1. Introduction
Chronic progressive pulmonary diseases, including chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILDs), should be managed according to symptom phases and activity limitations in individual patients [
1,
2,
3,
4]. Many previous studies have demonstrated that it is critical to capture the effects of dyspnea on living activities and when daily life becomes more restrictive [
5,
6,
7,
8]. Furthermore, living disabilities due to dyspnea could guide the introduction of pharmacological and non-pharmacological approaches, and severe daily life restrictions may provide an important starting phase for consultation on advanced care planning with patients and their families [
9,
10,
11].
The nature of dyspnea is reliant on patients’ self-reported symptoms. However, many COPD patients self-limit their living behaviors to avoid experiencing dyspnea, and therefore, underreport the severity of their dyspnea, which makes assessments difficult [
8,
12,
13]. The inaccurate assessment of dyspnea severity is particularly problematic in end-stage COPD when patients are most affected by dyspnea [
1]. However, their dyspnea is often assessed subjectively by physician observation only. There are several validated assessment tools for dyspnea, namely the modified Medical Research Council (mMRC) scale [
14] and the COPD Assessment Test (CAT) [
15]. The mMRC is a five-level scale of activity limitation due to dyspnea, while lacks the evaluation of self-limiting their living behaviors. Although the CAT includes a self-limiting living behaviors factor, it is a five-point Likert scale and is abstract. Physicians should discuss self-limiting behaviors with patients and evaluate the causes affecting them in a multifaceted manner, and intervene appropriately. Therefore, the lack of a simplified, specific and comprehensive patient-reported outcome measure remains a major challenge.
The unmet needs for dyspnea-related behavior and activity limitations in patients with COPD that have been described so far are similar to the measures of the frailty assessment for patients with COPD. Frailty is associated with geriatric syndromes that are recognized to represent a clinical state of physical, psychological, and social vulnerability and an increased risk for healthcare requirements [
16]. Frailty is closely associated with COPD, which shares common risk factors such as aging, smoking, inflammation, and endocrine disorders [
17]. As dyspnea increases, fatigue, muscle weakness, physical inactivity, and osteoporosis progress. These manifestations are similar to the symptoms of frailty. Once frailty progresses, the exacerbation of COPD, as a new physiological stressor, can result in increasingly worsening conditions ranging from disability and morbidity to death. The frequent coexistence of frailty and COPD seems to suggest a common underlying pathophysiological mechanism [
18]. Actually, patients with COPD have a greater risk for frailty than patients without COPD [
19,
20]. Moreover, patients with comorbid COPD and frailty have even worse outcomes (exacerbations, hospitalization, mortality, and worse quality of life) [
18,
21,
22,
23,
24,
25]. Therefore, there is strong linkage of COPD, dyspnea and frailty. Although it is important to identify frailty in patients with COPD, the diagnosis of frailty requires comprehensive assessment and is not easily implemented in a busy daily clinical practice.
We developed a new dyspnea evaluation system that focuses on patients’ perceptions of dyspnea and their living disabilities, and named the evaluation tool “patient-reported outcome measures for dyspnea-related behavior and activity limitation (PROMs-D).” This study aimed to validate PROMs-D in patient with COPD and verify whether PROMs-D can identify frailty status.
4. Discussion
We have demonstrated the distribution of PROMs-D in patients with COPD; and although SDS showed a bias towards Lv.0, there was a positive correlation between SDS and ADS. After reviewing the relationship between PROMs-D and frailty status, PROMs-D was demonstrated to be a useful frailty screening tool for patients with COPD. Moreover, we propose the importance of capturing the troublesome nature of changing clothes and bathing due to dyspnea because it may trigger that physicians consider a new pharmacological and nonpharmacological COPD management.
