Therapeutic Exercise and Pain Neurophysiology Education in Female Patients with Fibromyalgia Syndrome: A Feasibility Study
Abstract
1. Introduction
2. Material and Methods
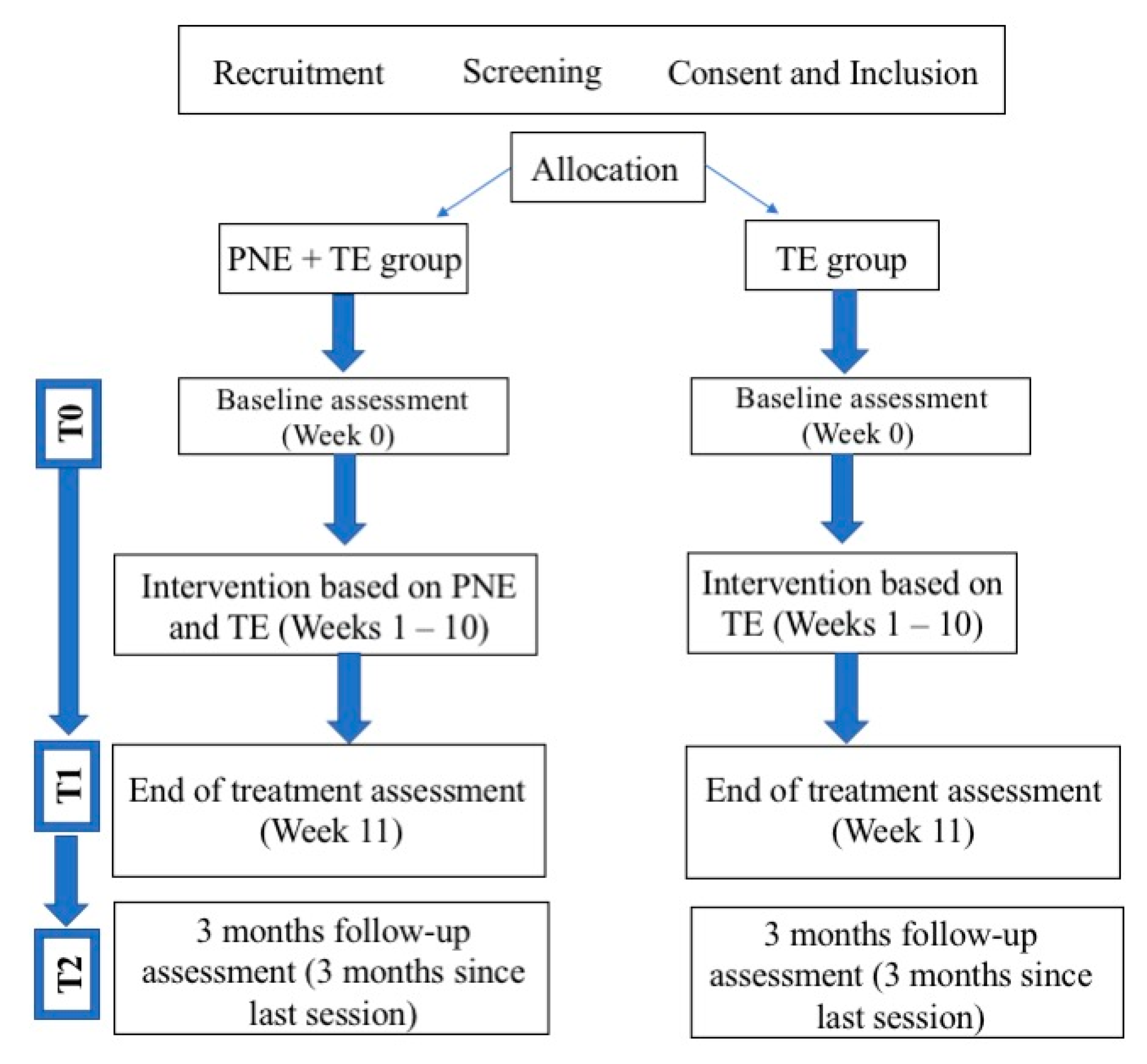
2.1. Study Design
2.2. Participants
2.3. Sample Size
2.4. Randomization
2.5. Interventions
2.6. Therapeutic Exercise
2.7. Pain Neurophysiology Education
2.8. Outcome Measures
2.9. Statistical Analysis
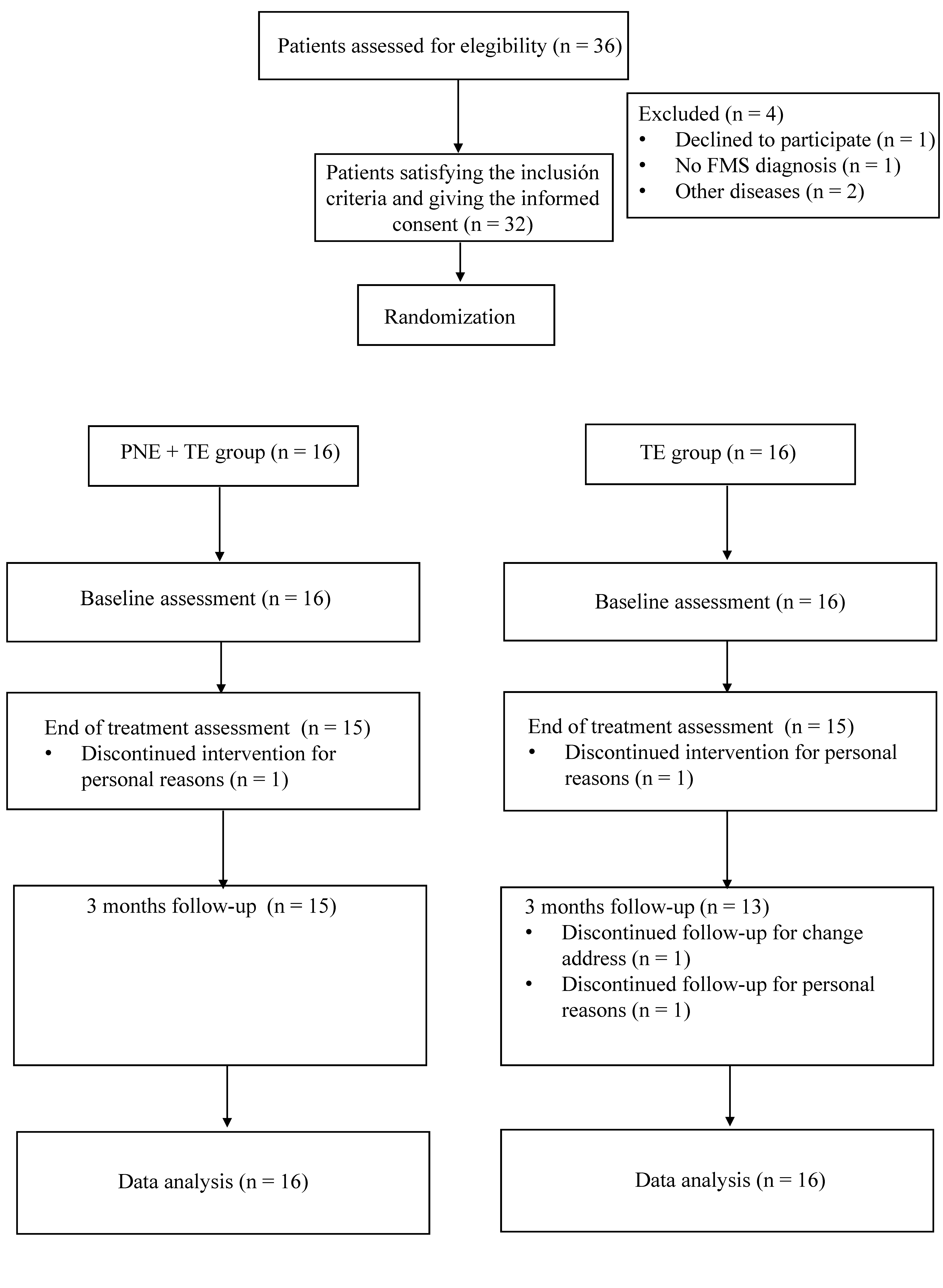
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Therapeutic Exercise Protocol
| Part 1. Warm-Up | Part 2. Central Part | Part 3. Cool Down |
| 10 min: Joint mobility exercises in the available range of motion of:
Submaximal repetitions of the strengthening exercises. | 40 min: Aerobic exercises (20 min): Exercises:
Low to moderate intensity (40–60% of maximum heart rate). Strengthening exercises (20 min): Circuits of 4 exercises with 3 sets of 10–12 repetitions or 3 sets of 30 s for planks. Exercises: Lower limb exercises:
The intensity was 50% of 1RM. The intensity was increased by 10% according to the individual tolerance. Progression: Adding soft elastic bands and dumbbells. | 10 min: Pulmonary exercises: Inspiration through the noise and expiration through the mouth, deep breathing and then expiration through the mouth slowly deep breathing. Stretching exercises: 3 sets of 30 s. Exercises:
|
References
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993, 20, 475–479. [Google Scholar] [PubMed]
- Mannerkorpi, K.; Burckhardt, C.S.; Bjelle, A. Physical performance characteristics of women with fibromyalgia. Arthritis Care Res. (Hoboken) 1994, 7, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Ferraz, M.B.; Sato, E.I.; Atra, E. Fibromyalgia versus rheumatoid arthritis: A longitudinal comparison of the quality of life. J. Rheumatol. 1995, 22, 270–274. [Google Scholar] [PubMed]
- Clauw, D.J. Fibromyalgia: An Overview. Am. J. Med. 2009, 122, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Sousa ADe Matsutani, L.A.; Lee, S.; Yuan, K.; Assumpc, A. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. 2017, 7, 356–363. [Google Scholar] [CrossRef]
- Kwiatek, R. Treatment of fibromyalgia. Aust. Prescr. 2017, 40, 179–183. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Ambrose, K.R.; Golightly, Y.M. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best Pract. Res. Clin. Rheumatol. 2015, 29, 120–130. [Google Scholar] [CrossRef]
