Accuracy of Fibrosis-4 Index in Identification of Patients with Cirrhosis Who Could Potentially Avoid Variceal Screening Endoscopy
Abstract
1. Introduction
2. Patients and Methods
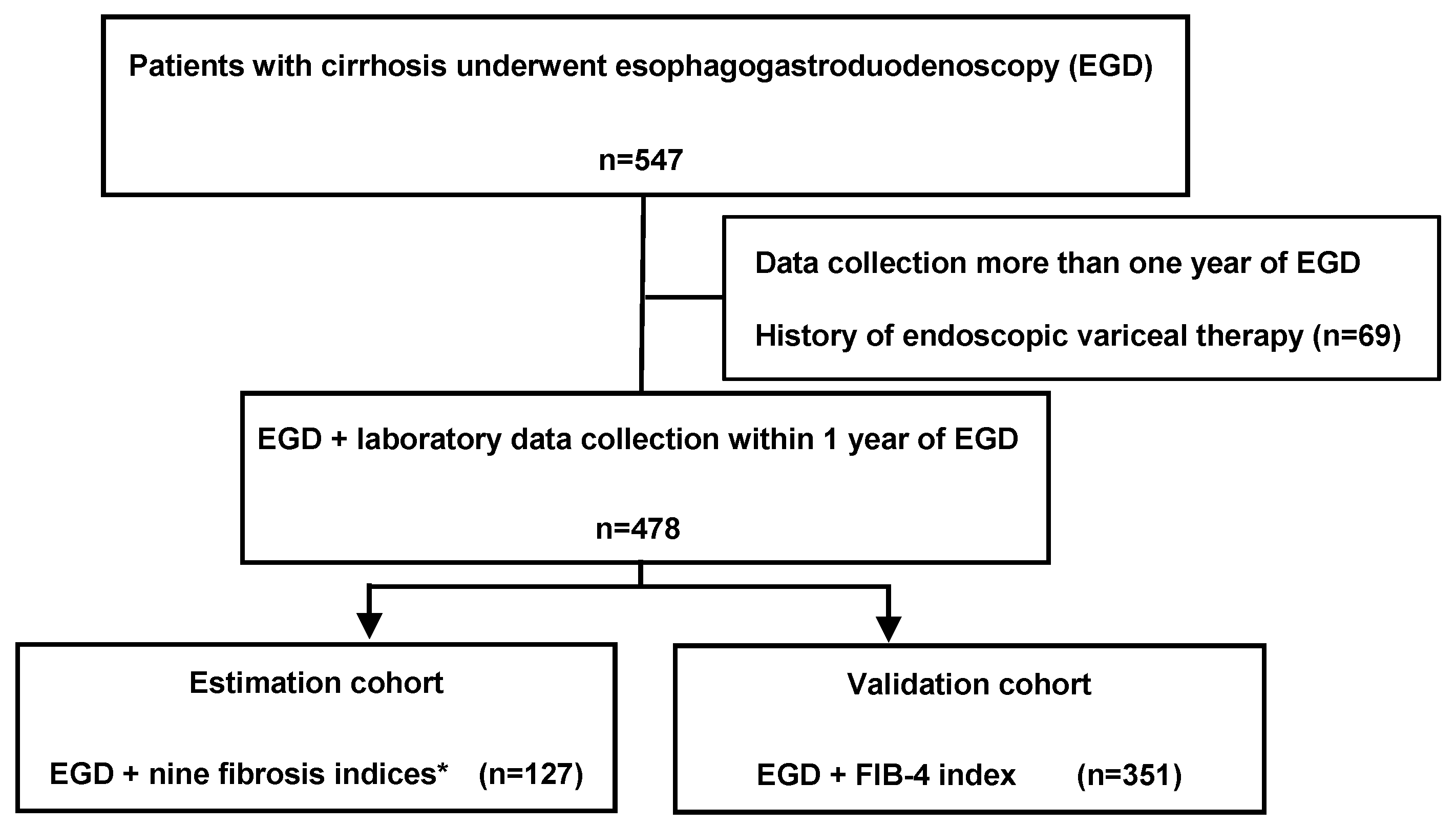
2.1. Patients and Study Protocol
2.2. Laboratory Data
2.3. Assessment of Varices and Bleeding Risk
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
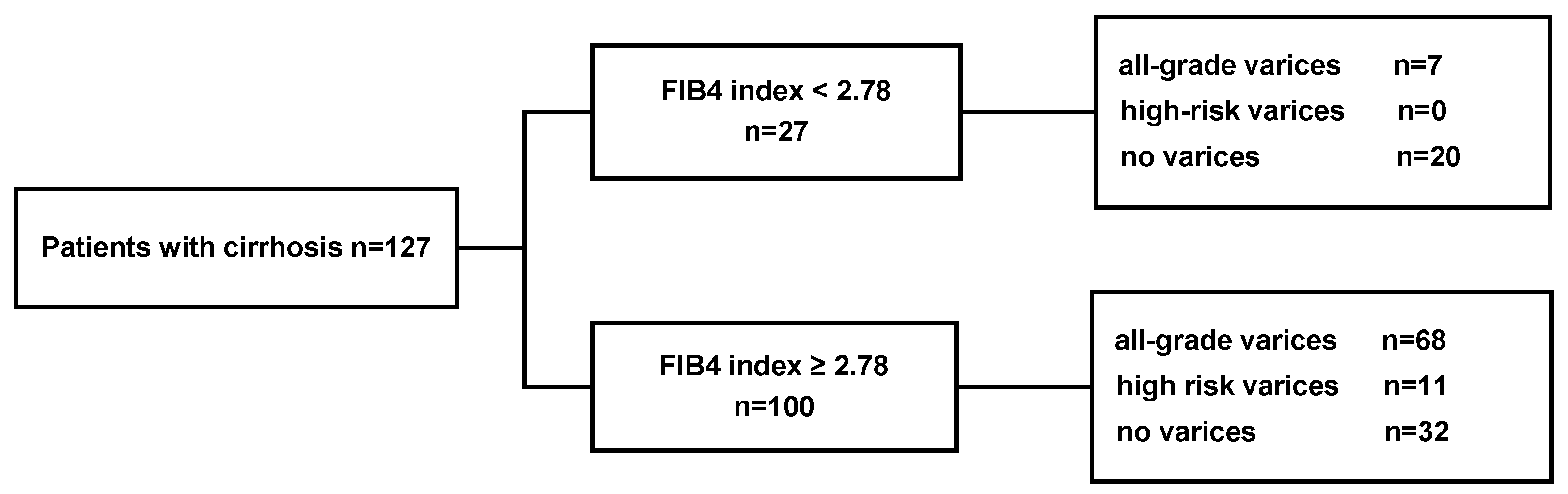
3.2. Performance of a FIB-4 Cutoff of 2.78 in the Estimation Cohort
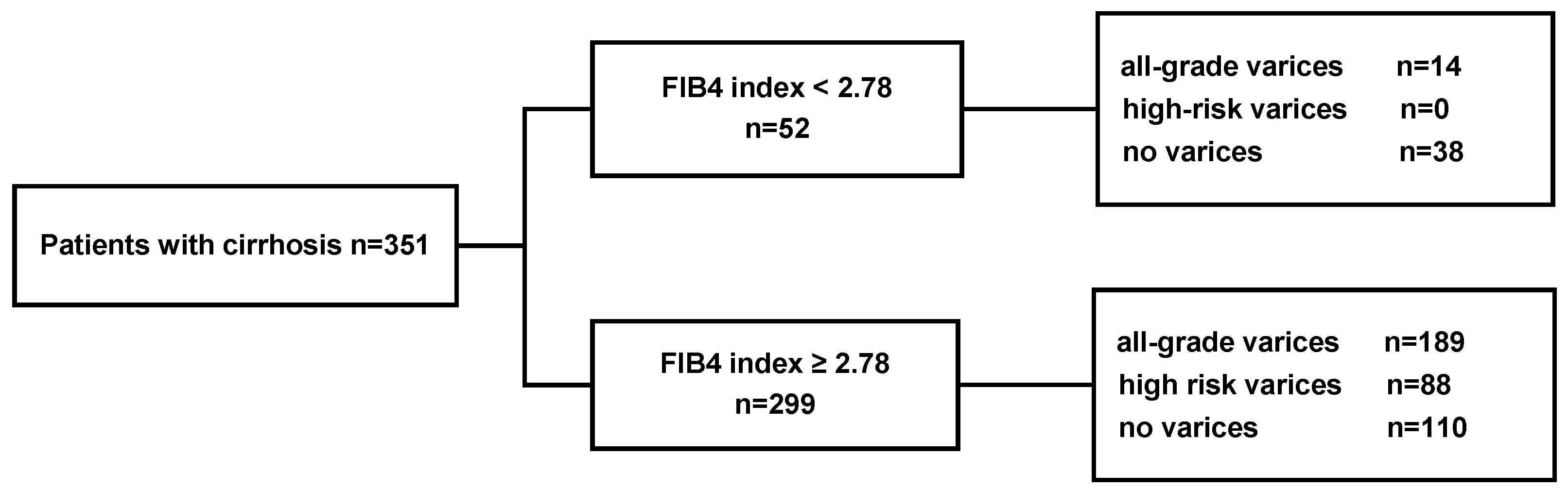
3.3. Performance of a FIB-4 Cutoff of 2.78 in the Validation Cohort
3.4. Performance of a FIB-4 Cutoff of 3.2 in the Estimation and Validation Cohort
3.5. Performance of a Combination of FIB-4 and the Other Fibrosis Indices
3.6. Performance of Other Fibrosis Indices
3.7. Univariate and Multivariate Analyses of Variables Associated with High-Risk Varices
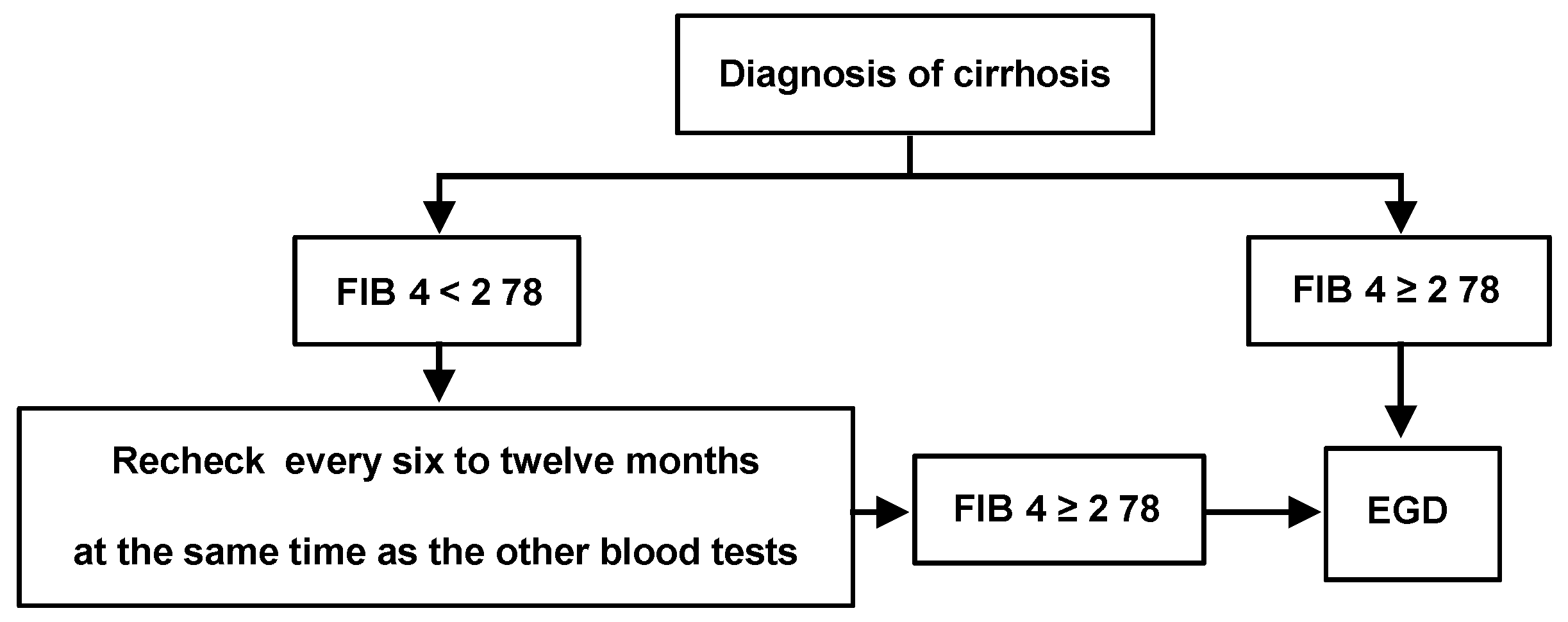
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsuji, S.; Uchida, Y.; Uemura, H.; Kouyama, J.I.; Naiki, K.; Nakao, M.; Motoya, D.; Sugawara, K.; Nakayama, N.; Imai, Y.; et al. Involvement of portosystemic shunts in impaired improvement of liver function after direct-acting antiviral therapies in cirrhotic patients with hepatitis C virus. Hepatol. Res. 2020, 50, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Practice Guidelines Committee of the American Association for the Study of Liver, Diseases; the Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Moriguchi, M.; Hara, T.; Kataoka, S.; Okuda, K.; Furuta, M.; Takemura, M.; Taketani, H.; Umemura, A.; Nishikawa, T.; et