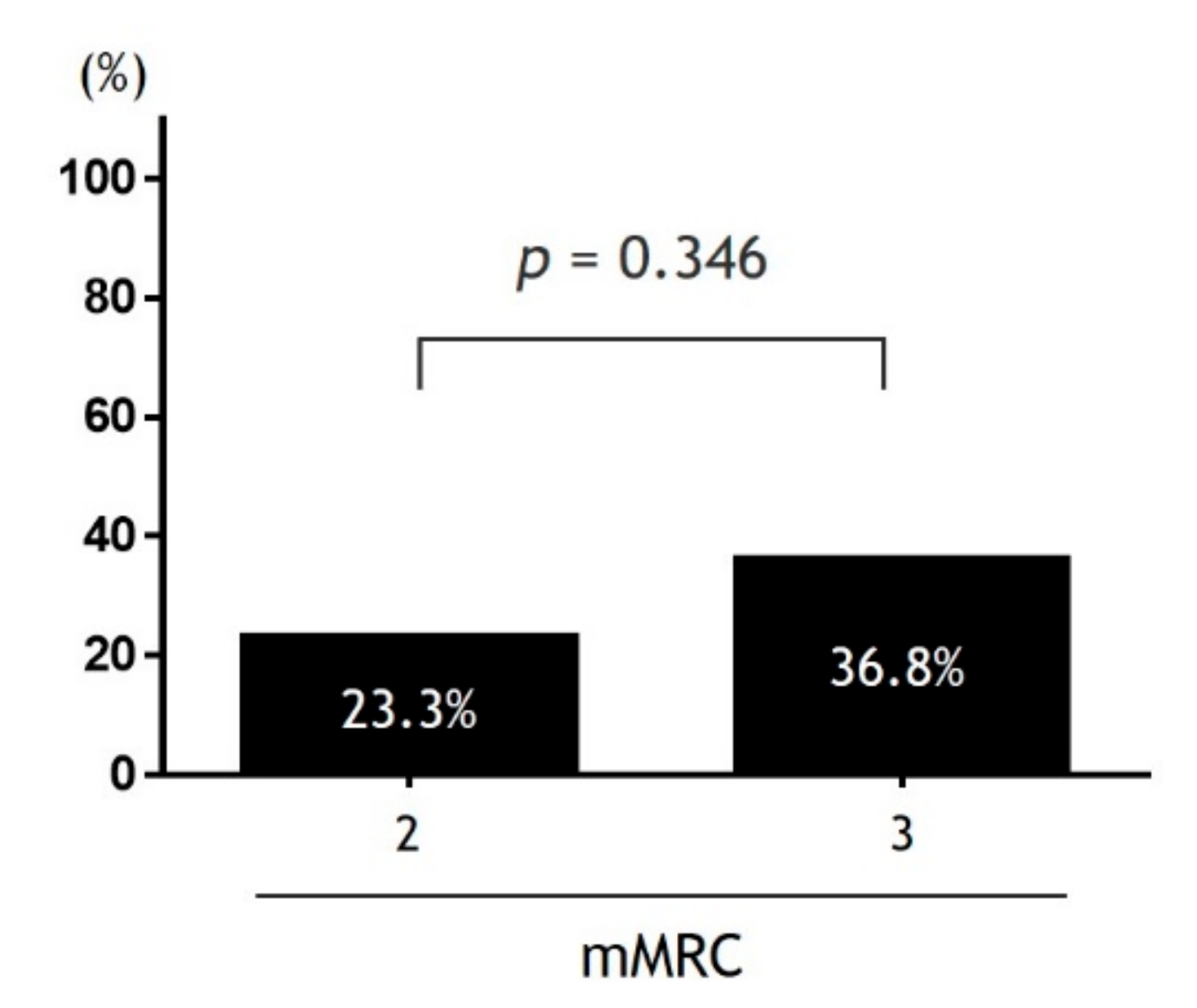
Based on the simplicity and diagnostic ability of identifying frailty, PROMs-D could be used as the first step for frailty screening in patients with COPD. Previous studies have reported that St. George’s Respiratory Questionnaire (SGRQ) relates to frailty in patients with COPD [
23]. However, because it takes too much time to measure, it is used as a research tool rather than in routine practice. The mMRC scale is a very simple and valid method for stratifying patients in terms of their risk of COPD exacerbation and airflow limitation [
36]. It has been suggested to be useful as a frailty screening tool [
22,
35,
37,
38]. On the other hand, a recent study showed that there was little difference in frailty prevalence between mMRC 0 and 1 and between 2 and 3 [
22], and our findings were consistent with this observation. According to the findings that focused on the prevalence of moderate or severe self-limitation between the patients with mMRC 2 and 3, there was no difference in this groups. Thus, we have identified that the assessment of mMRC alone may miss patients with self-limitation that require new therapeutic intervention such as self-management education, lung rehabilitation, and adequate assist use of short-acting β-agonist. It is possible to reduce the physician’s underestimation of self-limitation by capturing the troublesome nature of living behaviors due to dyspnea during the patient review even if the consultation time is limited. Therefore, it would be more useful for frailty screening to combine a three-level activity limitation assessment with the self-limitation and comorbidity assessment, rather than strictly dividing activity limitations into five levels. Our proposal makes sense because the mechanisms of dyspnea and the concept of frailty are multifactorial and encompass physiological, psychological, emotional, and social factors [
16,
39]. Of course, there are other easily applied frailty tests, such as the FRAIL scale, which that is 5 component patient-reported (yes/no) questions: Fatigue, Resistance, Ambulation, Illness, and Loss of weight [
40]. The question on the FRAIL scale that applies to self-limitation is Fatigue; “How much time during the previous 4 weeks did you feel tired?”. Compared with the FRAIL scale, the SDS questions on the PROMs-D are highly specific recall questions. If physicians ask the patient’s self-limitation when using abstract questions, the patient may underestimate. Therefore, we believe that highly specific recall questions are more effective in identifying self-limitation than abstract questions. Furthermore, the SDS questions are very simple and take almost no time. If the answer to SDS questions is “yes”, physicians can share the patient’s specific self-limitation and consider interventions to resolve it. This is an advantage over other tools.