- Gavi, M.B.R.O.; Vassalo, D.V.; Amaral, F.T.; Macedo, D.C.F.; Gava, P.L.; Dantas, E.M.; Valim, V. Strengthening Exercises Improve Symptoms and Quality of Life but Do Not Change Autonomic Modulation in Fibromyalgia: A Randomized Clinical Trial. PLoS ONE 2014, 9, e90767. [Google Scholar] [CrossRef] [PubMed]
- Bircan, Ç.; Karasel, S.A.; Akgün, B.; El, Ö.; Alper, S. Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol. Int. 2008, 28, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hooten, W.M.; Qu, W.; Townsend, C.O.; Judd, J.W. Effects of strength vs. aerobic exercise on pain severity in adults with fibromyalgia: A randomized equivalence trial. Pain 2012, 153, 915–923. [Google Scholar] [CrossRef]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-Ljungar, I.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—A randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef]
- Sañudo, B.; Carrasco, L.; de Hoyo, M.; Figueroa, A.; Saxton, J.M. Vagal modulation and symptomatology following a 6-month aerobic exercise program for women with fibromyalgia. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 88), S41–S45. [Google Scholar]
- Gowans, S.E.; DeHueck, A.; Voss, S.; Silaj, A.; Abbey, S.E.; Reynolds, W.J. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001, 45, 519–529. [Google Scholar] [CrossRef]
- Busch, A.J.; Schachter, C.L.; Overend, T. Exercise for Fibromyalgia: A Systematic Review. J. Rheumatol. 2008, 35, 1130–1144. [Google Scholar]
- Sosa-Reina, M.D.; Nunez-Nagy, S.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Monserrat, J.; Álvarez-Mon, M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomed. Res. Int. 2017, 2017, 2356346. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A. Is Aerobic Exercise Training Beneficial for Adults with Fibromyalgia? Am. J. Phys. Med. Rehabil. 2019, 98, 169–170. [Google Scholar] [CrossRef]
- Sañudo, B.; Galiano, D.; Carrasco, L.; Blagojevic, M.; de Hoyo, M.; Saxton, J. Aerobic exercise versus combined exercise therapy in women with fibromyalgia syndrome: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2010, 91, 1838–1843. [Google Scholar] [CrossRef]
- Colleary, G.; O’Sullivan, K.; Griffin, D.; Ryan, C.G.; Martin, D.J. Effect of pain neurophysiology education on physiotherapy students’ understanding of chronic pain, clinical recommendations and attitudes towards people with chronic pain: A randomised controlled trial. Physiotherapy 2017, 103, 423–429. [Google Scholar] [CrossRef]
