The Influence of Gender in The Prognostic Impact of Diabetes mellitus in acute Pulmonary Embolism
Abstract
1. Introduction
2. Material and Methods
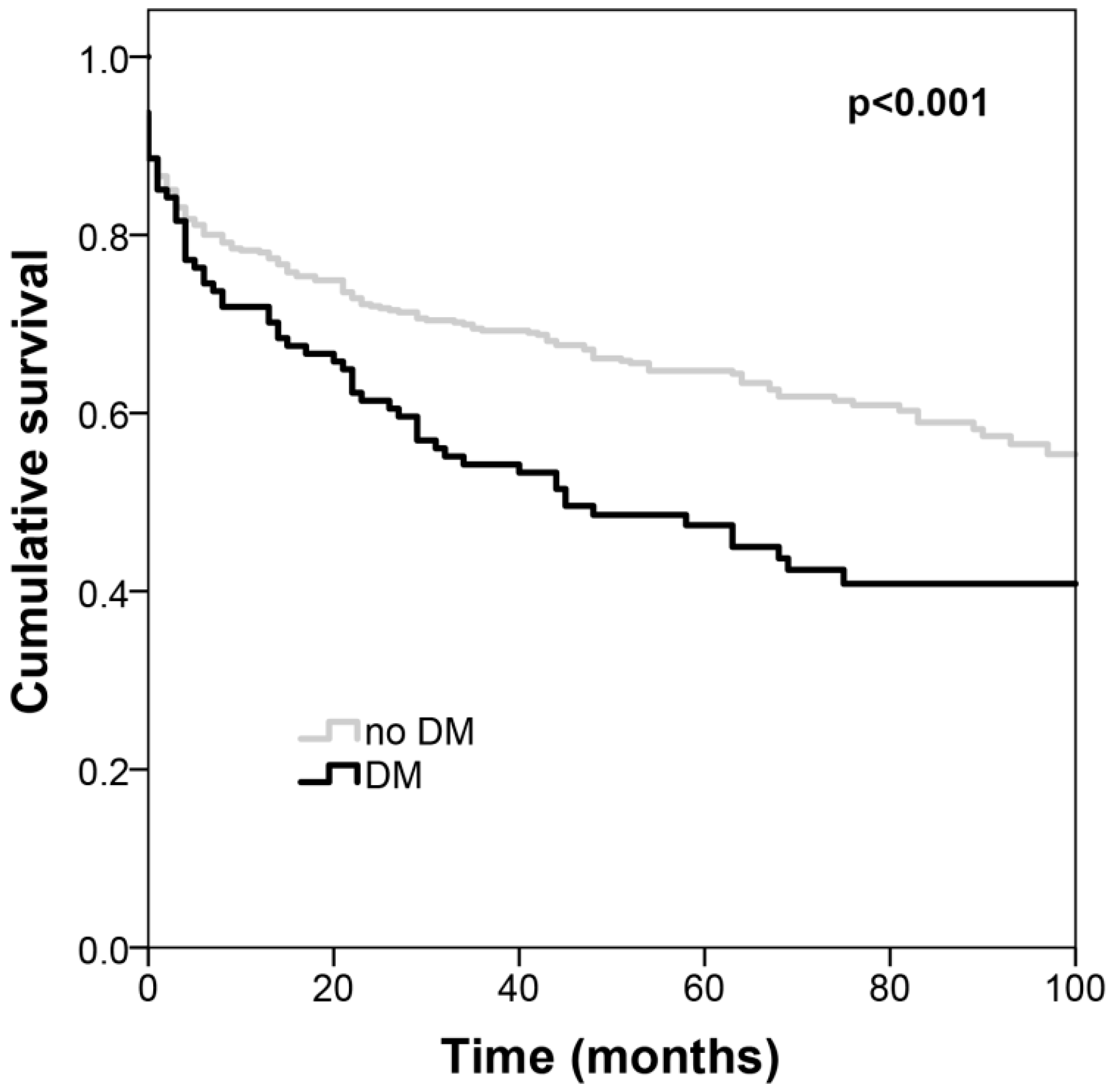
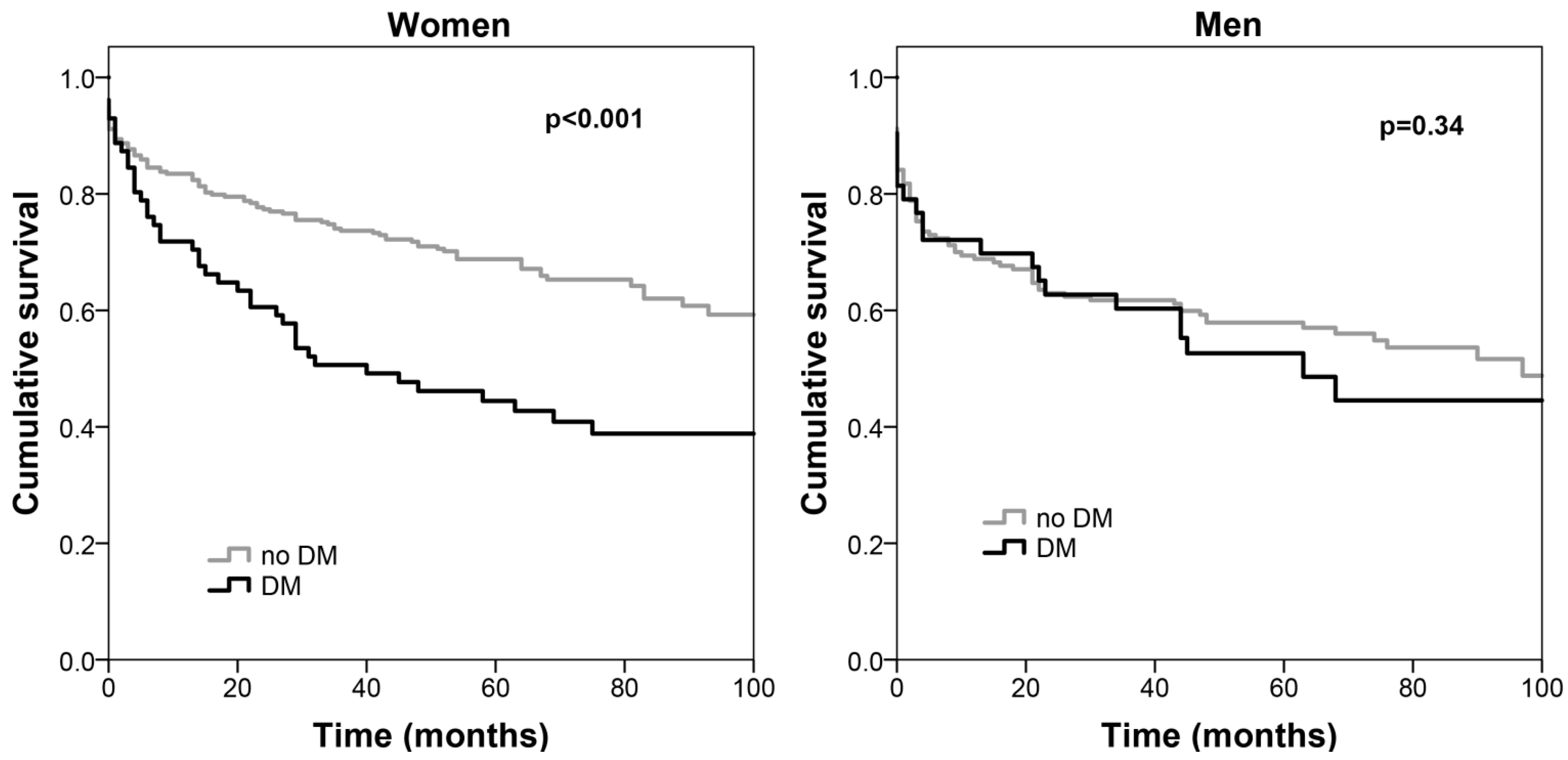
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Ba, Y.; Cai, R.-C.; Xing, Q. Association between diabetes mellitus and the risk for major cardiovascular outcomes and all-cause mortality in women compared with men: A meta-analysis of prospective cohort studies. BMJ Open 2019, 9, 024935. [Google Scholar] [CrossRef]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Elling, D.L.; Surkan, P.J.; Enayati, S.; El-Khatib, Z. Sex differences and risk factors for diabetes mellitus—An international study from 193 countries. Glob. Health 2018, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, J.; Green, J.B.; Ms, S.R.S.; Reed, S.D.; Armstrong, P.W.; Bethel, M.A.; Engel, S.S.; McGuire, D.K.; Van De Werf, F.; Hramiak, I.; et al. Sex differences in management and outcomes of patients with type 2 diabetes and cardiovascular disease: A report from TECOS. Diabetes Obes. Metab. 2018, 20, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, N.; Ivy, J.S.; Payton, F.C.; Norman, J. Diabetes and the hospitalized patient: A cluster analytic framework for characterizing the role of sex, race and comorbidity from 2006 to 2011. Health Care Manag. Sci. 2018, 21, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Muntner, P.; Woodward, M. Sex Differences in the Prevalence of, and Trends in, Cardiovascular Risk Factors, Treatment, and Control in the United States, 2001 to 2016. Circulation 2019, 139, 1025–1035. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetology 2014, 57, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Palau, P.; Bertomeu-González, V.; Sanchis, J.; Soler, M.; De La Espriella, R.; Domínguez, E.; Santas, E.; Núñez, E.; Chorro, F.J.; Miñana, G.; et al. Differential prognostic impact of type 2 diabetes mellitus in women and men with heart failure with preserved ejection fraction. Revista Española de Cardiología (Engl. Ed.) 2020, 73, 463–470. [Google Scholar] [CrossRef]
