Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Exposure
2.3. Outcome
2.4. Statistical Analysis
3. Results
3.1. Study Population
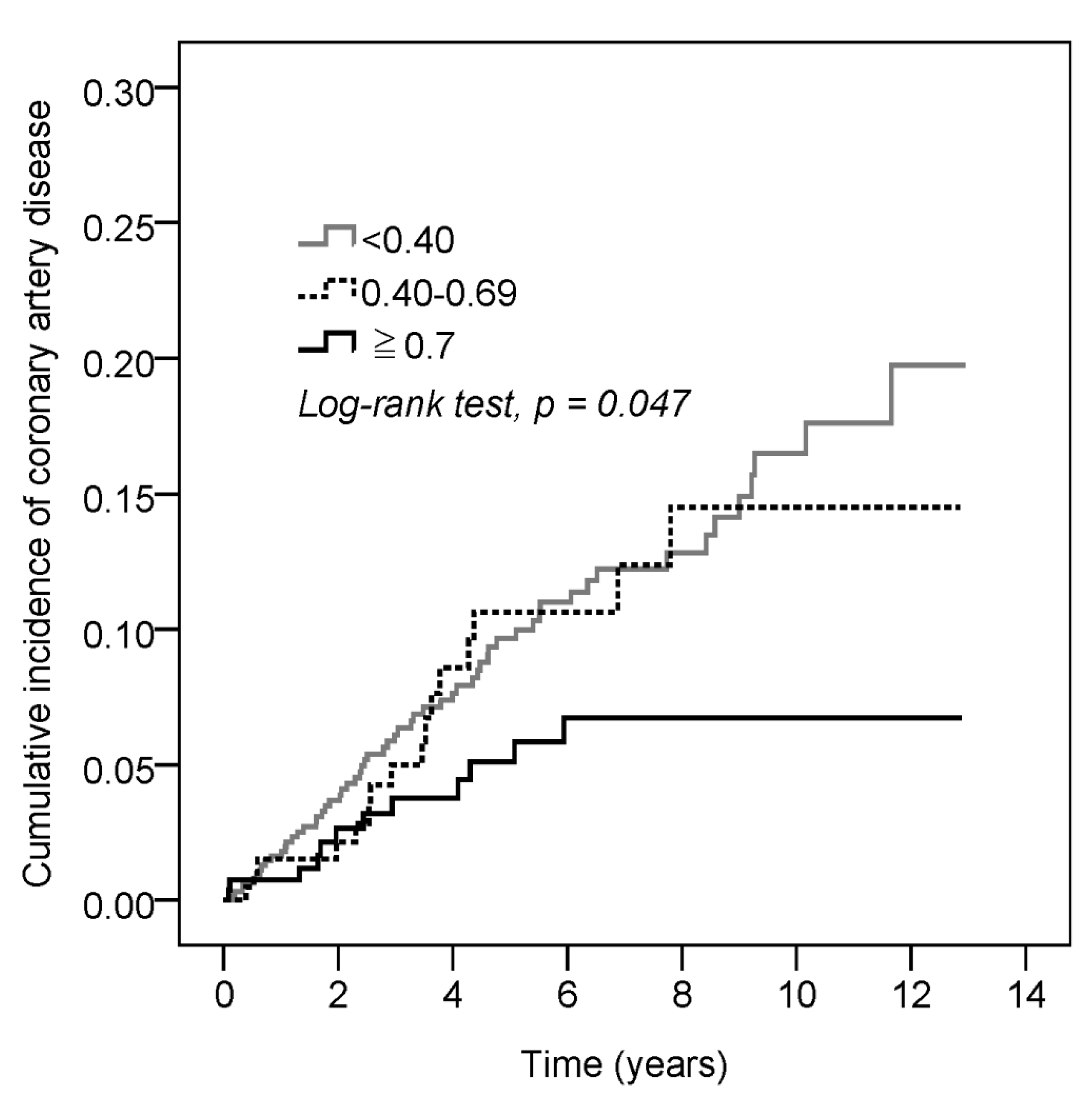
3.2. A Higher Incidence of CAD Developed in the SS Patients with a Lower MPR (<0.40) of HCQ, and There Was Significant Decreasing Cumulative Incidence of CAD in the SS Patients with a Higher MPR (≥0.70) of HCQ
3.3. Significant Cardiovascular Protection of HCQ in the 40–64 Age Group among the Patients with SS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariette, X.; Criswell, L.A. Primary Sjogren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Leone, M.C.; Giacomelli, R.; Gerli, R.; Carubbi, F. Lymphoma and Lymphomagenesis in Primary Sjogren’s Syndrome. Front. Med. 2018, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, E.; Alunno, A.; Valentini, V.; Valentini, E.; La Paglia, G.C.M.; Leone, M.C.; Cafaro, G.; Marcucci, E.; Bonifacio, A.F.; Luccioli, F.; et al. The prevalence and relevance of traditional cardiovascular risk factors in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 113–120. [Google Scholar] [PubMed]
- Perez-De-Lis, M.; Akasbi, M.; Siso, A.; Diez-Cascon, P.; Brito-Zeron, P.; Diaz-Lagares, C.; Ortiz, J.; Perez-Alvarez, R.; Ramos-Casals, M.; Coca, A. Cardiovascular risk factors in primary Sjogren’s syndrome: A case-control study in 624 patients. Lupus 2010, 19, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.C.; Sanguankeo, A.; Upala, S. Association between primary Sjogren’s syndrome, arterial stiffness, and subclinical atherosclerosis: A systematic review and meta-analysis. Clin. Rheumatol. 2019, 38, 447–455. [Google Scholar] [CrossRef]
- Tully, P.J.; Harrison, N.J.; Cheung, P.; Cosh, S. Anxiety and Cardiovascular Disease Risk: A Review. Curr. Cardiol. Rep. 2016, 18, 120. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhang, L.W.; Wei, P.; Hua, H. Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 186. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Ravaud, P.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Goeb, V.; Larroche, C.; Dubost, J.J.; Rist, S.; Saraux, A.; et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjogren syndrome: The JOQUER randomized clinical trial. JAMA 2014, 312, 249–258. [Google Scholar] [CrossRef]
