Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients
Abstract
:1. Introduction
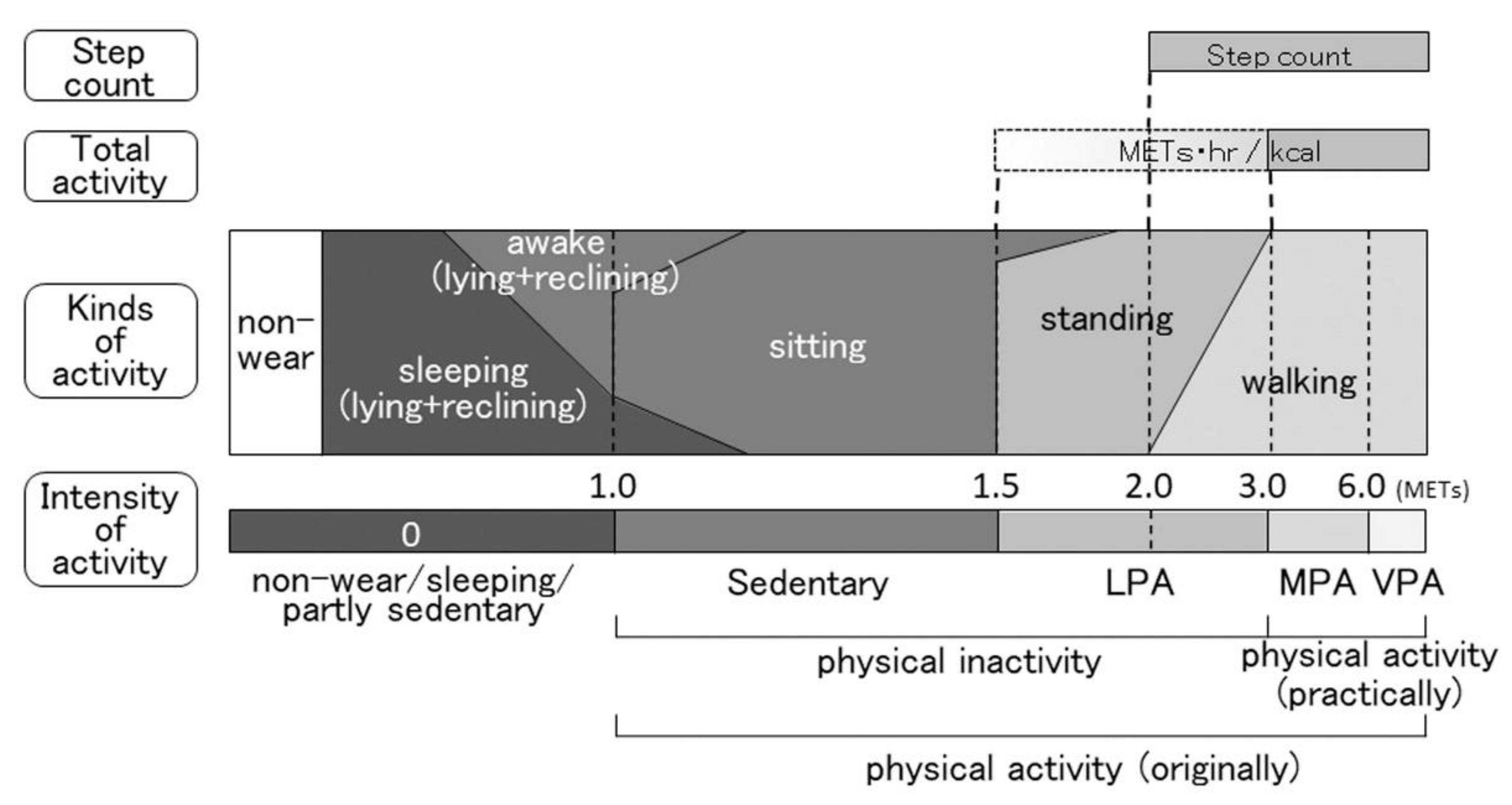
2. Indicators of PA
2.1. Duration of MVPA
2.2. Duration of LPA with MVPA
2.3. Duration of Walking and/or Standing
2.4. Total Activity
2.5. Step Count
2.6. Sedentary Time
3. Factors Influencing the Reproducibility of Data
3.1. Non-Wear Time
3.1.1. Definition of Non-Wear Time
3.1.2. Minimum Required Wearing Time
3.2. Days of Special Behavior
3.2.1. Days with Uncommon Activities
3.2.2. First and Last Days of Measurement
3.3. Environmental Factors
3.3.1. Weather
3.3.2. Season
3.3.3. Air Pollution
3.3.4. Holidays
3.4. Number of Valid Days Required
4. Effects of Bronchodilators on Physical Activity
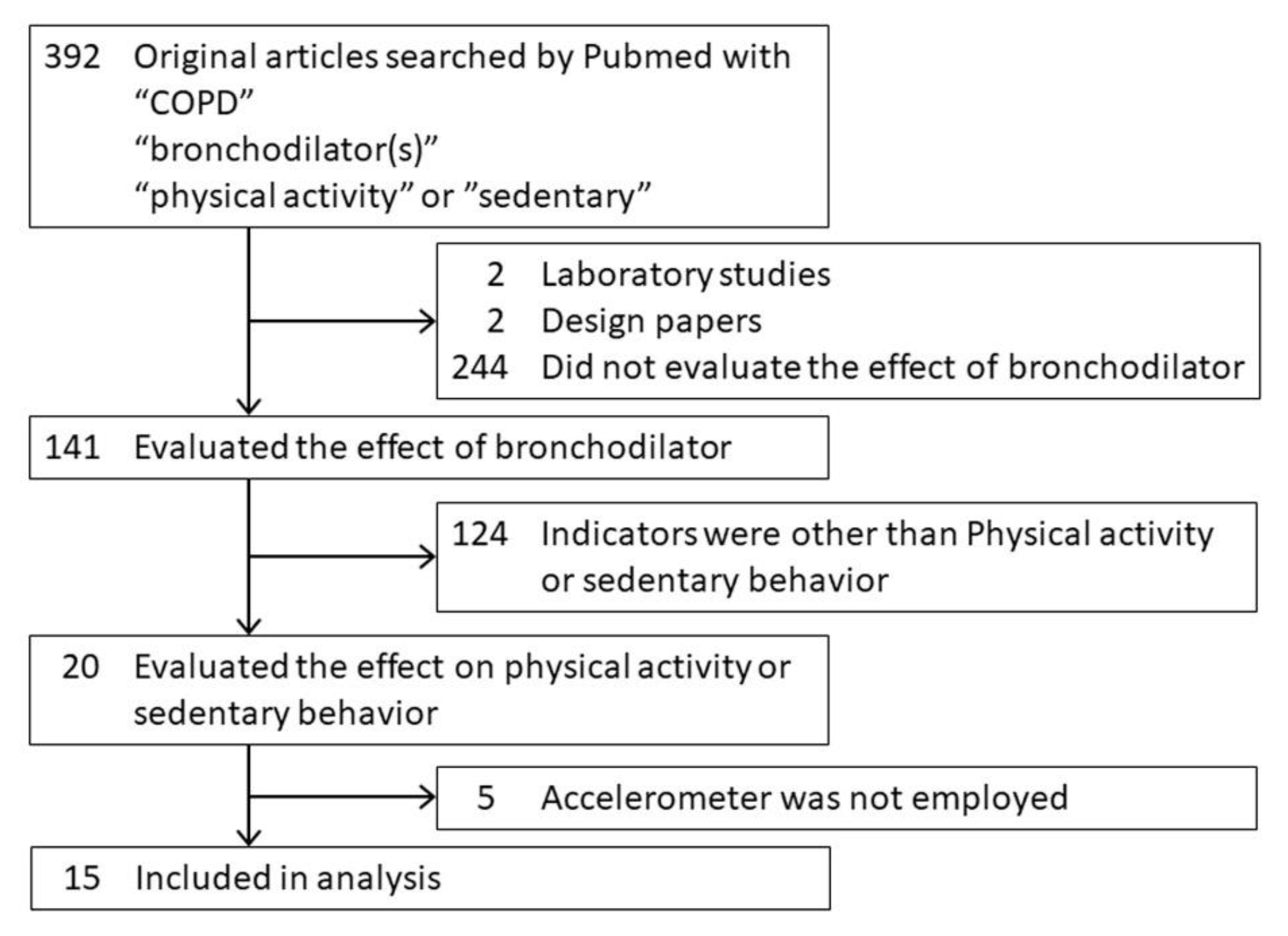
4.1. Methods
4.2. Effectiveness in COPD
4.2.1. Studies Reporting that Bronchodilators Were Effective
4.2.2. Studies in Which the Success of Bronchodilators Depended on the Indicator
4.2.3. Studies Reporting that Bronchodilators Were Ineffective
4.3. Effectiveness and Influencing Factors
4.4. Possible Characteristics of Patients in Whom Bronchodilators Are Effective
5. Sedentary Time as a New Indicator
5.1. Importance of Sedentary Time for Other Conditions
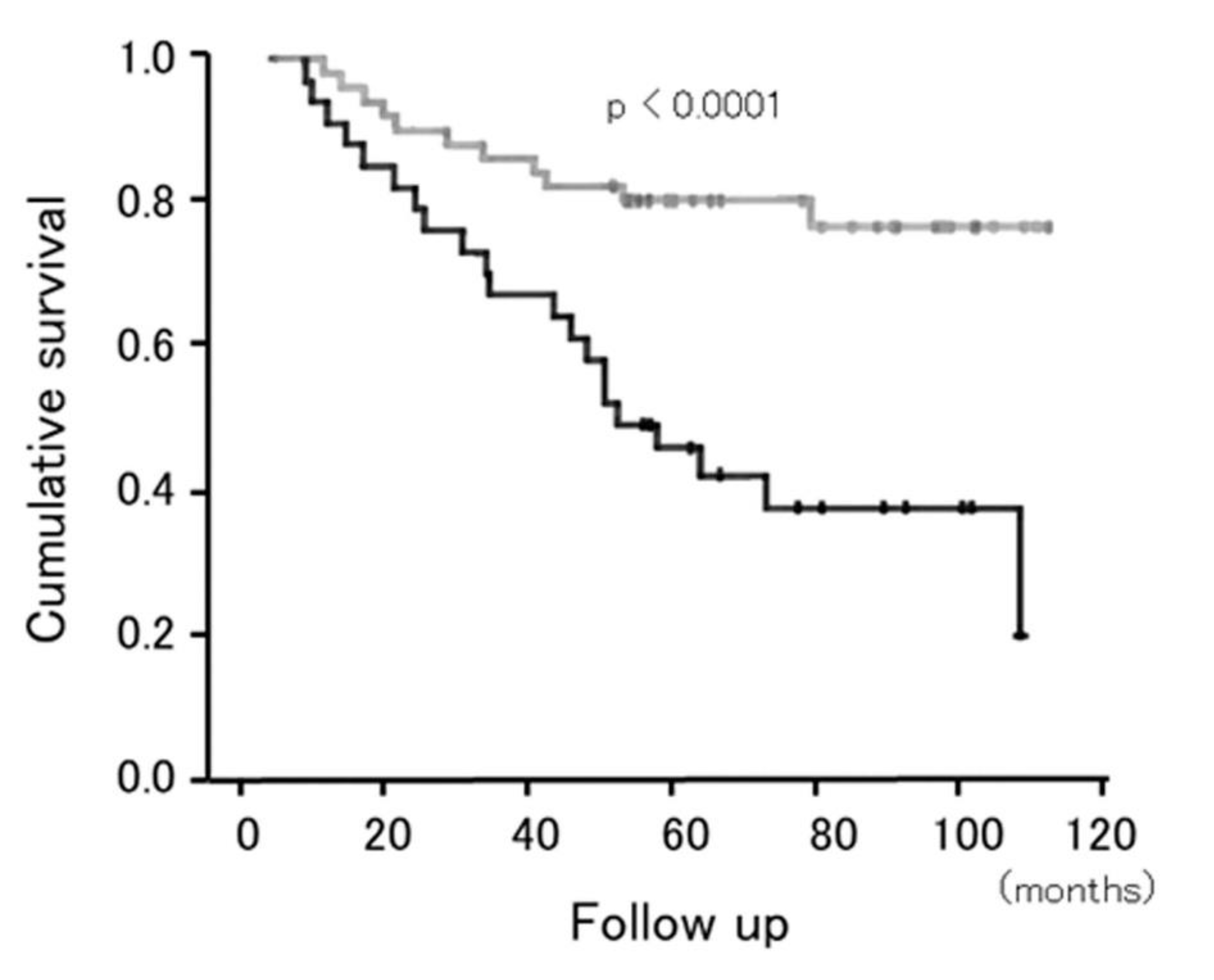
5.2. Importance of Sedentary Time in Patients with COPD
5.3. Effect of Bronchodilators on Sedentary Time
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020. Available online: www.goldcopd.org/ (accessed on 25 August 2020).
- Watz, H.; Pitta, F.; Rochester, C.L.; Garcia-Aymerich, J.; ZuWallack, R.; Troosters, T.; Vaes, A.W.; Puhan, M.A.; Jehn, M.; Polkey, M.I.; et al. An official European Respiratory Society statement on physical activity in COPD. Eur. Respir. J. 2014, 44, 1521–1537. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Baldwin, R.C.; Connolly, M. Mortality predictors in disabling chronic obstructive pulmonary disease in old age. Age Ageing 2002, 31, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Anto, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Rojo, B.; Casitas, R.; Lores, V.; Madero, R.; Romero, D.; Galera, R.; Villasante, C. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012, 142, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Moy, M.L.; Teylan, M.; Weston, N.A.; Gagnon, D.R.; Garshick, E. Daily Step Count Predicts Acute Exacerbations in a US Cohort with COPD. PLoS ONE 2013, 8, e60400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crook, S.; Busching, G.; Keusch, S.; Wieser, S.; Turk, A.; Frey, M.; Puhan, M.A.; Frei, A. The association between daily exacerbation symptoms and physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2018, 13, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Alahmari, A.D.; Patel, A.R.C.; Kowlessar, B.S.; Mackay, A.J.; Singh, R.; Wedzicha, J.A.; Donaldson, G.C. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeyer, H.; Costilla-Frias, M.; Louvaris, Z.; Gimeno-Santos, E.; Tabberer, M.; Rabinovich, R.A.; de Jong, C.; Polkey, M.I.; Hopkinson, N.S.; Karlsson, N.; et al. Both moderate and severe exacerbations accelerate physical activity decline in COPD patients. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Jakes, R.W.; Day, N.E.; Patel, B.; Khaw, K.T.; Oakes, S.; Luben, R.; Welch, A.; Bingham, S.; Wareham, N.J. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk Prospective Population Study. Am. J. Epidemiol. 