Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Clinical Examination and Fluid Biomarkers Collection
2.3. Anthropometric Assessment
2.4. NAFLD Assessment
2.5. Statistics
3. Results
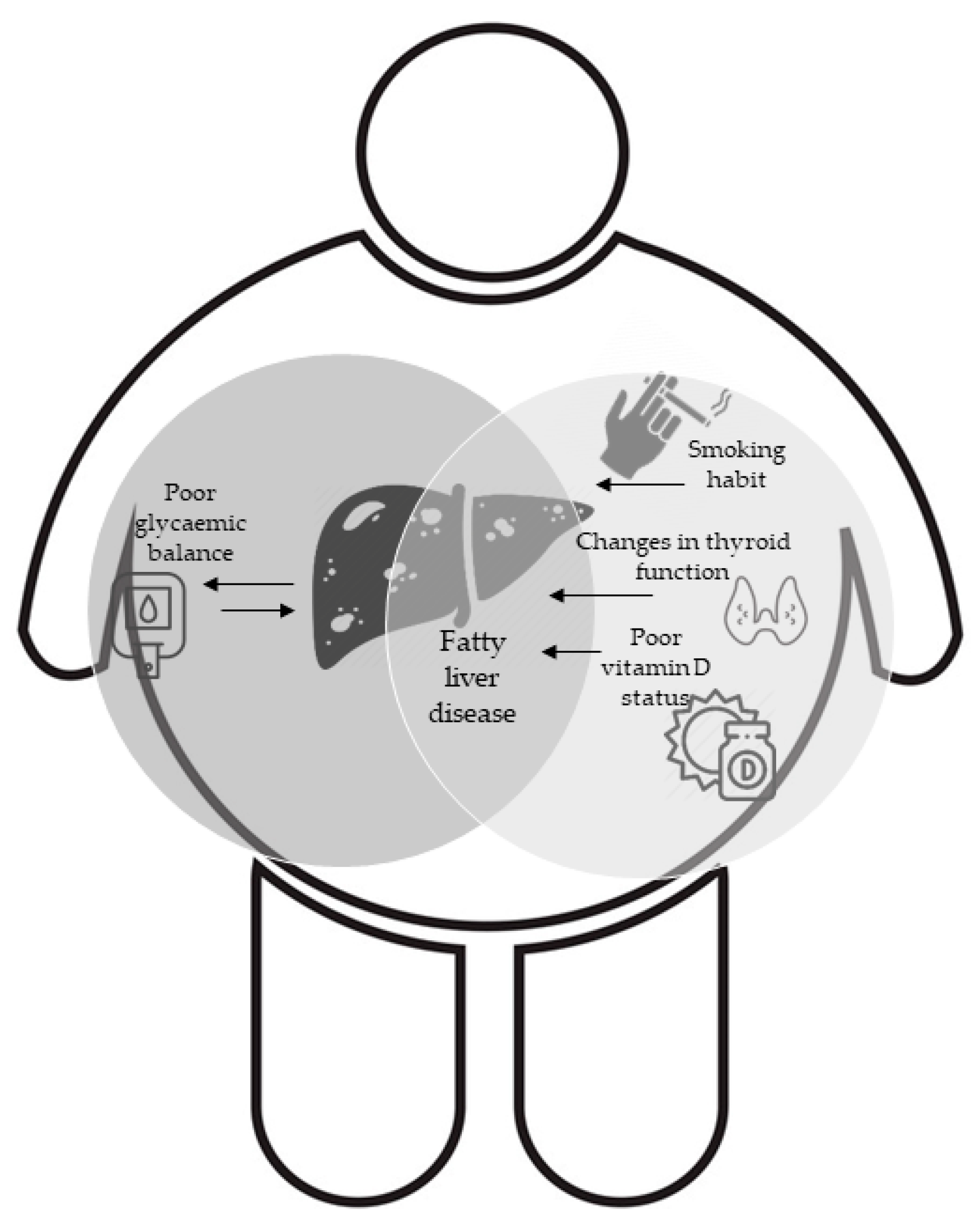
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Cheah, M.C.; McCullough, A.J.; Goh, G.B.-B. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2017, 5, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes: The British Women’s Heart and Health Study and meta-analysis. Diabetes 2009, 32, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Cho, Y.K.; Park, J.H.; Kim, H.J.; Park, D.I.; Sohn, C.I.; Jeon, W.K.; Kim, B.I. Abnormal glucose tolerance in young male patients with nonalcoholic fatty liver disease. Liver Int. 2009, 29, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, L.; Xue, H.; Lin, H.; Li, Y.; Chan, S.-O. Association of glycated hemoglobin with the risk of advanced fibrosis in non-alcoholic fatty liver disease patients without diabetes. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Sardone, R.; Scicchitano, P.; Lampignano, L.; Ciccone, M.M.; Triggiani, V.; Guastamacchia, E.; Giannelli, G.; De Pergola, G. Impaired fasting plasma glucose is a risk indicator of interventricular septum thickening among non-diabetic subjects with obesity. Diabetes Res. Clin. Pract. 2020, 169, 108436. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.-H.; Lee, H.-S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Warnick, G.R.; Knopp, R.H.; Fitzpatrick, V.; Branson, L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990, 36, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ulijaszek, S.J. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. pp. 252. (World Health Organization, Geneva, 2000.) SFr 56.00, ISBN 92-4-120894-5, paperback. J. Biosoc. Sci. 2003, 35, 624–625. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Jung, H.-S.; Chang, Y.; Kwon, M.-J.; Sung, E.; Yun, K.E.; Cho, Y.K.; Shin, H.; Ryu, S. Smoking and the Risk of Non-Alcoholic Fatty Liver Disease: A Cohort Study. Am. J. Gastroenterol. 2019, 114, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.S.; Hollenbeck, C.B.; Jeppesen, J.; Chen, Y.D.; Reaven, G.M. Insulin resistance and cigarette smoking. Lancet 1992, 339, 1128–1130. [Google Scholar] [CrossRef]
- Attvall, S.; Fowelin, J.; Lager, I.; Von Schenck, H.; Smith, U. Smoking induces insulin resistance--a potential link with the insulin resistance syndrome. J. Intern. Med. 1993, 233, 327–332. [Google Scholar] [CrossRef]