The frailty group in the patients with PROMs-D score 1–2 had a higher CCI score than the non-frailty group. Since comorbid conditions in COPD are closely related to respiratory symptoms, exacerbations, and physical inactivity, it is important to identify therapeutic targets for these unmet medical needs [
25]. Several studies have reported that comorbidities in COPD are associated with an increased risk of frailty [
21,
22]. We realized the importance of focusing on comorbidities and considering the possibility of frailty, even in patients with COPD without self-limitations due to dyspnea.
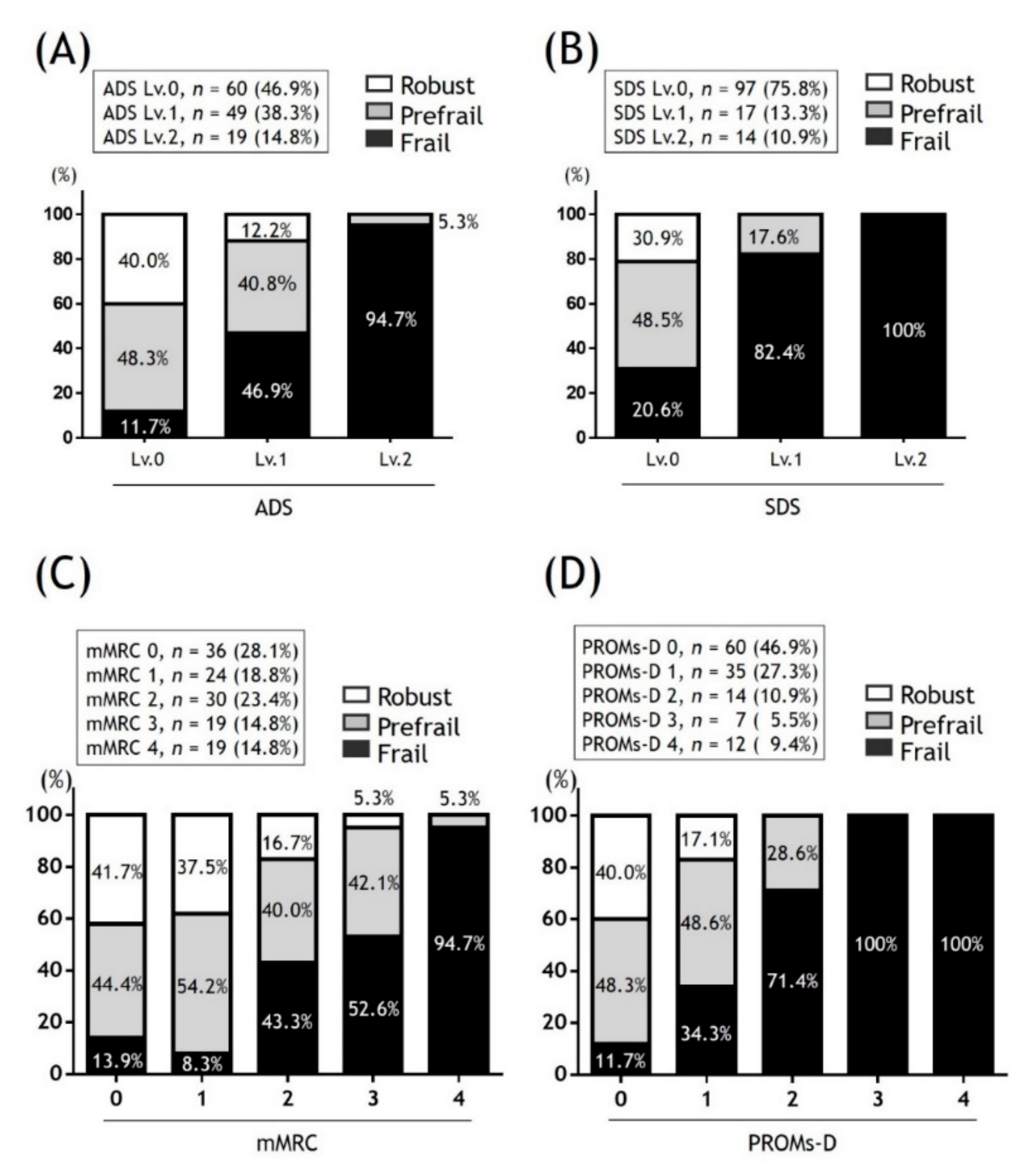
There are various tools that assess frailty in elderly patients, with the Fried frailty phenotype (FFP) being the most widely used. FFP consists of five components (weakness, slowness, unintentional weight loss, exhaustion, and low physical activity) that classify individuals as robust, prefrail, or frail, depending on the number of components affected, respectively, zero, one or two, and three or more [
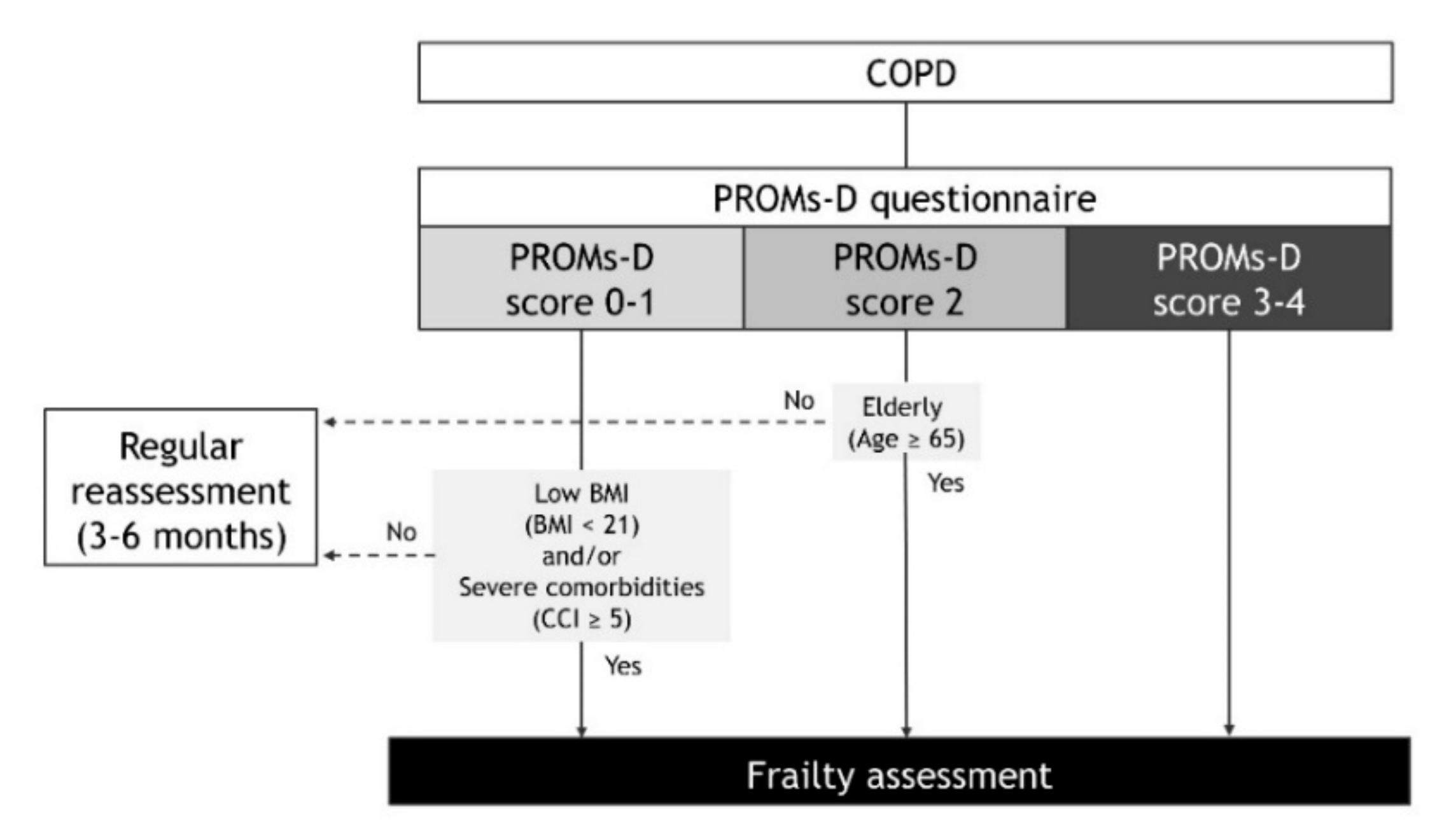
41]. From the findings of the present study, we propose the following strategies in patients with COPD that consider frailty in daily clinical practice (