- Ammirati, E.; Bozzolo, E.P.; Contri, R.; Baragetti, A.; Palini, A.G.; Cianflone, D.; Banfi, M.; Uboldi, P.; Bottoni, G.; Scotti, I.; et al. Cardiometabolic and immune factors associated with increased common carotid artery intima-media thickness and cardiovascular disease in patients with systemic lupus erythematosus. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 751–759. [Google Scholar] [CrossRef]
- Yang, D.H.; Leong, P.Y.; Sia, S.K.; Wang, Y.H.; Wei, J.C.C. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2019, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Rempenault, C.; Combe, B.; Barnetche, T.; Gaujoux-Viala, C.; Lukas, C.; Morel, J.; Hua, C. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2018, 77, 98–103. [Google Scholar] [CrossRef]
- Cai, X.; Luo, J.; Wei, T.; Qin, W.; Li, X.; Wang, X. Risk of Cardiovascular Involvement in Patients with Primary Sjogren’s Syndrome: A large-scale cross-sectional cohort study. Acta Reumatol. Port. 2019, 44, 71–77. [Google Scholar]
- Bartoloni, E.; Baldini, C.; Schillaci, G.; Quartuccio, L.; Priori, R.; Carubbi, F.; Bini, V.; Alunno, A.; Bombardieri, S.; De Vita, S.; et al. Cardiovascular disease risk burden in primary Sjogren’s syndrome: Results of a population-based multicentre cohort study. J. Intern. Med. 2015, 278, 185–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, L.; Li, Q.; Yang, P.; Kong, X.; Duan, X.; Zhang, M.; Li, X.; Wang, Y.; et al. Association between comorbidities and extraglandular manifestations in primary Sjogren’s syndrome: A multicenter cross-sectional study. Clin. Rheumatol. 2020, 39, 2677–2688. [Google Scholar] [PubMed]
- Bartoloni, E.; Alunno, A.; Bistoni, O.; Caterbi, S.; Luccioli, F.; Santoboni, G.; Mirabelli, G.; Cannarile, F.; Gerli, R. Characterization of circulating endothelial microparticles and endothelial progenitor cells in primary Sjogren’s syndrome: New markers of chronic endothelial damage? Rheumatology 2015, 54, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, V.A.; Moyssakis, I.; Boki, K.A.; Moutsopoulos, H.M. Is the heart affected in primary Sjogren’s syndrome? An echocardiographic study. Clin. Exp. Rheumatol. 2008, 26, 109–112. [Google Scholar] [PubMed]