- Chen, H.; Hansen, M.J.; Jones, J.E.; Vlahos, R.; Anderson, G.P.; Morris, M.J. Detrimental metabolic effects of combining long-term cigarette smoke exposure and high-fat diet in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1564–E1571. [Google Scholar] [CrossRef][Green Version]
- Zeidel, A.; Beilin, B.; Yardeni, I.; Mayburd, E.; Smirnov, G.; Bessler, H. Immune response in asymptomatic smokers. Acta Anaesthesiol. Scand. 2002, 46, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.P. The impact of cigarette smoking on metabolic syndrome. Biomed. Environ. Sci. 2013, 26, 947–952. [Google Scholar] [PubMed]
- Sinha-Hikim, A.P.; Sinha-Hikim, I.; Friedman, T.C. Connection of Nicotine to Diet-Induced Obesity and Non-Alcoholic Fatty Liver Disease: Cellular and Mechanistic Insights. Front. Endocrinol. 2017, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Lampignano, L.; Lattanzio, A.; Mariano, F.; Osella, A.R.; Bonfiglio, C.; Giannelli, G.; De Pergola, G. Association between adherence to the Mediterranean Diet and circulating Vitamin D levels. International journal of food sciences and nutrition. Int. J. Food Sci. Nutr. 2020, 71, 884–890. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Martino, T.; Zupo, R.; Caccavo, D.; Pecorella, C.; Paradiso, S.; Silvestris, F.; Triggiani, V. 25 Hydroxyvitamin D Levels are Negatively and Independently Associated with Fat Mass in a Cohort of Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 838–844. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Carotti, S.; Bertoccini, L.; Baroni, M.G.; Vespasiani-Gentilucci, U.; Cavallo, M.-G.; Morini, S. Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3407–3417. [Google Scholar] [CrossRef]
- Bennett, C.M.; Guo, M.; Dharmage, S.C. HbA(1c) as a screening tool for detection of Type 2 diabetes: A systematic review. Diabet. Med. 2007, 24, 333–343. [Google Scholar] [CrossRef]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Z.; Mao, Y.; Xu, Y.; Du, J.; Tang, X.; Cao, H. HbA1c may contribute to the development of non-alcoholic fatty liver disease even at normal-range levels. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Van den Berg, E.H.; van Tienhoven-Wind, L.J.N.; Amini, M.; Schreuder, T.C.M.A.; Faber, K.N.; Blokzijl, H.; Dullaart, R.P.F. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: The Lifelines Cohort Study. Metabolism 2017, 67, 62–71. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yu, X.; Qi, X. Thyroid Function and Risk of Non-Alcoholic Fatty Liver Disease in Euthyroid Subjects. Ann. Hepatol. 2018, 17, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. Thyroid Function and the Risk of Non-Alcoholic Fatty Liver Disease in Morbid Obesity. Front. Endocrinol. 2020, 11, 572128. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Sackett, D.L. Bias in analytic research. J. Chronic Dis. 1979, 32, 51–63. [Google Scholar] [CrossRef]