2002, 156, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Pelkonen, M.; Notkola, I.L.; Lakka, T.; Tukiainen, H.O.; Kivinen, P.; Nissinen, A. Delaying decline in pulmonary function with physical activity: A 25-year follow-up. Am. J. Respir. Crit. Care Med. 2003, 168, 494–499. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Macera, C.A.; Addy, C.L.; Sy, F.S.; Wieland, D.; Blair, S.N. Effects of physical activity on exercise tests and respiratory function. Br. J. Sports Med. 2003, 37, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Anto, J.M. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: A population-based cohort study. Am. J. Respir. Crit. Care Med. 2007, 175, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Lange, P.; Serra, I.; Schnohr, P.; Antó, J.M. Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: An application of the marginal structural model. Ann. Epidemiol. 2008, 18, 775–783. [Google Scholar] [CrossRef]
- Thyregod, M.; Bodtger, U. Coherence between self-reported and objectively measured physical activity in patients with chronic obstructive lung disease: A systematic review. Int. J. Chronic Obs. Pulm. Dis. 2016, 11, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Sievi, N.A.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Kohler, M.; Clarenbach, C.F. Accelerometer- versus questionnaire-based assessment of physical activity and their changes over time in patients with COPD. Int. J. Chronic Obs. Pulm. Dis. 2017, 12, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Tremblay, M.S.; Colley, R.C.; Saunders, T.J.; Healy, G.N.; Owen, N. Physiological and health implications of a sedentary lifestyle. Appl. Physiol. Nutr. Metab. 2010, 35, 725–740. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Network, S.B.R. Letter to the editor: Standardized use of the terms "sedentary" and "sedentary behaviours". Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef] [Green Version]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karner, C.; Chong, J.; Poole, P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Melani, A.S. Long-acting muscarinic antagonists. Expert Rev. Clin. Pharmacol. 2015, 8, 479–501. [Google Scholar] [CrossRef]
- D’Urzo, A.; Ferguson, G.T.; van Noord, J.A.; Hirata, K.; Martin, C.; Horton, R.; Lu, Y.; Banerji, D.; Overend, T. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: The GLOW1 trial. Respir. Res. 2011, 12, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerwin, E.; Hébert, J.; Gallagher, N.; Martin, C.; Overend, T.; Alagappan, V.K.; Lu, Y.; Banerji, D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: The GLOW2 study. Eur. Respir. J. 2012, 40, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Beeh, K.M.; Singh, D.; Di Scala, L.; Drollmann, A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: The GLOW3 trial. Int. J. Chronic Obs. Pulm. Dis. 2012, 7, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, R.; Richard, N.; Mehta, R.; Church, A. Umeclidinium in patients with COPD: A randomised, placebo-controlled study. Eur. Respir. J. 2014, 43, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Beeh, K.M.; Watz, H.; Puente-Maestu, L.; de Teresa, L.; Jarreta, D.; Caracta, C.; Gil, E.G.; Magnussen, H. Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: A randomized, placebo-controlled, crossover trial. BMC Pulm. Med. 2014, 14, 209. [Google Scholar] [CrossRef]