al. Presence of varices in patients after hepatitis C virus eradication predicts deterioration in the FIB-4 index. Hepatol. Res. 2019, 49, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Seijo, S.; Arena, U.; Abraldes, J.G.; Vizzutti, F.; Garcia-Pagan, J.C.; Pinzani, M.; Bosch, J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013, 144, 102–111. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Shang, L.; Hosseini, M.; Liu, X.; Kisseleva, T.; Brenner, D.A. Human hepatic stellate cell isolation and characterization. J. Gastroenterol. 2018, 53, 6–17. [Google Scholar] [CrossRef]
- Yoneda, M.; Imajo, K.; Takahashi, H.; Ogawa, Y.; Eguchi, Y.; Sumida, Y.; Yoneda, M.; Kawanaka, M.; Saito, S.; Tokushige, K.; et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J. Gastroenterol. 2018, 53, 181–196. [Google Scholar] [CrossRef]
- Schwabl, P.; Bota, S.; Salzl, P.; Mandorfer, M.; Payer, B.A.; Ferlitsch, A.; Stift, J.; Wrba, F.; Trauner, M.; Peck-Radosavljevic, M.; et al. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015, 35, 381–390. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Y.; Ma, X.; Wang, G.; Wu, H.; Xie, X.; Zhang, C.; Zhu, Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS ONE 2017, 12, e0182969. [Google Scholar] [CrossRef]
- Shirabe, K.; Bekki, Y.; Gantumur, D.; Araki, K.; Ishii, N.; Kuno, A.; Narimatsu, H.; Mizokami, M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 2018, 53, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Qi, X.; Guo, X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015, 94, e1795. [Google Scholar] [CrossRef]