| Prop. (%) | FLI < 60 | FLI ≥ 60 | p Value * |
|---|---|---|---|
| 366 (29.70) | 866 (70.30) | ||
| Age (years) | 37.63 ± 12.48 | 40.11 ± 12.74 | <0.01 |
| Sex | |||
| Females | 325 (37.70) | 536 (62.30) | <0.01χ2 |
| Males | 41 (11.10) | 330 (88.90) | |
| BMI (kg/m2) | 28.85 ± 2.49 | 36.06 ± 5.91 | <0.01 |
| Smoking (Yes) | 60 (16.90) | 189 (22.70) | 0.03χ2 |
| Fatty Liver index (%) | 37.41 ± 14.62 | 85.1 ± 11.78 | <0.01 |
| Waist Circumference (cm) | 95.1 ± 6.63 | 114.29 ± 12.39 | <0.01 |
| SBP (mmHg) | 120.88 ± 13.97 | 127.82 ± 13.98 | <0.01 |
| DBP (mmHg) | 78.4 ± 9.17 | 82.77 ± 9.75 | <0.01 |
| FPG (mg/dL) | 86.86 ± 8.57 | 93.57 ± 13.34 | <0.01 |
| Insulin (μU/mL) | 15.78 ± 8.73 | 26.27 ± 17.21 | <0.01 |
| Homa-IR | 3.41 ± 1.94 | 6.16 ± 4.38 | <0.01 |
| HbA1c (%) | 5.27 ± 0.36 | 5.42 ± 0.51 | <0.01 |
| Triglycerides (mg/dL) | 69.28 ± 30.27 | 123.19 ± 63.01 | <0.01 |
| HDL Cholesterol (mg/dL) | 54.43 ± 13.51 | 45.59 ± 10.91 | <0.01 |
| Total Cholesterol (mg/dL) | 183.62 ± 36.92 | 196.41 ± 38.06 | <0.01 |
| LDL Cholesterol (mg/dL) | 115.01 ± 31.62 | 127.28 ± 33.2 | <0.01 |
| Metabolic Syndrome (yes) | 27 (7.40) | 352 (41.20) | <0.01χ2 |
| TSH (mU/L) | 1.95 ± 1.21 | 1.98 ± 1.25 | 0.43 |
| FT3 (pg/mL) | 3.12 ± 0.42 | 3.16 ± 0.42 | 0.30 |
| FT4 (pg/mL) | 10.49 ± 1.41 | 10.63 ± 1.43 | 0.17 |
| FT3/FT4 ratio | 0.30 ± 0.05 | 0.30 ± 0.04 | 0.72 |
| Vitamin D (ng/mL) | 22.02 ± 8.01 | 19.5 ± 8.1 | <0.01 |
| Uric acid (mg/dL) | 4.01 ± 1.09 | 5.01 ± 1.44 | <0.01 |
| AST (U/L) | 19.15 ± 5.67 | 24.06 ± 9.8 | <0.01 |
| ALT (U/L) | 33.67 ± 11.55 | 46.82 ± 23.08 | <0.01 |
| GGT (U/L) | 20.92 ± 7.42 | 38.54 ± 27.07 | <0.01 |
| FLI < 60 | FLI ≥ 60 | p Value * | |
|---|---|---|---|
| Prop. (%) | 366 (50.00) | 366 (50.00) | |
| Age (years) | 37.62 ± 12.47 | 42.63 ± 13.69 | <0.01 |
| Sex | |||
| Females | 325 (88.80) | 36 (9.80) | <0.01 |
| Males | 41 (11.20) | 330 (90.20) | |
| BMI (Kg/m2) | 28.85 ± 2.47 | 34.77 ± 5.80 | <0.01 |
| Smoking (Yes) | 60 (16.90) | 91 (25.90) | 0.03 |
| Fatty Liver index (%) | 37.41 ± 14.62 | 87.24 ± 11.00 | <0.01 |
| Waist Circumference (cm) | 95.10 ± 6.62 | 116.15 ± 12.61 | <0.01 |
| SBP (mmHg) | 120.88 ± 13.96 | 132.28 ± 13.54 | <0.01 |
| DBP (mmHg) | 78.39 ± 9.16 | 85.64 ± 9.793 | <0.01 |
| FBG (mg/dL) | 86.86 ± 8.57 | 95.90 ± 14.53 | <0.01 |