- Rennard, S.I.; Anderson, W.; ZuWallack, R.; Broughton, J.; Bailey, W.; Friedman, M.; Wisniewski, M.; Rickard, K. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, M.A.; Aizawa, H.; Fukuchi, Y.; Mishima, M.; Nishimura, M.; Ichinose, M. Efficacy and safety of inhaled formoterol 4.5 and 9 μg twice daily in Japanese and European COPD patients: Phase III study results. BMC Pulm. Med. 2011, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, J.F.; Fogarty, C.; Lotvall, J.; Mahler, D.A.; Worth, H.; Yorgancioglu, A.; Iqbal, A.; Swales, J.; Owen, R.; Higgins, M.; et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: Indacaterol versus tiotropium. Am. J. Respir. Crit. Care Med. 2010, 182, 155–162. [Google Scholar] [CrossRef]
- Buhl, R.; Dunn, L.J.; Disdier, C.; Lassen, C.; Amos, C.; Henley, M.; Kramer, B. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur. Respir. J. 2011, 38, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Kristufek, P.; Levine, B.E.; Thomson, M.H.; Till, D.; Kottakis, J.; Della Cioppa, G. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest 2002, 121, 1058–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, W.D.; Mustfa, N.; Nikoletou, D.; Kaul, S.; Hart, N.; Rafferty, G.F.; Donaldson, N.; Polkey, M.I.; Moxham, J. Effect of salmeterol on respiratory muscle activity during exercise in poorly reversible COPD. Thorax 2004, 59, 471–476. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, D.E.; Voduc, N.; Fitzpatrick, M.; Webb, K.A. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur. Respir. J. 2004, 24, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Watz, H.; Krippner, F.; Kirsten, A.; Magnussen, H.; Vogelmeier, C. Indacaterol improves lung hyperinflation and physical activity in patients with moderate chronic obstructive pulmonary disease--a randomized, multicenter, double-blind, placebo-controlled study. BMC Pulm. Med. 2014, 14, 158. [Google Scholar] [CrossRef] [Green Version]
- Watz, H.; Troosters, T.; Beeh, K.M.; Garcia-Aymerich, J.; Paggiaro, P.; Molins, E.; Notari, M.; Zapata, A.; Jarreta, D.; Garcia Gil, E. ACTIVATE: The effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int. J. Chronic Obs. Pulm. Dis. 2017, 12, 2545–2558. [Google Scholar] [CrossRef] [Green Version]
- Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Nakamura, S.; Anzai, T.; Hirata, K.; Ichinose, M. Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: Post hoc analysis of the VESUTO((R)) study. Int. J. Chronic Obs. Pulm. Dis. 2019, 14, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Hataji, O.; Naito, M.; Ito, K.; Watanabe, F.; Gabazza, E.C.; Taguchi, O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2013, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Minakata, Y.; Morishita, Y.; Ichikawa, T.; Akamatsu, K.; Hirano, T.; Nakanishi, M.; Matsunaga, K.; Ichinose, M. Effects of pharmacologic treatment based on airflow limitation and breathlessness on daily physical activity in patients with chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2015, 10, 1275–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, T.; Nakamura, H.D.; Nanki, N.D.; Minakata, Y.D.; Matsunaga, K.D.; Mori, Y.D. Clinical benefit of two-times-per-day aclidinium bromide compared with once-a-day tiotropium bromide hydrate in COPD: A multicentre, open-label, randomised study. BMJ Open 2019, 9, e024114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Matsunaga, K.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Oishi, K.; Asami, M.; Edakuni, N.; Ogawa, H.; et al. Combination of assist use of short-acting beta-2 agonists inhalation and guidance based on patient-specific restrictions in daily behavior: Impact on physical activity of Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2019, 57, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, Y.; Hiramatsu, T.; Kojima, E.; Tabira, K. Effect of pulmonary rehabilitation with assistive use of short-acting β2 agonist in COPD patients using long-acting bronchodilators. Physiother. Theory Pract. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Mailander, C.; Baier, M.; Kirsten, A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: A randomised, placebo-controlled, crossover study (The MOVE Study). BMC Pulm. Med. 2016, 16, 95. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, Y.; Minami, S.; Yamamoto, S.; Ogata, Y.; Koba, T.; Futami, S.; Komuta, K. Influence of indacaterol on daily physical activity in patients with untreated chronic obstructive pulmonary disease. Int. J. Chronic Obs. Pulm. Dis. 2015, 10, 439–444. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, D.E.; Casaburi, R.; Vincken, W.; Puente-Maestu, L.; Swales, J.; Lawrence, D.; Kramer, B. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir. Med. 2011, 105, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Troosters, T.; Sciurba, F.C.; Decramer, M.; Siafakas, N.M.; Klioze, S.S.; Sutradhar, S.C.; Weisman, I.M.; Yunis, C. Tiotropium in patients with moderate COPD naive to maintenance therapy: A randomised placebo-controlled trial. NPJ Prim. Care Respir. Med. 2014, 24, 14003. [Google Scholar] [CrossRef]
- Ichinose, M.; Minakata, Y.; Motegi, T.; Ueki, J.; Gon, Y.; Seki, T.; Anzai, T.; Nakamura, S.; Hirata, K. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int. J. Chronic Obs. Pulm. Dis. 2018, 13, 1407–1419. [Google Scholar] [CrossRef] [Green Version]
- Troosters, T.; Maltais, F.; Leidy, N.; Lavoie, K.L.; Sedeno, M.; Janssens, W.; Garcia-Aymerich, J.; Erzen, D.; De Sousa, D.; Korducki, L.; et al. Effect of Bronchodilation, Exercise Training, and Behavior Modification on Symptoms and Physical Activity in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1021–1032. [Google Scholar] [CrossRef]
- Cavalheri, V.; Straker, L.; Gucciardi, D.F.; Gardiner, P.A.; Hill, K. Changing physical activity and sedentary behaviour in people with COPD. Respirology 2016, 21, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Arbillaga-Etxarri, A.; Gimeno-Santos, E.; Barberan-Garcia, A.; Benet, M.; Borrell, E.; Dadvand, P.; Foraster, M.; Marin, A.; Monteagudo, M.; Rodriguez-Roisin, R.; et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax 2017, 72, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Van Remoortel, H.; Hornikx, M.; Demeyer, H.; Langer, D.; Burtin, C.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013, 68, 962–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.Q.; Chu, L.; Amy Liu, I.L.; Lee, J.S.; Suh, D.; Korotzer, B.; Yuen, G.; Desai, S.; Coleman, K.J.; Xiang, A.H.; et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Burge, A.T.; Cox, N.S.; Abramson, M.J.; Holland, A.E. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2020, 4, Cd012626. [Google Scholar] [CrossRef]
- Hunt, T.; Williams, M.T.; Olds, T.S. Reliability and validity of the multimedia activity recall in children and adults (MARCA) in people with chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e81274. [Google Scholar] [CrossRef] [Green Version]
