Observational study | Adults (age not reported) | 87% | via online channels (clinic website, social media, newsletters) | Migraine: - suffering from migraine or headaches | 1464 | 1. Intervention group: Migraine app with medication reminder, expert chats, relaxation, education, couching | No | not specified | max. 12 months (no primary endpoint defined) | Pain VAS | Germany |
| Guetin 2016 | Observational study | Patients (7–88 years) | 79% | via clinic | Different chronic pain conditions | 53 | 1. Intervention group: Music-care app receptive music intervention (max. 7 sessions) | No | not specified | After use of app (min. 1 session and max. 7 sessions) | Pain VAS | France |
| Guillory 2015 | Pilot RCT (Observational study) | Adults (18–80 years) | 75% | Via clinic | Chronic non-cancer pain: - pain on most days for >3 months | 82 | 1. Intervention group: Pain tracking app usage + twice daily text messages reminder | YES- daily reminder to use the app plus twice-daily supportive text messages for encouragement | 4 weeks | 4 weeks | Pain NRS | United States |
| Huber 2017 | retrospective study | Adults (mean age of 33.9) | 58% | via online channels (FB, Google ads, company homepage) | Unspecific low back pain: - declaration of medical treatment of back pain | 180 | 1. Intervention group: Kaia mobile app that digitalizes multidisciplinary pain treatment | NO | not specified | 12 weeks | Pain NRS | Germany |
| Irvine 2015 | RCT (Comparison App vs. Control) | Adults (18–65 years) | 60% | via online channels (FB, Google ads, company homepage) | Non-specific low back pain: - low back pain within the past 3 months | 597 | 1. Intervention group: FitBack app; 2. Control group: Usual care with reminder E-mails; 3. Alternative care group: 8 E-mails with link to resources | YES: Weekly E-Mail reminder | not specified | 16 weeks | Pain intensity (1–7) | United States |
| Jamison 2016 | Observational study | Adults (>18 years) | 64% | via clinic | chronic pain: - chronic pain for >6 months | 90 | 1. Intervention group: Pain coping app + Fitbit | No: only technical support was offered | 12 weeks | 12 weeks | Brief pain inventory (BPI) -> Pain intensity (0–10) | United States |
| Kravitz 2018 | RCT | Adults (18–75 years) | 47% | via research institutions | CMSP: - musculo-skeletal pain for >6 weeks at the time of screening | 215 | 1. Intervention group: Mobile health app (choice of e.g., drug or alternative treatments); 2. Control group: TAU + self-management booklet | YES: Reminder phone calls or e-mail + self-management booklet | not specified | 48 weeks | Pain intensity (PROMIS 3a short form) (0–100) | United States |
| Kristjánsdóttir 2013 | RCT | Women (>18 years) | 100% | via clinic | CWP: - having suffered from CWP for more than 6 months | 140 | 1. Intervention group: Smartphone intervention with diaries and daily feedback; 2. Control group: Informational website with self-help material | YES: Access to an informational website with self-help pain-management material | 4 weeks | 4 weeks | Pain VAS | Norway |
| Lee 2017 | RCT | Adult office worker (25–35 years) | 45% | via research intuition | Chronic neck pain: - pain for more than 6 months | 20 | 1. Intervention group: App with self-feedback for exercises; 2. Control group: Brochure and one education session on care their neck pain | YES: Both groups received text messages once a week about caring for their pain | not specified | 8 weeks | Pain VAS | Korea |
| Lo 2018 | Observational study | Adults (18–65 years) | 25% | via homepage invitation of clinic | Chronic neck and back pain: - pain within the past 3 months | 161 | 1. Intervention group: Artificial intelligence (AI) embedded smartphone app | No: But contact function via in-app messaging function | not specified | 4 weeks | Pain NRS | China |
| Mollard 2018 | Pilot study two group experimental design | Adults (>18 years) | n/a | via clinic | rheumatoid arthritis (RA): - actively seeing a rheumatology provider at the researchers’ university rheumatology clinic | 36 | 1. Intervention group: Live with Arthritis app to monitor progression of rheumatoid arthritis inflammation using optical imaging; 2. Control group: TAU | No | not specified | 6 months | Pain VAS | United States |