- Gerli, R.; Bartoloni Bocci, E.; Vaudo, G.; Marchesi, S.; Vitali, C.; Shoenfeld, Y. Traditional cardiovascular risk factors in primary Sjogren’s syndrome--role of dyslipidaemia. Rheumatology 2006, 45, 1580–1581. [Google Scholar] [CrossRef]
- Patrono, C. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Michaud, K. The Risk of Cardiovascular Events Associated With Disease-modifying Antirheumatic Drugs in Rheumatoid Arthritis. J. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Miranda, S.; Billoir, P.; Damian, L.; Thiebaut, P.A.; Schapman, D.; Le Besnerais, M.; Jouen, F.; Galas, L.; Levesque, H.; Le Cam-Duchez, V.; et al. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: Role of reduced inflammation and endothelial dysfunction. PLoS ONE 2019, 14, e0212614. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lin, C.H.; Lan, T.H.; Chen, H.H.; Chang, S.N.; Chen, Y.H.; Wang, J.S.; Hung, W.T.; Lan, J.L.; Chen, D.Y. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: A population-based cohort study. Rheumatology 2015, 54, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.J.; Wasko, M.C.M.; Antohe, J.L.; Sartorius, J.A.; Kirchner, H.L.; Dancea, S.; Bili, A. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. 2011, 63, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.; Aujero, M.; Richards, J.; Sayles, H.; Davis, L.; Cannon, G.; Caplan, L.; Michaud, K.; Mikuls, T. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: Pharmacologic implications. Arthritis Care Res. 2014, 66, 1619–1626. [Google Scholar] [CrossRef]
- Urbanski, G.; Caillon, A.; Poli, C.; Kauffenstein, G.; Begorre, M.A.; Loufrani, L.; Henrion, D.; Belizna, C. Hydroxychloroquine partially prevents endothelial dysfunction induced by anti-beta-2-GPI antibodies in an in vivo mouse model of antiphospholipid syndrome. PLoS ONE 2018, 13, e0206814. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Zampeli, E.; Moutsopoulos, H.M. IgG4-related sialadenitis and Sjogren’s syndrome. Oral Dis. 2017, 23, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, M.; Mancuso, G.; Della-Torre, E. Advances in the diagnosis and management of IgG4 related disease. BMJ 2020, 369, m1067. [Google Scholar] [CrossRef]
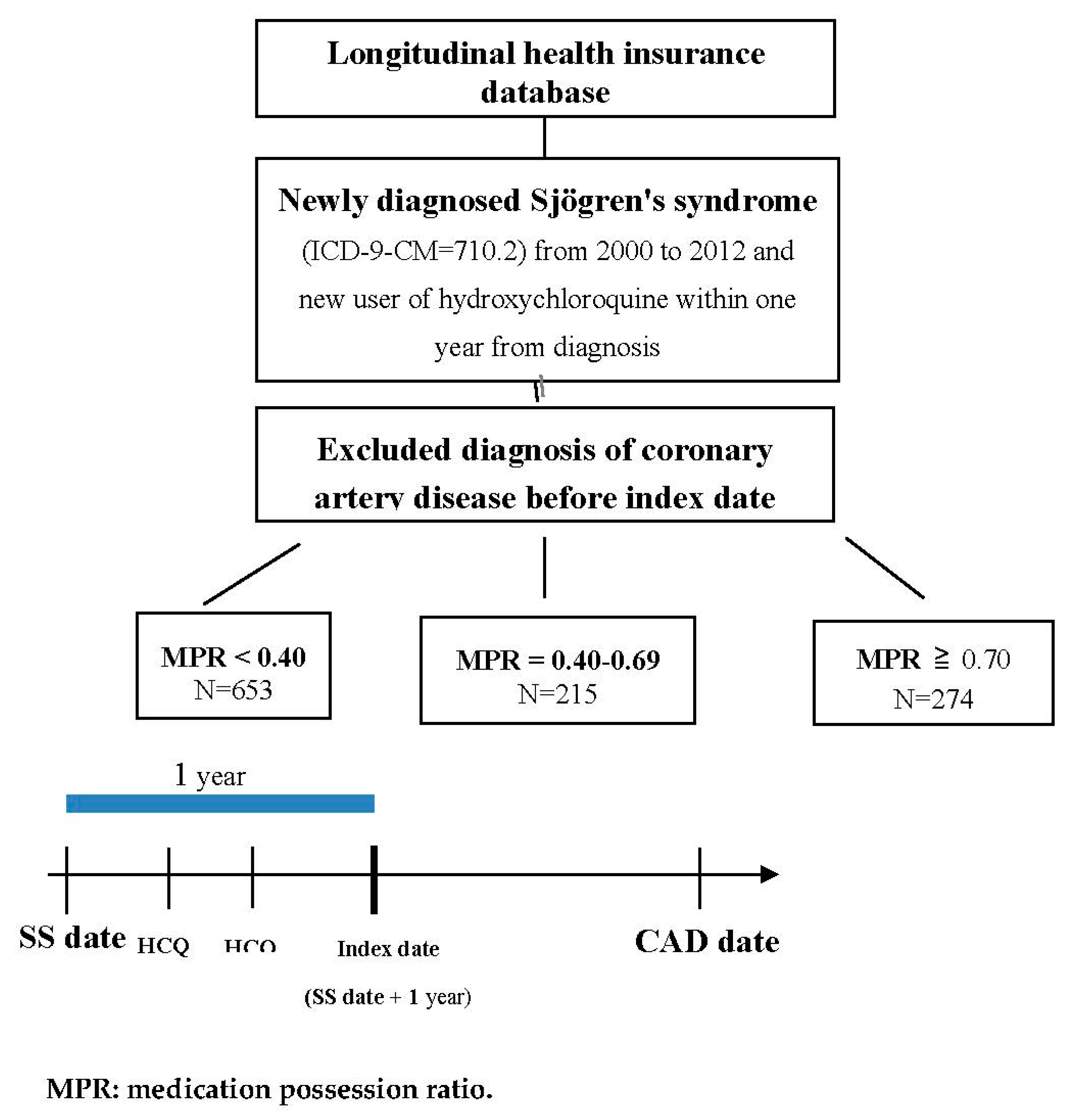