- van Remoortel, H.; Camillo, C.A.; Langer, D.; Hornikx, M.; Demeyer, H.; Burtin, C.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Moderate intense physical activity depends on selected Metabolic Equivalent of Task (MET) cut-off and type of data analysis. PLoS ONE 2013, 8, e84365. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Byrom, B.; Rowe, D.A. Measuring free-living physical activity in COPD patients: Deriving methodology standards for clinical trials through a review of research studies. Contemp. Clin. Trials 2016, 47, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Watz, H.; Waschki, B.; Boehme, C.; Claussen, M.; Meyer, T.; Magnussen, H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: A cross-sectional study. Am. J. Respir. Crit. Care Med. 2008, 177, 743–751. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evenson, K.R.; Terry, J.W., Jr. Assessment of differing definitions of accelerometer nonwear time. Res. Q. Exerc. Sport 2009, 80, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Choi, L.; Ward, S.C.; Schnelle, J.F.; Buchowski, M.S. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med. Sci. Sports Exerc. 2012, 44, 2009–2016. [Google Scholar] [CrossRef] [Green Version]
- Sugino, A.; Minakata, Y.; Kanda, M.; Akamatsu, K.; Koarai, A.; Hirano, T.; Sugiura, H.; Matsunaga, K.; Ichinose, M. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration 2012, 83, 300–307. [Google Scholar] [CrossRef]
- Alahmari, A.D.; Mackay, A.J.; Patel, A.R.; Kowlessar, B.S.; Singh, R.; Brill, S.E.; Allinson, J.P.; Wedzicha, J.A.; Donaldson, G.C. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir. Res. 2015, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Minakata, Y.; Azuma, Y.; Kawabe, K.; Ono, H.; Yanagimoto, R.; Suruda, T. Verification of a Motion Sensor for Evaluating Physical Activity in COPD Patients. Can. Respir. J. 2018, 2018, 8343705. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; Thomas-Ollivier, V.; Hug, F.; Bernady, A.; Le Blanc, C.; de Bisschop, C.; Chambellan, A. Translation and Cultural Adaptation of PROactive Instruments for COPD in French and Influence of Weather and Pollution on Its Difficulty Score. Int. J. Chronic Obs. Pulm. Dis. 2020, 15, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balish, S.M.; Dechman, G.; Hernandez, P.; Spence, J.C.; Rhodes, R.E.; McGannon, K.; Blanchard, C. The Relationship Between Weather and Objectively Measured Physical Activity Among Individuals With COPD. J. Cardiopulm. Rehabil. Prev. 2017, 37, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sewell, L.; Singh, S.J.; Williams, J.E.; Morgan, M.D. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J. Cardiopulm. Rehabil. Prev. 2010, 30, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, K.C.; Demeyer, H.; Sant’anna, T.; Hernandes, N.A.; Camillo, C.A.; Pons, I.S.; Gosselink, R.; Troosters, T.; Pitta, F. Physical Activity of Patients with COPD from Regions with Different Climatic Variations. Copd 2017, 14, 276–283. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Goldring, J.J.; Wedzicha, J.A. Influence of season on exacerbation characteristics in patients with COPD. Chest 2012, 141, 94–100. [Google Scholar] [CrossRef]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R., Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Burkett, L.; Reis, J.P.; Ainsworth, B.E.; Macera, C.A.; Wilson, D.K. How many days of pedometer monitoring predict weekly physical activity in adults? Prev. Med. 2005, 40, 293–298. [Google Scholar] [CrossRef]
- Gretebeck, R.J.; Montoye, H.J. Variability of some objective measures of physical activity. Med. Sci. Sports Exerc. 1992, 24, 1167–1172. [Google Scholar] [CrossRef]