Figure 5). In the case of PROMs-D scores of 0-1, although the probability of frailty is low, we recommend that frailty assessment may be considered when BMI is low (BMI < 21) and/or comorbidities are severe (CCI ≥ 5). In the case of a PROMs-D score 2, we suggest frailty assessment unless younger patients (age < 65 years). In the case of PROMs-D scores of 3–4, we strongly suggest that the frailty assessment should be performed immediately, due to the extremely high probability of frailty. In addition, it is necessary to routinely reassess PROMs-D after 3–6 months, even if the frailty assessment was not performed in patients with PROMs-D scores of 0–2. This is because patients with COPD also have a high prevalence of prefrailty, which is likely to move towards frailty.
The comprehensive management of patients with COPD with frailty that includes multidisciplinary teamwork and a community-based integrated care system, is crucial for the realization of a long healthy life expectancy society [
42,
43,
44,
45,
46,
47]. Since COPD is a very common disease, it is ideal for the frailty assessment to be comprehensively and easily assessed by primary care providers, pulmonary specialists, and other physicians. From this viewpoint, our proposal could contribute to improving the overall management of COPD.
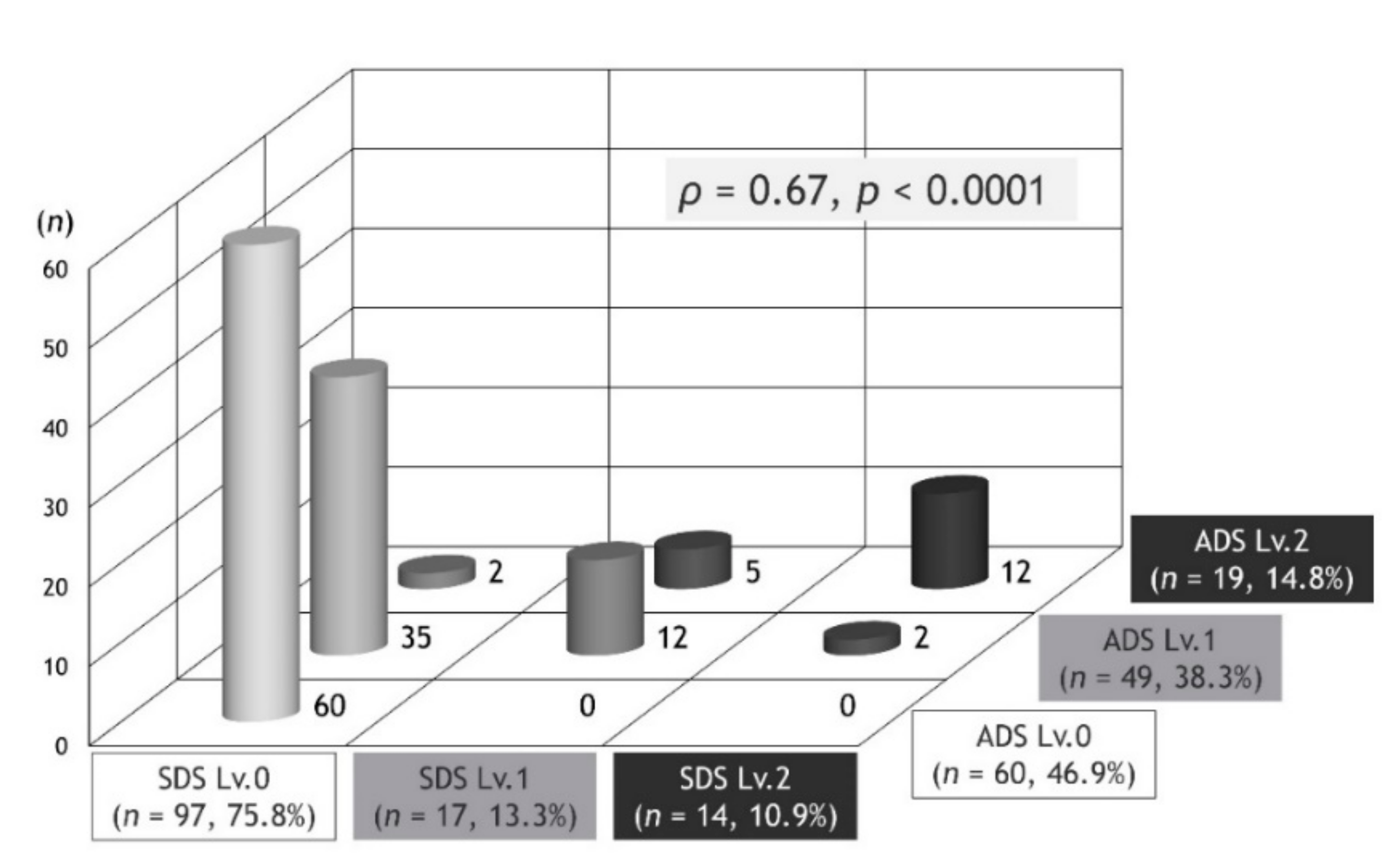
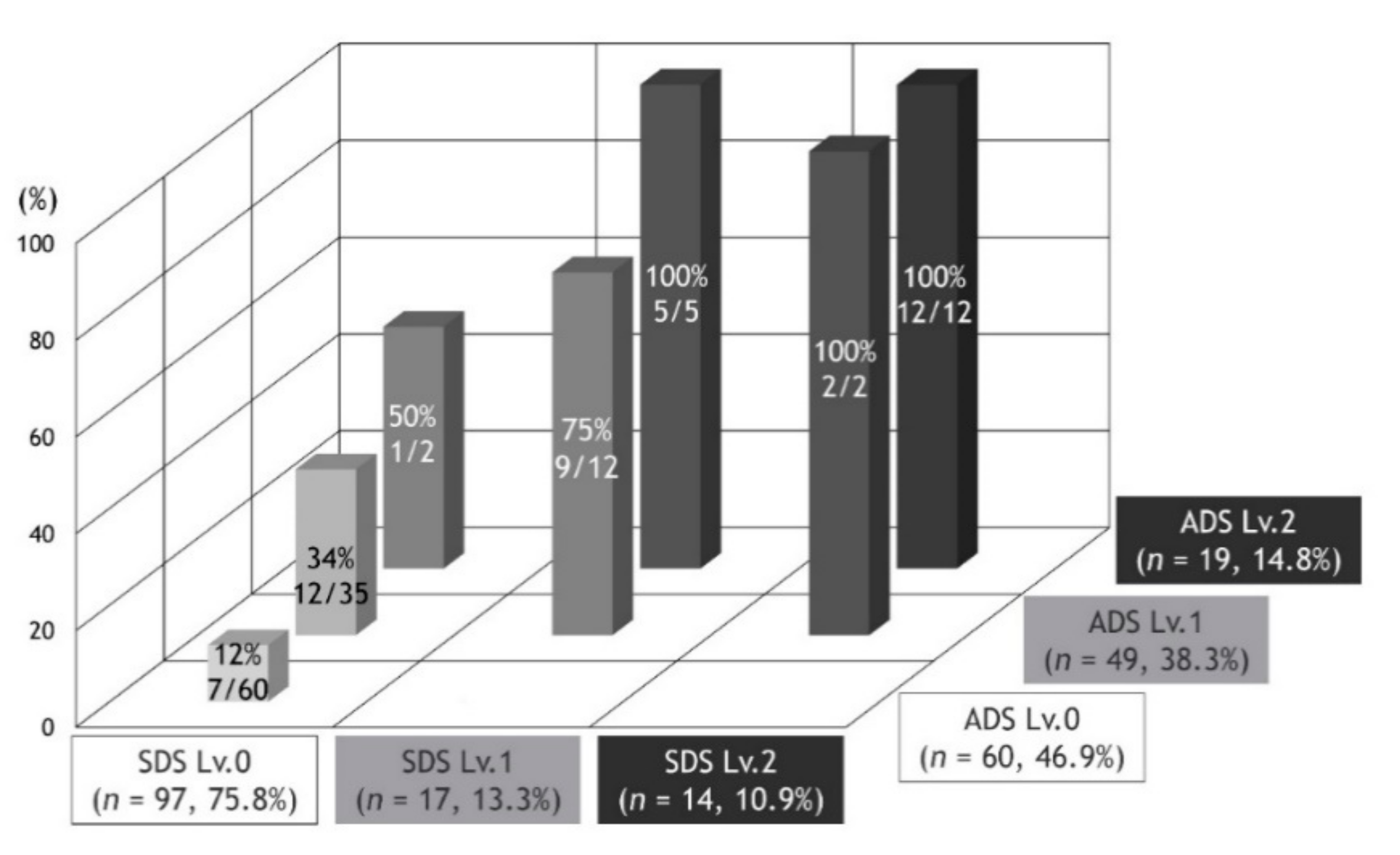
In the present study, there was a positive correlation between SDS and ADS. In fact, all patients with ADS Lv.0 were SDS Lv.0, and the cohort with ADS Lv.2 had the highest number of patients with SDS Lv.2. However, at the time of ADS development, we assumed that there would be a significant number of patients with SDS Lv.1 and Lv.2. Against assumptions, SDS showed a bias towards Lv.0, and SDS Lv.0 accounted for three-quarters of the total number of subjects, with SDS Lv.0 being the most common among patients with ADS Lv.1. The level for troublesome changing of clothes and bathing due to dyspnea may refer to a higher level of self-limitation than what was estimated. This survey will continue to require validation and modifications for SDS. For example, it may be meaningful to capture the troublesome nature of certain living behaviors other than changing clothes and bathing.