- Crossan, C.; Tsochatzis, E.A.; Longworth, L.; Gurusamy, K.; Davidson, B.; Rodriguez-Peralvarez, M.; Mantzoukis, K.; O’Brien, J.; Thalassinos, E.; Papastergiou, V.; et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: Systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 1–409. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- Inadomi, C.; Takahashi, H.; Ogawa, Y.; Oeda, S.; Imajo, K.; Kubotsu, Y.; Tanaka, K.; Kessoku, T.; Okada, M.; Isoda, H.; et al. Accuracy of the Enhanced Liver Fibrosis test, and combination of the Enhanced Liver Fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2020, 50, 682–692. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.B.; Brodkin, E.; Arnold, F.; Navaratnam, A.; Paine, H.; Khawar, S.; Dhar, A.; Patch, D.; O’Beirne, J.; Mookerjee, R.; et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J. Hepatol. 2016, 65, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Jangouk, P.; Turco, L.; De Oliveira, A.; Schepis, F.; Villa, E.; Garcia-Tsao, G. Validating, deconstructing and refining Baveno criteria for ruling out high-risk varices in patients with compensated cirrhosis. Liver Int. 2017, 37, 1177–1183. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Hissar, S.; Jain, P.; Rastogi, A.; Kumar, D.; Sakhuja, P.; Sarin, S.K. Hepatic venous pressure gradient as a predictor of fibrosis in chronic liver disease because of hepatitis B virus. Liver Int. 2008, 28, 690–698. [Google Scholar] [CrossRef]