- Ohkuma, T.; Komorita, Y.; Peters, S.A.E.; Woodward, M. Diabetes as a risk factor for heart failure in women and men: A systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetology 2019, 62, 1550–1560. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Shikata, K.; Ninomiya, T.; Kiyohara, Y. Diabetes mellitus and cancer risk: Review of the epidemiological evidence. Cancer Sci. 2012, 104, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Stavros, V.K.; Guy, M.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group, 2019. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 543–603. [Google Scholar]
- De Miguel-Díez, J.; Andrés, A.L.-D.; Jiménez-Trujillo, I.; Hernández-Barrera, V.; Jiménez-García, R.; Lorenzo, A.; Pedrajas, J.M.; Visonà, A.; López-Miguel, P.; Jiménez-García, R. Mortality after pulmonary embolism in patients with diabetes. Findings from the RIETE registry. Eur. J. Intern. Med. 2019, 59, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Kotecha, A.; Raghavan, D.; Yadav, S.K.; Sule, A.A.; Arsene, C. New insights on patient-related risk factors for venous thromboembolism in patients with solid organ cancers. Int. J. Hematol. 2020, 112, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Al-Salameh, A.; Chanson, P.; Bucher, S.; Ringa, V.; Becquemont, L. Cardiovascular Disease in Type 2 Diabetes: A Review of Sex-Related Differences in Predisposition and Prevention. Mayo Clin. Proc. 2019, 94, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef]
- Díaz, A.; Lopez-Grueso, R.; Gambini, J.; Monleon, D.; Mas-Bargues, C.; Abdelaziz, K.M.; Viña, J.; Borras, C. Sex Differences in Age-Associated Type 2 Diabetes in Rats-Role of Estrogens and Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 6734836–13. [Google Scholar] [CrossRef]