| Rini 2015 | RCT | Adults (>18 years) | 81% | via research institution | Osteoarthritis pain: - confirmed radiographically (Kellgren & Lawrence grade ≥ 2, with pain in the affected joint); - Osteoarthritis pain pain > 3 months | 113 | 1. Intervention group: PainCOACH app including coping skills training, guided instructions, individualized feedback, interactive feedback and demonstrations; 2. Control group: Assessment only | YES: Brief regular phone calls phoned to encourage continued use of the program | 11 weeks | 11 weeks | Pain (AIMS2) ->pain in the prior month (1 = severe –5 = none) | United States |
| Shebib 2019 | RCT | Adults (>18 years) | 41% | via participating employers across 12 locations in the US | Unspecific low back pain: - pain for at least 6 weeks in the past 12 months | 177 | 1. Intervention group: App including personal coaching in a team to provide peer support; 2. Control group: Three digital education articles from the intervention + TAU | YES: Intervention participants received a tablet and two Bluetooth wearable motion-sensors to be placed along the lower back and torso during the in-app exercise therapy + TAU | 12 weeks | 12 weeks | Pain VAS | United States |
| Skrepnik 2017 | RCT | Adults (30–80 years) | 50% | via selected private community-based practices | Knee Osteoarthritis: - knee OA whom the physician investigator decided to treat with one 6-mL injection of hylan G-F 20 | 211 | 1. Intervention group: App “OA GO” including motivational messages, pain and mood tracking; 2. Control group: regular follow-up + wearable activity monitor | YES: Regular follow-ups as per standard-of-care following Hylan G-F 20 treatment + wearable activity monitor | 90 days | 90 days | Pain NRS | United States |
| Suso-Ribera 2018 | Feasibility Study | Adults (18–65 years) | 53% | via clinic | Heterogenous chronic pain: - pain for more than 6 months prior to the study | 38 | 1. Intervention group: Ecological momentary assessment (EMA) monitoring app with protocol for pain, mood and medication (e.g., side effects) | YES: Weekly phone calls to assess recalled pain intensity and mood | 30 days | 30 days | Brief Pain Inventory (BPI) -> Pain NRS | Spain |
| Toelle 2019 | RCT | Adults (18–65 years) | 70% | via clinic | Unspecific low back pain: - non-specific low back pain; - pain had to be ongoing for the last 6 weeks up to 12 months | 101 | 1. Intervention group: Kaia App including modules: (1) education, (2) physiotherapy, and (3) relaxation; 2. Control group: Six face-to-face sessions of standard physiotherapy once a week + weekly E-mails with online resources | No | 12 weeks | 12 weeks | Pain NRS | Germany |
| Yang 2019 | RCT | Adults (>18 years) | 50% | via clinic | Chronic low back pain: - confirmed diagnosis of chronic low back pain (>3 months) by physicians; - no musculo-skeletal origins | 8 | 1. Intervention group: Self- management app (Pain Care); 2. Control group: Physiotherapy | YES: Physiotherapy | 4 weeks | 4 weeks | Pain VAS | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, A.-C.; Uddin, R.; Schröder-Pfeifer, P.; Holl, F.; Swoboda, W.; Schiltenwolf, M. Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. J. Clin. Med. 2020, 9, 3557. https://doi.org/10.3390/jcm9113557
Pfeifer A-C, Uddin R, Schröder-Pfeifer P, Holl F, Swoboda W, Schiltenwolf M. Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. Journal of Clinical Medicine. 2020; 9(11):3557. https://doi.org/10.3390/jcm9113557
Chicago/Turabian StylePfeifer, Ann-Christin, Riaz Uddin, Paul Schröder-Pfeifer, Felix Holl, Walter Swoboda, and Marcus Schiltenwolf. 2020. "Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness" Journal of Clinical Medicine 9, no. 11: 3557. https://doi.org/10.3390/jcm9113557
APA StylePfeifer, A.-C., Uddin, R., Schröder-Pfeifer, P., Holl, F., Swoboda, W., & Schiltenwolf, M. (2020). Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. Journal of Clinical Medicine, 9(11), 3557. https://doi.org/10.3390/jcm9113557