| Total | MPR < 0.40 (N = 653) | MPR = 0.40–0.69 (N = 215) | MPR ≥ 0.70 (N = 274) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p | |
| Age | 0.891 | ||||||||
| <40 | 302 | 26.4 | 178 | 27.3 | 51 | 23.7 | 73 | 26.6 | |
| 40–64 | 677 | 59.3 | 384 | 58.8 | 131 | 60.9 | 162 | 59.1 | |
| ≥65 | 163 | 14.3 | 91 | 13.9 | 33 | 15.3 | 39 | 14.2 | |
| Mean ± SD | 49.5 ± 14.5 | 49.4 ± 14.4 | 49.8 ± 15.3 | 49.5 ± 13.9 | 0.935 | ||||
| Gender | 0.234 | ||||||||
| Female | 997 | 87.3 | 562 | 86.1 | 188 | 87.4 | 247 | 90.1 | |
| Male | 145 | 12.7 | 91 | 13.9 | 27 | 12.6 | 27 | 9.9 | |
| Hypertension | 143 | 12.5 | 89 | 13.6 | 29 | 13.5 | 25 | 9.1 | 0.149 |
| Hyperlipidemia | 79 | 6.9 | 51 | 7.8 | 19 | 8.8 | 9 | 3.3 | 0.022 |
| Diabetes | 68 | 6.0 | 35 | 5.4 | 19 | 8.8 | 14 | 5.1 | 0.139 |
| COPD | 22 | 1.9 | 15 | 2.3 | 2 | 0.9 | 5 | 1.8 | 0.445 |
| Stroke | 33 | 2.9 | 16 | 2.5 | 5 | 2.3 | 12 | 4.4 | 0.239 |
| Corticosteroids | 451 | 39.5 | 159 | 28.8 | 104 | 48.4 | 188 | 58.0 | <0.001 |
| NSAIDs | 787 | 68.9 | 434 | 66.5 | 149 | 69.3 | 204 | 74.5 | 0.056 |
| No. of CAD | Observed Person-Years | Incidence Density (Per 1000 Person-Years) | Crude HR | 95% C.I. | Adjusted HR † | 95% C.I. | |
|---|---|---|---|---|---|---|---|
| MPR of HCQ | |||||||
| <0.40 | 60 | 653 | 91.9 | 1 | 1 | ||
| 0.40–0.69 | 16 | 215 | 74.4 | 0.88 | 0.51–1.53 | 0.93 | 0.53–1.63 |
| ≥0.70 | 12 | 274 | 43.8 | 0.46 | 0.25–0.86 | 0.49 | 0.26–0.94 |
| Age | |||||||
| <40 | 8 | 1680 | 4.8 | 1 | 1 | ||
| 40–64 | 53 | 3270 | 16.2 | 3.37 | 1.60–7.10 | 2.99 | 1.41–6.35 |
| ≥65 | 27 | 624 | 43.3 | 8.93 | 4.04–19.7 | 4.69 | 1.99–11.03 |
| Gender | |||||||
| Female | 72 | 4850 | 14.8 | 1 | 1 | ||
| Male | 16 | 723 | 22.1 | 1.49 | 0.87–2.57 | 1.15 | 0.65–2.05 |
| Hypertension | |||||||
| No | 65 | 5071 | 12.8 | 1 | 1 | ||
| Yes | 23 | 502 | 45.8 | 3.49 | 2.16–5.65 | 1.98 | 1.17–3.36 |
| Hyperlipidemia | |||||||
| No | 74 | 5331 | 13.9 | 1 | 1 | ||
| Yes | 14 | 242 | 57.8 | 3.96 | 2.22–7.06 | 2.27 | 1.18–4.38 |
| Diabetes | |||||||
| No | 78 | 5327 | 14.6 | 1 | 1 | ||
| Yes | 10 | 246 | 40.6 | 2.70 | 1.39–5.22 | 1.41 | 0.69–2.89 |
| COPD | |||||||
| No | 81 | 5470 | 14.8 | 1 | 1 | ||
| Yes | 7 | 103 | 68.0 | 4.70 | 2.17–10.18 | 3.57 | 1.51–8.46 |