- Harreiter, J.; Kautzky, W.A. Sex and Gender Differences in Prevention of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 220. [Google Scholar] [CrossRef]
- Jiménez, D.; Bikdeli, B.; Quezada, A.; Muriel, A.; Lobo, J.L.; De Miguel-Diez, J.; Jara-Palomares, L.; Ruiz-Artacho, P.; Yusen, R.D.; Monreal, M. Hospital volume and outcomes for acute pulmonary embolism: Multinational population based cohort study. BMJ 2019, 366, l4416. [Google Scholar] [CrossRef]
- Liang, Y.; Nie, S.; Wang, X.; Thomas, A.; Thompson, E.; Zhao, G.-Q.; Han, J.; Wang, J.; Griffiths, M.J.D. Role of Pulmonary Embolism Response Team in patients with intermediate- and high-risk pulmonary embolism: A concise review and preliminary experience from China. J. Geriatr. Cardiol. 2020, 17, 510–518. [Google Scholar] [PubMed]
- Lee, J.H.; Lee, J.-W.; Youn, Y.J.; Ahn, M.S.; Ahn, S.G.; Kim, J.Y.; Lee, S.-H.; Yoon, J.H.; Oh, J.; Kang, S.-M.; et al. Prognostic impact of preexisting hypertension and high systolic blood pressure at admission in patients hospitalized for systolic heart failure. J. Cardiol. 2016, 67, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.-F.; Streja, E.; Zahmatkesh, G.; Streja, D.; Kashyap, M.L.; Moradi, H.; Molnar, M.Z.; Reddy, U.; Amin, A.N.; Kovesdy, C.P.; et al. Reverse Epidemiology of Traditional Cardiovascular Risk Factors in the Geriatric Population. J. Am. Med. Dir. Assoc. 2015, 16, 933–939. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All (n = 577) | Women (n = 364) | Men (n = 213) | p-Value |
|---|---|---|---|---|
| Male gender, n (%) | 213 (36.9) | |||
| Age (years), mean (SD) | 65 (19) | 67 (18) | 62 (19) | 0.003 |
| Diabetes mellitus, n (%) | 114 (19.8) | 71 (19.5) | 43 (20.2) | 0.84 |
| Arterial Hypertension, n (%) | 318 (55.1) | 221 (60.7) | 97 (45.5) | <0.001 |
| Haemoglobin (g/dL), mean (SD) | 12.5 (2.3) | 12.1 (2.0) | 13.1 (2.2) | <0.001 |