| Insulin (μU/mL) | 15.78 ± 8.72 | 27.61 ± 19.22 | <0.01 |
| Homa-IR | 3.41 ± 1.94 | 6.61 ± 4.87 | <0.01 |
| HbA1c (%) | 5.26 ± 0.36 | 5.45 ± 0.45 | <0.01 |
| Triglycerides (mg/dL) | 69.28 ± 30.27 | 144.97 ± 71.65 | <0.01 |
| HDL Cholesterol (mg/dL) | 54.43 ± 13.51 | 41.83 ± 10.30 | <0.01 |
| Total Cholesterol (mg/dL) | 183.62 ± 36.92 | 198.95 ± 37.67 | <0.01 |
| LDL Cholesterol (mg/dL) | 115.00 ± 31.62 | 129.57 ± 33.28 | <0.01 |
| Metabolic Syndrome (yes) | 27 (7.40) | 194 (53.70) | <0.01 |
| TSH (mU/L) | 1.94 ± 1.20 | 1.87 ± 1.18 | 0.49 |
| FT3 (pg/mL) | 3.11 ± 0.42 | 3.19 ± 0.421 | 0.04 |
| FT4 (pg/mL) | 10.49 ± 1.40 | 10.59 ± 1.41 | 0.47 |
| FT3/FT4 ratio | 0.30 ± 0.05 | 0.30 ± 0.05 | 0.39 |
| Vitamin D (ng/mL) | 22.01 ± 8.00 | 20.68 ± 8.23 | 0.08 |
| Uric acid (mg/dL) | 4.01 ± 1.08 | 5.81 ± 1.41 | <0.01 |
| AST (U/L) | 19.15 ± 5.66 | 26.82 ± 10.63 | <0.01 |
| ALT (U/L) | 33.66 ± 11.54 | 53.86 ± 26.20 | <0.01 |
| GGT (U/L) | 20.92 ± 7.41 | 46.87 ± 28.88 | <0.01 |
| Raw model | ||
| Odds Ratio | CI 95% | |
| (Intercept) | 0.275 | 0.091 to 0.831 |
| FT3 | 1.506 | 1.064 to 2.131 |
| Adjusted model | ||
| (Intercept) | 0.002 | 0 to 0.144 |
| FT3 | 1.557 | 0.784 to 3.089 |
| HbA1c (%) | 2.32 | 1.193 to 4.512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupo, R.; Castellana, F.; Panza, F.; Castellana, M.; Lampignano, L.; Cincione, R.I.; Triggiani, V.; Giannelli, G.; Dibello, V.; Sardone, R.; et al. Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes. J. Clin. Med. 2021, 10, 1695. https://doi.org/10.3390/jcm10081695
Zupo R, Castellana F, Panza F, Castellana M, Lampignano L, Cincione RI, Triggiani V, Giannelli G, Dibello V, Sardone R, et al. Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes. Journal of Clinical Medicine. 2021; 10(8):1695. https://doi.org/10.3390/jcm10081695
Chicago/Turabian StyleZupo, Roberta, Fabio Castellana, Francesco Panza, Marco Castellana, Luisa Lampignano, Raffaele Ivan Cincione, Vincenzo Triggiani, Gianluigi Giannelli, Vittorio Dibello, Rodolfo Sardone, and et al. 2021. "Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes" Journal of Clinical Medicine 10, no. 8: 1695. https://doi.org/10.3390/jcm10081695
APA StyleZupo, R., Castellana, F., Panza, F., Castellana, M., Lampignano, L., Cincione, R. I., Triggiani, V., Giannelli, G., Dibello, V., Sardone, R., & De Pergola, G. (2021). Non Alcoholic Fatty Liver Disease Is Positively Associated with Increased Glycated Haemoglobin Levels in Subjects without Diabetes. Journal of Clinical Medicine, 10(8), 1695. https://doi.org/10.3390/jcm10081695