- Procopet, B.; Berzigotti, A. Diagnosis of cirrhosis and portal hypertension: Imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol. Rep. (Oxf.) 2017, 5, 79–89. [Google Scholar] [CrossRef]
- Hassan, E.M.; Omran, D.A.; El Beshlawey, M.L.; Abdo, M.; El Askary, A. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol. Hepatol. 2014, 37, 58–65. [Google Scholar] [CrossRef] [PubMed]
| Estimation Cohort (n = 127) | Validation Cohort (n = 351) | p Value | |
|---|---|---|---|
| Gender (male/female) | 82/45 | 217/134 | 0.67 |
| Etiology (HBV/HCV/alcohol/NASH/other) | 13/50/35/12/17 | 42/131/92/30/56 | 0.52 |
| Child-pugh classification (A/B/C) | 83/37/7 | 202/122/27 | 0.29 |
| Age (years) a | 71 (63–76.5) | 69 (62.5–76) | 0.42 |
| AST (IU/L) a | 40 (28–54.5) | 39 (28–55) | 0.81 |
| ALT (IU/L) a | 28 (19–43) | 27 (18–43) | 0.51 |
| ALB (g/dL) a | 3.9 (3.2–4.3) | 3.6 (3.1–4.1) | 0.01 |
| Total Bilirubin (mg/dl) a | 1.68 (0.8–1.68) | 1.0 (0.7–1.4) | 0.21 |
| Prothrombin time (%) a | 74 (63.5–85) | 75 (64–87) | 0.32 |
| Platelet (103/μL) a | 11 (8.25–14.5) | 10.1 (7.1–14.3) | 0.09 |
| Model for End-Stage Liver Disease (MELD) scores a | 8 (5–11.5) | 7 (4–10.3) | 0.47 |
| Hyaluronic acid (ng/mL) a | 402 (172.2–724.6) | - | |
| 7S collagen (ng/mL) a | 7.6 (5.25–11.05) | - | |
| PIIINP (ng/mL) a | 0.9 (0.6–1.1) | - | |
| TIMP-1 (ng/mL) a | 348.1 (269.2–486.3) | - | |
| M2BPGi (COI) a | 3.18 (1.36–6.56) | - | |
| FIB-4 index a | 4.54 (3.00–7.32) | 5.2 (3.5–8.2) | 0.065 |
| APRI a | 1.16 (0.80–1.86) | 1.37 (0.77–2.28) | 0.29 |
| ELF score a | 11.7 (10.8–12.6) | - | |
| The form (F) of esophageal varices F0/F1/F2-F3 | 52/64/11 | 150/115/86 | p < 0.001 |