| Creatinine (mg/dL), median (IQR) | 0.93 (0.72–1.30) | 0.90 (0.70–1.30) | 1.00 (0.80–1.30) | 0.003 |
| Lymphocytes (cells/µL), median (IQR) | 1600 (1015–2270) | 1740 (1110–2340) | 1410 (950–2030) | 0.001 |
| Platelets | 205 (159–267) | 220 (167–287) | 187 (146–239) | <0.001 |
| C–Reactive Protein (mg/L), median (IQR) | 39.8 (15.7–93.7) | 34.8 (14.2–79.6) | 51.9 (17.7–115.4) | 0.005 |
| BNP (pg/mL), median (IQR) | 283.0 (85.9–744.6) | 284.8 (88.9–759.1) | 278.8 (80.8–679.5) | 0.87 |
| Central/bilateral PE, n (%) | 175 (30.4) | 115 (31.8) | 60 (28.2) | 0.36 |
| idiopathic PE, n (%) | 376 (65.2) | 237 (65.1) | 139 (65.3) | 0.97 |
| Thrombolytic therapy, n (%) | 67 (11.6) | 45 (12.4) | 22 (10.3) | 0.46 |
| All–cause death, n (%) | 240 (41.6) | 140 (38.5) | 100 (46.9) | 0.05 |
| Characteristics | HR (95% CI) | p-Value |
|---|---|---|
| Diabetes mellitus | 2.33 (1.51–3.61) | <0.001 |
| Male gender | 1.84 (1.27–2.68) | 0.001 |
| Arterial hypertension history | 0.67 (0.47–0.97) | 0.03 |
| Central and bilateral Pulmonary embolism | 0.72 (0.51–1.02) | 0.06 |
| C–reactive protein (per 10 mg/L) | 0.98 (0.96–1.01) | 0.15 |
| B–type natriuretic peptide (per 100 pg/mL) | 1.02 (1.01–1.03) | <0.001 |
| Anemia | 1.22 (0.89–1.67) | 0.23 |
| Creatinine >1.5 mg/dL | 1.76 (1.24–2.52) | 0.002 |
| Lymphocyte counts <1500 cells/µL | 1.84 (1.34–2.53) | <0.001 |
| Platelets (×100) | 0.91 (0.76–1.09) | 0.31 |
| Age >65 years | 3.06 (2.05–4.57) | <0.001 |
| Thrombolytic therapy | 0.73 (0.45–1.20) | 0.22 |
| Interaction gender * Diabetes mellitus | 0.04 |
| Women | Men | |||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Diabetes mellitus | 2.31 (1.45––3.65) | <0.001 | 1.10 (0.59–2.04) | 0.77 |
| Arterial hypertension history | 0.64 (0.39–1.04) | 0.07 | 0.75 (0.44–1.27) | 0.29 |
| Central/bilateral PE | 0.72 (0.47–1.12) | 0.15 | 0.74 (0.39–1.38) | 0.33 |
| C–reactive protein (per 10 mg/L) | 0.98 (0.95–1.01) | 0.22 | 1.00 (0.96–1.03) | 0.81 |