- Steele, B.G.; Holt, L.; Belza, B.; Ferris, S.; Lakshminaryan, S.; Buchner, D.M. Quantitating physical activity in COPD using a triaxial accelerometer. Chest 2000, 117, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Lee, H.; Cardinal, B.J. Evidence to support including lifestyle light-intensity recommendations in physical activity guidelines for older adults. Am. J. Health Promot. 2015, 29, 277–284. [Google Scholar] [CrossRef]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, l4570. [Google Scholar] [CrossRef] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.M.; Duran, A.T.; Colabianchi, N.; Judd, S.E.; Howard, V.J.; Hooker, S.P. Potential Effects on Mortality of Replacing Sedentary Time With Short Sedentary Bouts or Physical Activity: A National Cohort Study. Am. J. Epidemiol. 2019, 188, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef]
- Grontved, A.; Hu, F.B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. JAMA 2011, 305, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Young, D.R.; Hivert, M.F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.T.; Lewis, C.E.; Owen, N.; Perry, C.K.; Siddique, J.; et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Howard, B.; Healy, G.N.; Owen, N. Too much sitting--a health hazard. Diabetes Res. Clin. Pract. 2012, 97, 368–376. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, P.C.; Owen, N.; Biddle, S.J.; Dunstan, D.W. Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Curr. Diabetes Rep. 2014, 14, 522. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Fitzsimons, C.; Jepson, R.; Saunders, D.H.; van der Ploeg, H.P.; Teixeira, P.J.; Gray, C.M.; Mutrie, N. Interventions with potential to reduce sedentary time in adults: Systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1056–1063. [Google Scholar] [CrossRef] [Green Version]
- Bakrania, K.; Edwardson, C.L.; Bodicoat, D.H.; Esliger, D.W.; Gill, J.M.; Kazi, A.; Velayudhan, L.; Sinclair, A.J.; Sattar, N.; Biddle, S.J.; et al. Associations of mutually exclusive categories of physical activity and sedentary time with markers of cardiometabolic health in English adults: A cross-sectional analysis of the Health Survey for England. BMC Public Health 2016, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Ukawa, S.; Tamakoshi, A.; Yatsuya, H.; Yamagishi, K.; Ando, M.; Iso, H. Association Between Average Daily Television Viewing Time and Chronic Obstructive Pulmonary Disease-Related Mortality: Findings From the Japan Collaborative Cohort Study. J. Epidemiol. 2015, 25, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Furlanetto, K.C.; Donaria, L.; Schneider, L.P.; Lopes, J.R.; Ribeiro, M.; Fernandes, K.B.; Hernandes, N.A.; Pitta, F. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects With COPD. Respir. Care 2017, 62, 579–587. [Google Scholar] [CrossRef]
- Donaire-Gonzalez, D.; Gimeno-Santos, E.; Balcells, E.; de Batlle, J.; Ramon, M.A.; Rodriguez, E.; Farrero, E.; Benet, M.; Guerra, S.; Sauleda, J.; et al. Benefits of physical activity on COPD hospitalisation depend on intensity. Eur. Respir. J. 2015, 46, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- McKeough, Z.; Cheng, S.W.M.; Alison, J.; Jenkins, C.; Hamer, M.; Stamatakis, E. Low leisure-based sitting time and being physically active were associated with reduced odds of death and diabetes in people with chronic obstructive pulmonary disease: A cohort study. J. Physiother. 2018, 64, 114–120. [Google Scholar] [CrossRef] [PubMed]
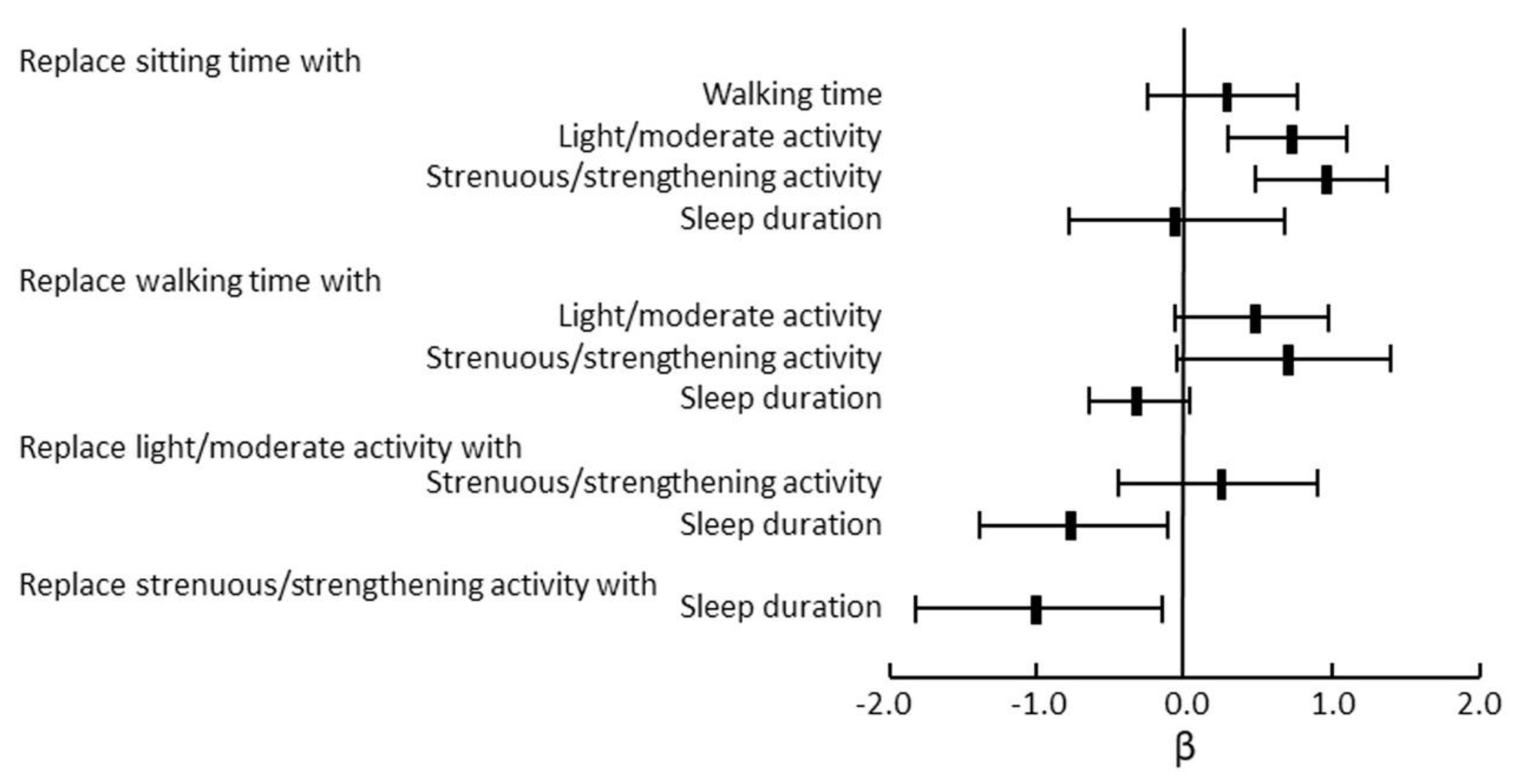