- Moseley, G.L.; Nicholas, M.K.; Hodges, P.W. A Randomized Controlled Trial of Intensive Neurophysiology Education in Chronic Low Back Pain. Clin. J. Pain. 2004, 20, 324–330. [Google Scholar] [CrossRef]
- Moseley, G.L. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur. J. Pain. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Moseley, G.L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust. J. Physiother. 2002, 48, 297–302. [Google Scholar] [CrossRef]
- Bodes Pardo, G.; Lluch Girbés, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jiménez Penick, V.; Pecos Martín, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients with Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis. Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef]
- Jones, K.D.; Liptan, G.L. Exercise Interventions in Fibromyalgia: Clinical Applications from the Evidence. Rheum. Dis. Clin. N. Am. 2009, 35, 373–391. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef]
- Butler, D.S.; Moseley, G.L. Explain Pain, 2nd ed.; Noigroup Publications: Adelaide City, Australia, 2013; p. 116. [Google Scholar]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test—Retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.; Hochberg, M.; Van Der Heijde, D.; et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: The minimal clinically important improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Vanderweeën, L.; Oostendorp, R.A.B.; Vaes, P.; Duquet, W. Pressure algometry in manual therapy. Man Ther. 1996, 1, 258–265. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Estévez-López, F.; Soriano-Maldonado, A.; Álvarez-Gallardo, I.C.; Delgado-Fernández, M.; Ruiz, J.R.; Aparicio, V.A. Gender Differences in Symptoms, Health-Related Quality of Life, Sleep Quality, Mental Health, Cognitive Performance, Pain-Cognition, and Positive Health in Spanish Fibromyalgia Individuals: The Al-Ándalus Project. Pain Res. Manag. 2016, 2016, 5135176. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.; García-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual. Life Outcomes 2013, 11, 132. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia. Med. Clin. (Barc) 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Terol-Cantero, M.C.; Cabrera-Perona, V.; Martín-Aragón, M. Revisión de estudios de la Escala de Ansiedad y Depresión Hospitalaria (HAD) en muestras españolas. An. Psicol. 2015, 31, 494. [Google Scholar] [CrossRef]