| Fibrosis Indices | All-Grade Varices | High-Risk Varices | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-Off | AUROC (95%CI) | Se | Sp | PPV | NPV | AUROC (95%CI) | Se | Sp | PPV | NPV | |
| FIB4-index | 2.78 | 0.69 (0.60–0.78) | 0.91 | 0.39 | 0.68 | 0.74 | 0.53 (0.40–0.66) | 1.00 | 0.28 | 0.12 | 1.00 |
| Hyaluronic acid (ng/mL) | 110.63 | 0.63 (0.53–0.73) | 0.95 | 0.29 | 0.66 | 0.79 | 0.50 (0.34–0.66) | 0.91 | 0.28 | 0.11 | 0.97 |
| Platelet (×103/μL) | 11.9 | 0.63 (0.61–0.79) | 0.69 | 0.65 | 0.74 | 0.60 | 0.56 (0.45–0.74) | 0.82 | 0.47 | 0.13 | 0.97 |
| ELF score | 11.75 | 0.63 (0.53–0.73) | 0.60 | 0.65 | 0.71 | 0.53 | 0.48 (0.33–0.64) | 0.91 | 0.21 | 0.10 | 0.96 |
| 7S collagen (ng/mL) | 6.1 | 0.66 (0.56–0.75) | 0.75 | 0.50 | 0.68 | 0.58 | 0.53 (0.39–0.67) | 0.82 | 0.36 | 0.11 | 0.96 |
| APRI | 0.89 | 0.66 (0.56–0.75) | 0.60 | 0.65 | 0.71 | 0.53 | 0.56 (0.42–0.70) | 0.73 | 0.53 | 0.13 | 0.95 |
| M2BPGi (COI) | 1.47 | 0.63 (0.53–0.73) | 0.85 | 0.44 | 0.69 | 0.68 | 0.53 (0.38–0.69) | 0.73 | 0.44 | 0.11 | 0.94 |
| PIIINP (ng/mL) | 0.60 | 0.54 (0.44–0.65) | 0.92 | 0.19 | 1.00 | 0.43 | 0.48 (0.31–0.66) | 0.09 | 1.00 | 1.00 | 0.92 |
| TIMP-1 (ng/mL) | 379.9 | 0.56 (0.48–0.68) | 0.52 | 0.65 | 0.67 | 0.50 | 0.48 (0.31–0.66) | 0.46 | 0.55 | 0.07 | 0.82 |
| Validation Set (n = 351) | High-Risk Varices | |||
|---|---|---|---|---|
| Se | Sp | PPV | NPV | |
| FIB 4-index cut-off 2.78 | 1.00 | 0.20 | 0.29 | 1.00 |
| Platelet cut-off 11.9 | 0.13 | 0.55 | 0.09 | 0.65 |
| APRI cut-off 0.89 | 0.91 | 0.37 | 0.33 | 0.93 |