- Dogra, S.; Good, J.; Gardiner, P.A.; Copeland, J.L.; Stickland, M.K.; Rudoler, D.; Buman, M.P. Effects of replacing sitting time with physical activity on lung function: An analysis of the Canadian Longitudinal Study on Aging. Health Rep. 2019, 30, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, H.; Effing, T.W.; Lenferink, A.; Olds, T.; Williams, M.T. Improving physical activity, sedentary behaviour and sleep in COPD: Perspectives of people with COPD and experts via a Delphi approach. PeerJ 2018, 6, e4604. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, R.; Spina, G.; Pitta, F.; Donaire-Gonzalez, D.; Deering, B.M.; Patel, M.S.; Mitchell, K.E.; Alison, J.; van Gestel, A.J.; Zogg, S.; et al. Physical activity patterns and clusters in 1001 patients with COPD. Chronic Respir. Dis. 2017, 14, 256–269. [Google Scholar] [CrossRef]
- Geidl, W.; Carl, J.; Cassar, S.; Lehbert, N.; Mino, E.; Wittmann, M.; Wagner, R.; Schultz, K.; Pfeifer, K. Physical Activity and Sedentary Behaviour Patterns in 326 Persons with COPD before Starting a Pulmonary Rehabilitation: A Cluster Analysis. J. Clin. Med. 2019, 8, 1346. [Google Scholar] [CrossRef] [Green Version]
- Demeyer, H.; Gimeno-Santos, E.; Rabinovich, R.A.; Hornikx, M.; Louvaris, Z.; de Boer, W.I.; Karlsson, N.; de Jong, C.; Van der Molen, T.; Vogiatzis, I.; et al. Physical Activity Characteristics across GOLD Quadrants Depend on the Questionnaire Used. PLoS ONE 2016, 11, e0151255. [Google Scholar] [CrossRef] [Green Version]

| Terms | Definitions |
|---|---|
| Physical activity | (Originally) Any bodily movement produced by skeletal muscles that results in energy expenditure [18] |
| (Practically) Physically active behavior that is comparable to MVPA [19,20] | |
| Physical inactivity | A PA level that is not sufficient for meeting the present PA recommendations [20,21] |
| Threshold: [20] The non-achievement of 150 min of MVPA per week or 75 min of VPA per week or an equivalent combination of moderate- and vigorous-intensity activity | |
| Sedentary behavior | Any waking behavior characterized by an energy expenditure ≤1.5 METs, while in a sitting, reclining or lying posture [20,22,23] |
| Authors/Year of Publication | Country | Bronchodilator | Study Design | Accelerometer | |
|---|---|---|---|---|---|
| Sensor Type | Product Name | ||||
| Effective | |||||
| Hataji 2013 [42] | Japan | Ind | observation | uniaxial | Lifecorder |
| Watz 2014 [39] | Germany | Ind/Tio/Plac | crossover | biaxial | SenseWear armband |
| Minakata 2015 [43] | Japan | BD | observation | triaxial | Actimarker |
| Watz 2017 [40] | Germany | Acl/For vs. Plac | parallel groups | triaxial | DynaPort MoveMonitor |
| Minakata 2019 [41] | Japan | Tio/Olo vs. Tio | Crossover (post-hoc) | triaxial | Active Style Pro HJA-750C |
| Kamei 2019 [44] | Japan | Acl, Tio | Observation (post-hoc) | triaxial | ActiGraph GT3X-BT |
| Hirano 2019 [45] | Japan | Procat | observation | triaxial | Actimarker |
| Tsujimura 2019 [46] | Japan | Procat | observation | uniaxial | Lifecorder |
| Dependent on Indicators | |||||
| Beeh 2014 [31] | Germany | Acl vs. Plac | crossover | biaxial | SenseWear Pro3 |
| Nishijima 2015 [48] | Japan | Ind | observation | uniaxial | Lifecorder |
| Watz 2016 [47] | Germany | Ind/Gly vs. Plac | crossover | biaxial | SenseWear armband |
| Ineffective | |||||
| O’Donnell 2011 [49] | Belgium, Canada, Denmark, Italy, Spain, USA | Ind vs. Plac | crossover | biaxial | SenseWear armband |
| Troosters 2014 [50] | Belgium, Canada, Czech Republic, Germany, Greece, Netherlands, Portugal, Ukraine, UK, USA | Tio vs. Plac | parallel groups | biaxial | SenseWear armband |
| Ichinose 2018 [51] | Japan | Tio/Olo vs. Tio | crossover | triaxial | Active Style Pro HJA-750C |