The current study has some limitations. First, the study included a small number of subjects, and the patients were characterized by older age, lower BMI, male majority, and low prevalence of current smokers. Although the characteristics of patients in the present study are comparable with other studies in Japan [
36,
48,
49], these patient characteristics may have influenced the findings, and there could be unmeasured confounders that could impact the results. Second, although we diagnosed frailty status using the KCL, there might be inconsistencies in frailty classification between various diagnostic tools. The prevalence of frailty was 37.5% of the subjects in the present study, which was higher than that of other studies [
19], although a direct comparison is difficult due to the differences in both criteria used and populations or settings studied. Third, our study did not measure the CAT. Because the CAT covers a broad variety of the health status of COPD and is related to frailty in COPD patients [
22,
23,
35,
38], a demonstration of the relationship between PROMs-D and CAT is also needed. Fourth, based on validation of the PROMs-D questionnaire, there were a few discrepancies between the answers in question 1 and questions 2–5. There was no contradiction between the patients answering “Yes” to question 1 and “No” to questions 2–5. This was because the patients had activity limitations or self-limitations than were captured by questions 2–5. However, there was a discrepancy between the patients answering “No” to question 1 and “Yes” to any of questions 2–5. One reason for this discrepancy is that for about 30% of the patients, 2–5 were highly specific recall questions, whereas question 1 was a somewhat abstract. Since several reports recommended asking as specific questions as possible about dyspnea measurement [
39,
50], underestimation may occur unless the physician ask the patient specific questions about dyspnea that impacts daily living. Thus, we did not exclude the patients with discrepancies in the analysis. Anyway, the PROMs-D questionnaire may continue to need further improvement to solve these problems, and comparison with current standard assessment tools. Finally, various therapies and comorbidities may have influenced the findings. Although we evaluated comorbidities with the updated CCI, we did not evaluate other important comorbid conditions in COPD, such as obstructive sleep apnea and gastroesophageal reflux. Further large-scale studies are required to validate these results.