| Stroke | |||||||
| No | 83 | 5447 | 15.2 | 1 | 1 | ||
| Yes | 5 | 127 | 39.4 | 2.48 | 0.78–6.13 | 1.17 | 0.44–3.10 |
| Corticosteroids | |||||||
| No | 43 | 2926 | 14.7 | 1 | 1 | ||
| Yes | 45 | 2647 | 17.0 | 1.18 | 0.78–1.80 | 1.23 | 0.79–1.93 |
| NSAIDs | |||||||
| No | 10 | 831 | 12.0 | 1 | 1 | ||
| Yes | 78 | 4742 | 16.4 | 1.47 | 0.75–2.86 | 1.36 | 0.68–2.70 |
| N | No. of CAD | Crude HR | 95% C.I. | Adjusted HR † | 95% C.I. | |
|---|---|---|---|---|---|---|
| Age <40 † | ||||||
| MPR of HCQ | ||||||
| <0.40 | 178 | 7 | 1 | 1 | ||
| 0.40–0.69 | 51 | 1 | 0.48 | 0.06–3.88 | 0.40 | 0.05–3.49 |
| ≥0.70 | 73 | 0 | NA | NA | NA | NA |
| Age = 40–64 † | ||||||
| MPR of HCQ | ||||||
| <0.40 | 384 | 39 | 1 | 1 | ||
| 0.40–0.69 | 131 | 8 | 0.65 | 0.31–1.40 | 0.58 | 0.27–1.25 |
| ≥0.70 | 162 | 6 | 0.34 | 0.14–0.79 | 0.28 | 0.12–0.68 |
| Age ≥ 65 † | ||||||
| MPR of HCQ | ||||||
| <0.40 | 91 | 14 | 1 | 1 | ||
| 0.40–0.69 | 33 | 7 | 1.67 | 0.67–4.15 | 1.84 | 0.71–4.73 |
| ≥0.70 | 39 | 6 | 1.37 | 0.52–3.59 | 1.46 | 0.52–4.13 |
| Female ‡ | ||||||
| MPR of HCQ | ||||||
| <0.40 | 562 | 49 | 1 | 1 | ||
| 0.40–0.69 | 188 | 15 | 1.03 | 0.58–1.84 | 0.99 | 0.55–1.80 |
| ≥0.70 | 247 | 8 | 0.37 | 0.17–0.77 | 0.36 | 0.16–0.77 |
| Male ‡ | ||||||
| MPR of HCQ | ||||||
| <0.40 | 91 | 11 | 1 | 1 | ||
| 0.40–0.69 | 27 | 1 | 0.24 | 0.03–1.91 | 0.30 | 0.03–2.80 |
| ≥0.70 | 27 | 4 | 1.06 | 0.34–3.36 | 0.85 | 0.22–3.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-H.; Wang, Y.-H.; Pan, L.-F.; Wei, J.C.-C. Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 3469. https://doi.org/10.3390/jcm9113469
Yang D-H, Wang Y-H, Pan L-F, Wei JC-C. Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome. Journal of Clinical Medicine. 2020; 9(11):3469. https://doi.org/10.3390/jcm9113469
Chicago/Turabian StyleYang, Deng-Ho, Yu-Hsun Wang, Lung-Fa Pan, and James Cheng-Chung Wei. 2020. "Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome" Journal of Clinical Medicine 9, no. 11: 3469. https://doi.org/10.3390/jcm9113469
APA StyleYang, D.-H., Wang, Y.-H., Pan, L.-F., & Wei, J. C.-C. (2020). Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome. Journal of Clinical Medicine, 9(11), 3469. https://doi.org/10.3390/jcm9113469