- Zigmond, A.; Snaith, R. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: A Review of Its History, Issues, Progress, and Documentation. J. Rheumatol. 2003, 30, 167–178. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Hillsdale, N., Ed.; Lawrence Erlbaum Associates Publishers: Hillsdale, NJ, USA, 1988; p. 459. [Google Scholar]
- Van Oosterwijck, J.; Meeus, M.; Paul, L.; De Schryver, M.; Pascal, A.; Lambrecht, L.; Nijs, J. Pain Physiology Education Improves Health Status and Endogenous Pain Inhibition in Fibromyalgia. Clin. J. Pain. 2013, 29, 873–882. [Google Scholar] [CrossRef]
- Amer-cuenca, J.J.; Pecos-mart, D.; Nijs, J.; Mart, P.; Lluch, E. How Much Is Needed? Comparison of the Effectiveness of Different Pain Education Dosages in Patients with Fibromyalgia. Pain Med. 2019, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elizagaray-Garcia, I.; Muriente-Gonzalez, J.; Gil-Martinez, A. Education for patients with fibromyalgia. A systematic review of randomised clinical trials. Rev. Neurol. 2016, 62, 49–60. [Google Scholar] [PubMed]
- Zusman, M. Forebrain-mediated sensitization of central pain pathways: ‘Non-specific’ pain and a new image for MT. Man Ther. 2002, 7, 80–88. [Google Scholar] [CrossRef]
- Nijs, J.; Van Houdenhove, B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: Application of pain neurophysiology in manual therapy practice. Man Ther. 2009, 14, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Van Ittersum, M.W.; van Wilgen, C.P.; van der Schans, C.P.; Lambrecht, L.; Groothoff, J.W.; Nijs, J. Written Pain Neuroscience Education in Fibromyalgia: A Multicenter Randomized Controlled Trial. Pain Pract. 2014, 14, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Van Houdenhove, B.; Luyten, P. Customizing treatment of chronic fatigue syndrome and fibromyalgia: The role of perpetuating factors. Psychosomatics 2008, 49, 470–477. [Google Scholar] [CrossRef]
- Harding, G.; Parsons, S.; Rahman, A.; Underwood, M. “It struck me that they didn’t understand pain”: The specialist pain clinic experience of patients with chronic musculoskeletal pain. Arthritis Care Res. 2005, 53, 691–696. [Google Scholar] [CrossRef] [PubMed]
| PNE + TE Group (n = 16) | TE Group (n = 16) | p-Value | |
|---|---|---|---|
| Age (years) | 52.13 ± 10.31 | 53.00 ± 10.68 | 0.818 |
| Height (cm) | 159.66 ± 5.08 | 159.35 ± 5.87 | 0.874 |
| Weight (Kg) | 76.56 ± 16.14 | 66.34 ± 12.34 | 0.052 |
| BMI (Kg/cm2) | 30.10 ± 6.69 | 26.17 ± 4.99 | 0.070 |
| Years since diagnosis | 12.37 ± 8.64 | 11.12 ± 8.10 | 0.676 |
| VAS last three days (cm) | 6.91 ± 1.62 | 7.11 ± 1.65 | 0.732 |