| BNP (per 100 pg/mL) | 1.01 (1.00–1.03) | 0.02 | 1.03 (1.01–1.04) | <0.001 |
| Anemia | 1.11 (0.73–1.67) | 0.64 | 1.39 (0.82–2.35) | 0.22 |
| Creatinine >1.5 mg/dL | 1.89 (1.20–2.99) | 0.006 | 1.42 (0.76–2.66) | 0.27 |
| Lymphocyte counts <1500 cell/µL | 1.87 (1.24–2.83) | 0.003 | 1.62 (0.95–2.76) | 0.08 |
| Platelets ×100 | 0.91 (0.73–1.13) | 0.41 | 0.85 (0.59–1.21)) | 0.36 |
| Age >65 years | 4.87 (2.57–9.26) | <0.001 | 2.20 (1.29–3.78) | 0.004 |
| Thrombolytic therapy | 0.75 (0.41–1.37) | 0.35 | 0.75 (0.30–1.88) | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, D.; Brito, T.; Elias, C.; Carreira, M.; Serino, M.; Guerreiro, I.; Magalhães, H.; Coelho, S.; Ferreira, S.; Araújo, E.; et al. The Influence of Gender in The Prognostic Impact of Diabetes mellitus in acute Pulmonary Embolism. J. Clin. Med. 2020, 9, 3511. https://doi.org/10.3390/jcm9113511
Oliveira D, Brito T, Elias C, Carreira M, Serino M, Guerreiro I, Magalhães H, Coelho S, Ferreira S, Araújo E, et al. The Influence of Gender in The Prognostic Impact of Diabetes mellitus in acute Pulmonary Embolism. Journal of Clinical Medicine. 2020; 9(11):3511. https://doi.org/10.3390/jcm9113511
Chicago/Turabian StyleOliveira, Diana, Teresa Brito, Catarina Elias, Marta Carreira, Mariana Serino, Inês Guerreiro, Helena Magalhães, Sara Coelho, Sara Ferreira, Emanuel Araújo, and et al. 2020. "The Influence of Gender in The Prognostic Impact of Diabetes mellitus in acute Pulmonary Embolism" Journal of Clinical Medicine 9, no. 11: 3511. https://doi.org/10.3390/jcm9113511
APA StyleOliveira, D., Brito, T., Elias, C., Carreira, M., Serino, M., Guerreiro, I., Magalhães, H., Coelho, S., Ferreira, S., Araújo, E., Ribeiro, A., & Lourenço, P. (2020). The Influence of Gender in The Prognostic Impact of Diabetes mellitus in acute Pulmonary Embolism. Journal of Clinical Medicine, 9(11), 3511. https://doi.org/10.3390/jcm9113511