| A Combination of FIB-4 and the other Fibrosis Indices | Se | Sp | PPV | NPV |
|---|---|---|---|---|
| Fib4 cut-off 2.78 | 1.0 | 0.284 | 0.117 | 1.0 |
| Fib4 < 2.78 and ELF < 11.75 | 1.0 | 0.172 | 0.103 | 1.0 |
| Fib4 < 2.78 and M2 < 1.47 | 1.0 | 0.155 | 0.101 | 1.0 |
| Fib4 < 2.78 and ELF < 11.75 and M2BPGi | 1.0 | 0.147 | 0.1 | 1.0 |
| Fib4 < 2.78 and HA < 110.63 | 1.0 | 0.103 | 0.096 | 1.0 |
| Fib4 < 2.78 and 7S collagen < 6.1 | 1.0 | 0.164 | 0.102 | 1.0 |
| Fib4 < 2.78 and P3P < 0.6 | 1.0 | 0.078 | 0.093 | 1.0 |
| Fib4 < 2.78 and 7S collagen < 6.1 and HA < 110.63 | 1.0 | 0.103 | 0.096 | 1.0 |
| Fib4 < 2.78 and 7S collagen < 6.1 and M2 < 1.47 | 1.0 | 0.155 | 0.0101 | 1.0 |
| Fib4 < 2.78 and ELF and 7S collagen < 6.1 | 1.0 | 0.147 | 0.1 | 1.0 |
| Fib4 < 2.78 and P3P < 0.6 and HA < 110.63 | 1.0 | 0.052 | 0.091 | 1.0 |
| Fib4 < 2.78 and P3P < 0.6 and M2 < 1.47 | 1.0 | 0.078 | 0.093 | 1.0 |
| Fib4 < 2.78 and P3P < 0.6 and 7S collagen < 6.1 | 1.0 | 0.078 | 0.093 | 1.0 |
| Fib4 < 2.776 and M2 < 1.47 and HA < 110.63 | 1.0 | 0.103 | 0.093 | 1.0 |
| Cut-Off | Rate of Ruling Out High-Risk Varices | Rate of Missing High-Risk Varices | |
|---|---|---|---|
| FIB-4 index | 2.78 | 27 (21.3%) | 0 (0%) |
| Hyaluronic acid (ng/mL) | 110.63 | 19 (15.9%) | 1 (5.3%) |
| Platelet (×103/μL) | 11.9 | 58 (45.7%) | 2 (3.5%) |
| ELF score | 11.75 | 64 (50.4%) | 5 (7.8%) |
| 7S collagen (ng/mL) | 6.1 | 44 (34.6%) | 2 (4.5%) |
| APRI | 0.89 | 43 (33.9%) | 2 (4.7%) |
| M2BPGi (COI) | 1.47 | 34 (26.8%) | 2 (5.9%) |
| PIIINP (ng/mL) | 0.60 | 16 (7.9%) | 1 (6.3%) |