| Troosters 2018 [52] | Australia, Austria, Belgium, Canada, Denmark, Germany, New Zealand, Poland, Portugal, UK, USA | Tio/Olo vs. Tio vs. Plac | parallel groups | triaxial | DynaPort MoveMonitor |
| Authors/Year of Publication | No. of Patients | Age | FEV1 % Pred | COPD Stage | Duration of Medication | Duration of Monitoring |
|---|---|---|---|---|---|---|
| Effective | ||||||
| Hataji 2013 [42] | 23 | 69.7 | 64.5 | I–IV | 4 W | 4 W |
| Watz 2014 [39] | 129 | 61.4 | 64 | II, III | 3 W | 1 W |
| Minakata 2015 [43] | 21 | 70.7 | 52.6 | I–IV | 6 W | 2 W |
| Watz 2017 [40] | 127 vs. 123 | 62.6 vs. 62.1 | 60.3 vs. 61.0 | II, III | 4 W | 1 W |
| Minakata 2019 [41] | 184 | 72.8 | 52.6 | II, III, IV | 6 W | 2 W |
| Kamei 2019 [44] | 22 vs. 22 | 72.3 vs. 70.9 | 60.1 vs. 57.6 | II, III | 8 W | 1 W |
| Hirano 2019 [45] | 14 | 72.1 | 55.6 | II, III, IV | 8 W | 2 W |
| Tsujimura 2019 [46] | 12 | 71.5 | FEV1% 34.5 | III, IV | 4, 12 W | 2 W |
| Dependent on indicators | ||||||
| Beeh 2014 [31] | 112 | 60.3 | 56.7 | II–III | 3 W | 1 W |
| Nishijima 2015 [48] | 18 | 74.2 | 55.2 | II, III, IV | 12 W | 1 W |
| Watz 2016 [47] | 194 | 62.8 | 61.6 | I–IV | 3 W | 1 W |
| Ineffective | ||||||
| O’Donnell 2011 [49] | 89 | 62.8 | 61.2 | I, II, III | 3 W | 5 days |
| Troosters 2014 [50] | 238 vs. 219 | 61.2 vs. 62.3 | 65.6 vs. 65.8 | II | 24 W | 1 W |
| Ichinose 2018 [51] | 184 | 72.8 | 52.6 | II, III, IV | 6 W | 2 W |
| Troosters 2018 [52] | 65 vs. 67 vs. 72 | 64.2 vs. 65.4 vs. 64.9 | 56 vs. 57 vs. 59 | I–IV | 12 W | 1 W |
| Authors/Year of Publication | Processing of Invalid Data | MVPA | Total Activity | (PA Level) | Steps | Sedentary | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-Wear | Special Behavior | Environmental Factors | Number of Valid Days | (min) Increase | (METs·h or kcal) Increase | (/resting) Increase | (steps) Increase | (min) Decrease | |
| Effective | |||||||||
| Hataji 2013 [42] | - | - | - | - | □ | □ | □ | ||
| Watz 2014 [39] | ☆ | - | - | ☆ | □ | □ | □ | ||
| Minakata 2015 [43] | - | ☆ | ☆ | ☆ | □ | □ | |||
| Watz 2017 [40] | ☆ | - | - | ☆ | □ | □ | □ | ||
| Minakata 2019 [41] | ☆ | - | ☆ | ☆ | □ | □ | |||
| Kamei 2019 [44] | - | - | - | - | □ | ||||
| Hirano 2019 [45] | - | ☆ | ☆ | ☆ | □ | □ | |||
| Tsujimura 2019 [46] | - | - | - | - | □ | □ | |||
| Dependent on indicators | |||||||||
| Beeh 2014 [31] | - | - | - | - | □ | □ | ■ | ■ | |
| Nishijima 2015 [48] | - | - | ☆ | ☆ | ■ | ■ | □ | ||
| Watz 2016 [47] | ☆ | ☆ | - | - | ■ | □ | □ | □ | |
| Ineffective | |||||||||
| O’Donnell 2011 [49] | - | - | - | - | ■ | ■ | |||
| Troosters 2014 [50] | - | - | - | - | ■ | ■ | |||
| Ichinose 2018 [51] | - | - | - | - | ■ | ■ | ■ | ||
| Troosters 2018 [52] | - | - | - | - | ■ | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minakata, Y.; Sasaki, S. Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients. J. Clin. Med. 2020, 9, 3497. https://doi.org/10.3390/jcm9113497
Minakata Y, Sasaki S. Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients. Journal of Clinical Medicine. 2020; 9(11):3497. https://doi.org/10.3390/jcm9113497
Chicago/Turabian StyleMinakata, Yoshiaki, and Seigo Sasaki. 2020. "Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients" Journal of Clinical Medicine 9, no. 11: 3497. https://doi.org/10.3390/jcm9113497
APA StyleMinakata, Y., & Sasaki, S. (2020). Data Reproducibility and Effectiveness of Bronchodilators for Improving Physical Activity in COPD Patients. Journal of Clinical Medicine, 9(11), 3497. https://doi.org/10.3390/jcm9113497