| Number tender points | 17.43 ± 1.63 | 17.37 ± 1.02 | 0.898 |
| Algometry (Kg/cm2) | 33.78 ± 12.70 | 33.57 ± 9.88 | 0.959 |
| FIQ-R | 60.98 ± 18.03 | 60.26 ± 15.05 | 0.982 |
| PCS | 24.75 ± 12.31 | 22.81 ± 8.51 | 0.609 |
| HADS | 18.06 ± 7.87 | 18.50 ± 7.17 | 0.871 |
| HAQ | 9.06 ± 4.65 | 9.88 ± 3.57 | 0.584 |
| Baseline (T0) | End of Treatment (T1) | Between-Group Score Changes | Between-Group p-Value | 3-Months Follow-Up (T2) | Between-Group Score Changes | Between-Group p-Value | |
|---|---|---|---|---|---|---|---|
| VAS last three days (0–10 cm) | |||||||
| PNE + TE group | 6.91 ± 1.62 | 3.99 ± 2.22 | −2.16 (−3.87, −0.45) | F = 6.64 | 4.21 ± 2.87 | −1.39 (−3.23, 0.44) | F = 2.39 |
| TE group | 7.11 ± 1.65 | 6.15 ± 2.50 | p = 0.015 | 5.61 ± 2.16 | p = 0.132 | ||
| Number of tender points | |||||||
| PNE + TE group | 17.43 ± 1.63 | 14.5 ± 4.11 | 0.63 (−0.92, 1.04) | F = 0.01 | 13.68 ± 4.12 | −1.25 (−3.94, 1.44) | F = 0.896 |
| TE group | 17.37 ± 1.02 | 15.56 ± 3.68 | p = 0.898 | 14.93 ± 3.29 | p = 0.351 | ||
| Algometer score (Kg/cm2) | |||||||
| PNE + TE group | 33.78 ±12.70 | 53.60 ± 19.10 | 7.56 (−6.21, 21.35) | F = 0.11 | 58.14 ± 22.38 | 11.35 (−2.88, 25.59) | F = 2.65 |
| TE group | 33.57 ± 9.88 | 46.03 ± 19.08 | p = 0.271 | 46.78 ± 16.83 | p = 0.114 | ||
| FIQ-R PNE + TE group TE group | 60.98 ± 18.03 60.26 ± 15.05 | 51.88 ± 21.91 52.23 ± 21.69 | −0.35(−16.09,15.39) | F = 0.03 p = 0.855 | 37.66 ± 20.89 48.56 ± 21.25 | −10.9 (−26.12,4.31) | F = 3.53 p = 0.07 |
| PCS Total score | |||||||
| PNE + TE group | 24.75 ± 12.31 | 18.69 ± 11.49 | −0.56 (−8.35, 7.23) | F = 0.02 | 17.75 ± 11.86 | −2.00 (−9.70, 5.70) | F = 0.28 |
| TE group | 22.81 ± 8.51 | 19.25 ± 10.03 | p = 0.884 | 19.75 ± 9.32 | p = 0.600 | ||
| PCS Rumiation | |||||||
| PNE + TE group | 8.62 ± 3.93 | 6.63 ± 4.17 | −0.5 (−3.36, 2.36) | F = 0.12 | 6.31 ± 4.06 | −1.00 (−5.70, 9.70) | F = 0.53 |
| TE group | 8.69 ± 3.36 | 7.13 ± 3.75 | p = 0.724 | 7.31 ± 3.66 | p = 0.470 | ||
| PCS Magnification | |||||||
| PNE + TE group | 5.06 ± 3.02 | 3.50 ± 2.85 | −0.31 (−2.31, 1.68) | F = 0.10 | 3.50 ± 3.07 | −0.31 (−2.27, 1.65) | F = 0.10 |
| TE group | 4.13 ± 2.39 | 3.81 ± 2.88 | p = 0.752 | 3.81 ± 2.31 | p = 0.748 | ||
| PCS Helplessness | |||||||
| PNE + TE group | 11.06 ± 5.96 | 8.56 ± 5.18 | 0.31 (−3.26, 3.89) | F = 0.03 | 7,94 ± 5.38 | −1.00 (−4.61, 2.61) | F = 0.32 |
| TE group | 10.00 ± 4.42 | 8.25 ± 4.72 | p = 0.860 | 8.94 ± 4.58 | p = 0.576 | ||
| HADS total score | |||||||