| TIMP-1 (ng/mL) | 379.9 | 69 (54.3%) | 5 (7.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, K.; Namisaki, T.; Murata, K.; Fujimoto, Y.; Takeda, S.; Enomoto, M.; Ogawa, H.; Takagi, H.; Tsuji, Y.; Kaya, D.; et al. Accuracy of Fibrosis-4 Index in Identification of Patients with Cirrhosis Who Could Potentially Avoid Variceal Screening Endoscopy. J. Clin. Med. 2020, 9, 3510. https://doi.org/10.3390/jcm9113510
Ishida K, Namisaki T, Murata K, Fujimoto Y, Takeda S, Enomoto M, Ogawa H, Takagi H, Tsuji Y, Kaya D, et al. Accuracy of Fibrosis-4 Index in Identification of Patients with Cirrhosis Who Could Potentially Avoid Variceal Screening Endoscopy. Journal of Clinical Medicine. 2020; 9(11):3510. https://doi.org/10.3390/jcm9113510
Chicago/Turabian StyleIshida, Koji, Tadashi Namisaki, Koji Murata, Yuki Fujimoto, Souichi Takeda, Masahide Enomoto, Hiroyuki Ogawa, Hirotetsu Takagi, Yuki Tsuji, Daisuke Kaya, and et al. 2020. "Accuracy of Fibrosis-4 Index in Identification of Patients with Cirrhosis Who Could Potentially Avoid Variceal Screening Endoscopy" Journal of Clinical Medicine 9, no. 11: 3510. https://doi.org/10.3390/jcm9113510
APA StyleIshida, K., Namisaki, T., Murata, K., Fujimoto, Y., Takeda, S., Enomoto, M., Ogawa, H., Takagi, H., Tsuji, Y., Kaya, D., Fujinaga, Y., Furukawa, M., Sawada, Y., Kitagawa, K., Sato, S., Nishimura, N., Takaya, H., Kaji, K., Shimozato, N., ... Yoshiji, H. (2020). Accuracy of Fibrosis-4 Index in Identification of Patients with Cirrhosis Who Could Potentially Avoid Variceal Screening Endoscopy. Journal of Clinical Medicine, 9(11), 3510. https://doi.org/10.3390/jcm9113510