| PNE + TE group | 18.06 ± 7.87 | 14.56 ± 9.18 | −1.43 (−7.83, 4.95) | F = 0.21 | 14.19 ± 7.95 | 1.31 (−3.58, 6.21) | F = 0.30 |
| TE group | 18.50 ± 7.17 | 16.00 ± 8.50 | p = 0.649 | 12.87 ± 5.36 | p = 0.588 | ||
| HADS anxiety | |||||||
| PNE + TE group | 9.75 ± 3.56 | 8.44 ± 4.41 | −0.43 (−3.54, 2.67) | F = 0.08 | 7.56 ± 3.66 | 0.37 (−2.07, 2.82) | F = 0.09 |
| TE group | 10.31 ± 3.45 | 8.88 ± 4.19 | p = 0.776 | 7.19 ± 3.08 | p = 0.756 | ||
| HADS depression | |||||||
| PNE + TE group | 8.31 ± 4.96 | 6.75 ± 5.38 | −1.00 (−4.50, 2.50) | F = 0.33 | 6.63 ± 4.99 | 0.93 (−2.04, 3.92) | F = 0.41 |
| TE group | 8.19 ± 4.29 | 7.75 ± 4.26 | p = 0.565 | 5.69 ± 3.04 | p = 0.526 | ||
| HAQ | |||||||
| PNE + TE group | 9.06 ± 4.65 | 7.00 ± 4.21 | −2.50 (−5.89,0.89) | F = 2.26 | 6.37 ± 5.45 | −2.13 (−5.67, 1.42) | F = 1.49 |
| TE group | 9.88 ± 3.57 | 9.50 ± 5.13 | p = 0.143 | 8.50 ± 4.32 | p = 0.231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos-Laita, L.; Mingo-Gómez, M.T.; Navas-Cámara, F.; Estébanez-de-Miguel, E.; Caudevilla-Polo, S.; Verde-Rello, Z.; Fernández-Araque, A.; Jiménez-del-Barrio, S. Therapeutic Exercise and Pain Neurophysiology Education in Female Patients with Fibromyalgia Syndrome: A Feasibility Study. J. Clin. Med. 2020, 9, 3564. https://doi.org/10.3390/jcm9113564
Ceballos-Laita L, Mingo-Gómez MT, Navas-Cámara F, Estébanez-de-Miguel E, Caudevilla-Polo S, Verde-Rello Z, Fernández-Araque A, Jiménez-del-Barrio S. Therapeutic Exercise and Pain Neurophysiology Education in Female Patients with Fibromyalgia Syndrome: A Feasibility Study. Journal of Clinical Medicine. 2020; 9(11):3564. https://doi.org/10.3390/jcm9113564
Chicago/Turabian StyleCeballos-Laita, Luis, María Teresa Mingo-Gómez, Francisco Navas-Cámara, Elena Estébanez-de-Miguel, Santos Caudevilla-Polo, Zoraida Verde-Rello, Ana Fernández-Araque, and Sandra Jiménez-del-Barrio. 2020. "Therapeutic Exercise and Pain Neurophysiology Education in Female Patients with Fibromyalgia Syndrome: A Feasibility Study" Journal of Clinical Medicine 9, no. 11: 3564. https://doi.org/10.3390/jcm9113564
APA StyleCeballos-Laita, L., Mingo-Gómez, M. T., Navas-Cámara, F., Estébanez-de-Miguel, E., Caudevilla-Polo, S., Verde-Rello, Z., Fernández-Araque, A., & Jiménez-del-Barrio, S. (2020). Therapeutic Exercise and Pain Neurophysiology Education in Female Patients with Fibromyalgia Syndrome: A Feasibility Study. Journal of Clinical Medicine, 9(11), 3564. https://doi.org/10.3390/jcm9113564





