Outcomes of COVID-19 Hospitalized Patients Previously Treated with Renin-Angiotensin System Inhibitors
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Patient Selection
2.2. Baseline Variables
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics at Baseline
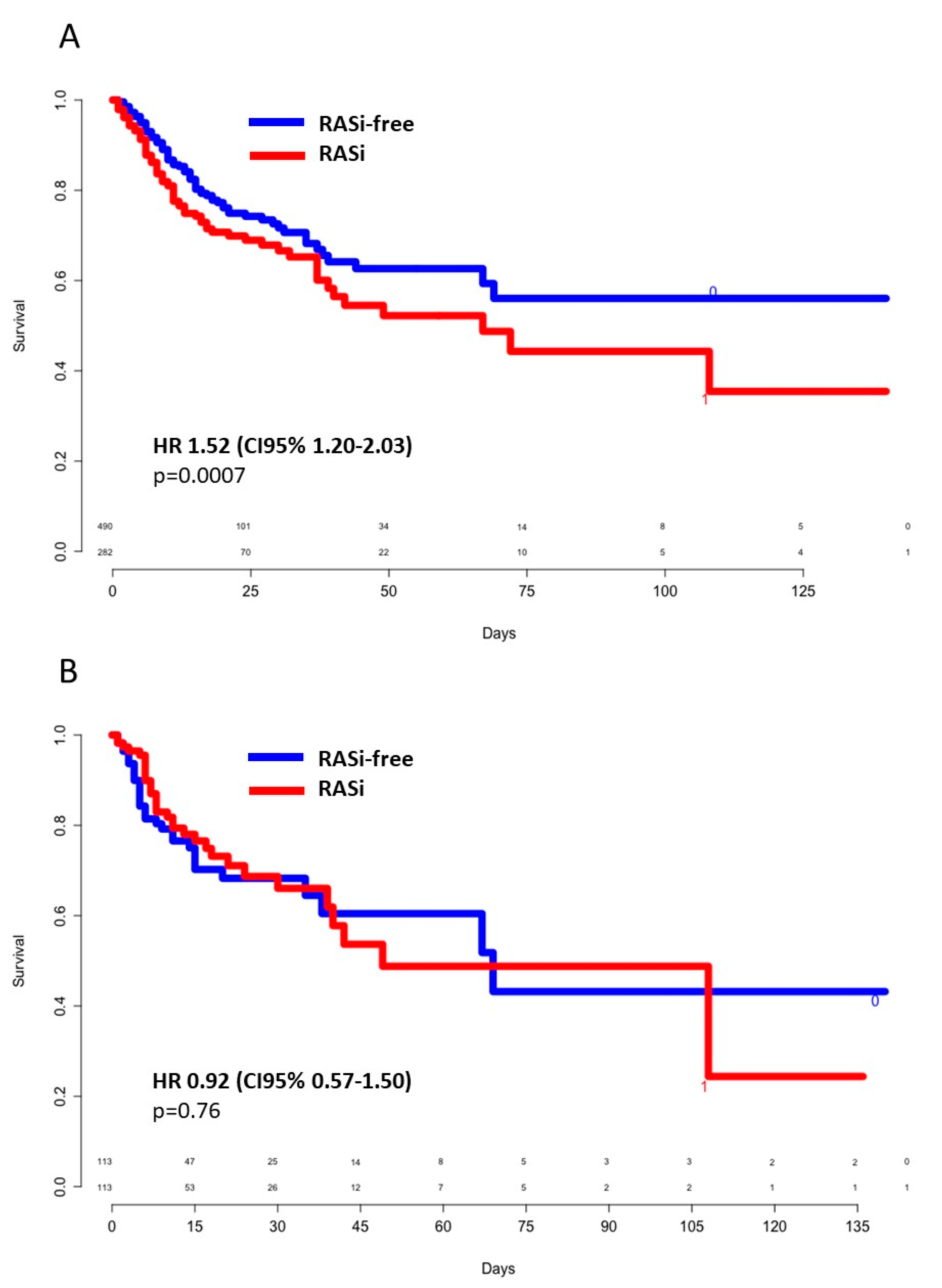
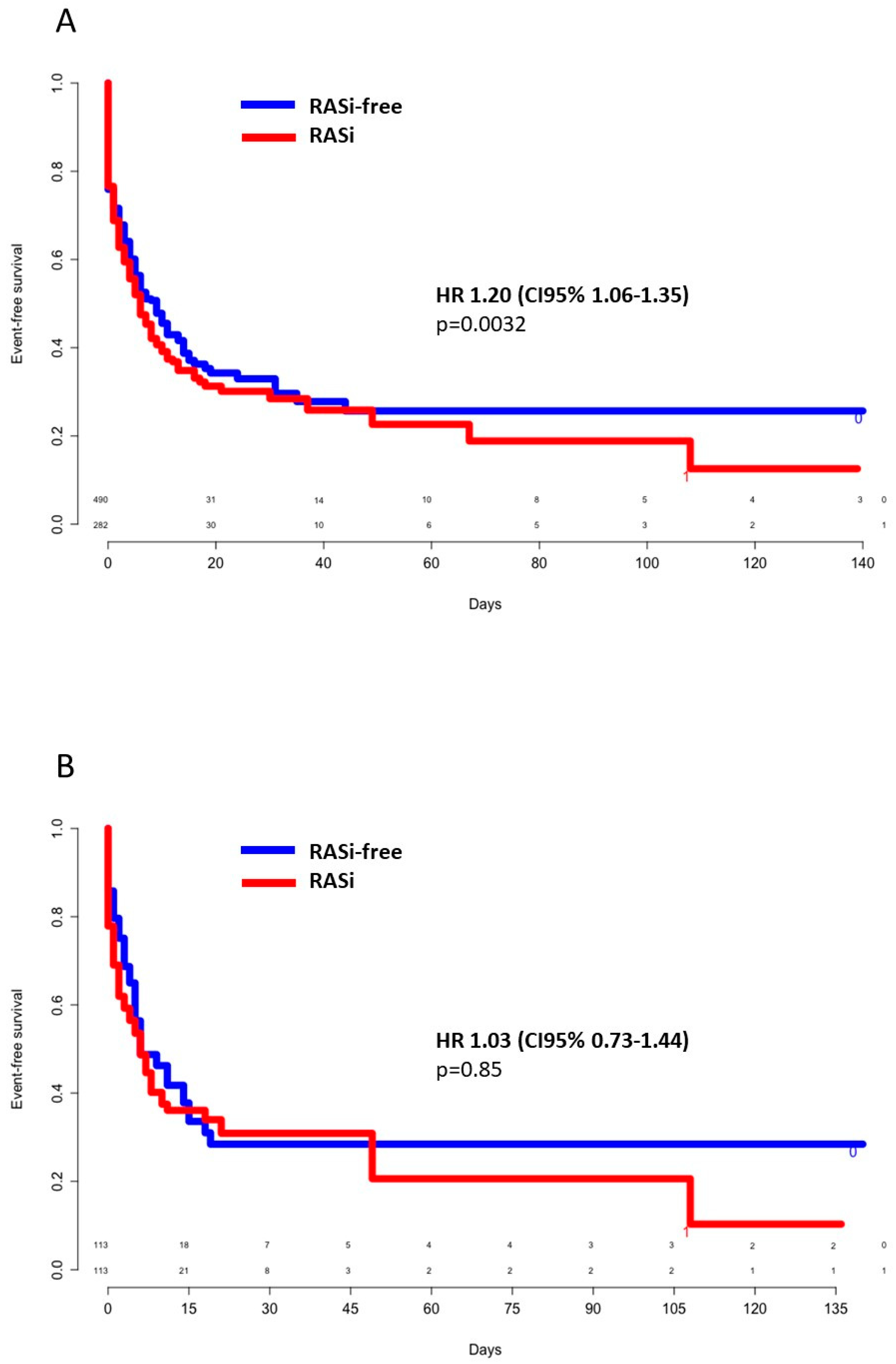
3.2. In-Hospital Outcomes
3.3. Survivors Versus Nonsurvivors
3.4. Mild Versus Severe Forms of COVID-19 Infection
3.5. ACEIs Versus ARBs and Poor Outcomes
4. Discussion
4.1. Main Results in Brief
4.2. Hypothetical Pathogenic Mechanisms of RASi Impact on COVID-19 Evolution
4.3. Poor Prognosis Risk Factors
4.4. ACEIs Versus ARBs
4.5. Evidence in the Available Literature
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Epidemiology of COVID-19 2020; European Centre for Disease Prevention and Control: Solna Municipality, Sweden, 2020. [Google Scholar]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ye, D.; E Mullick, A.; Li, Z.; Danser, A.J.; Daugherty, A.; Lu, H.S. Effects of Renin-Angiotensin Inhibition on ACE2 and TMPRSS2 Expression: Insights into COVID-19. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Rea, F.; Ludergnani, M.; Apolone, G.; Corrao, G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2431–2440. [Google Scholar] [CrossRef]
- Trifirò, G.; Massari, M.; Da Cas, R.; Ippolito, F.M.; Sultana, J.; Crisafulli, S.; Rossi, P.G.; Marino, M.; Zorzi, M.; ITA-COVID-19: RAAS Inhibitor Group; et al. Renin—Angiotensin—Aldosterone System Inhibitors and Risk of Death in Patients Hospitalised with COVID-19: A Retrospective Italian Cohort Study of 43,000 Patients. Drug Saf. 2020, 1–12. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef]
- Yang, G.; Tan, Z.; Zhou, L.; Yang, M.; Peng, L.; Liu, J.; Cai, J.; Yang, R.; Han, J.; Huang, Y.; et al. Effects of Angiotensin II Receptor Blockers and ACE (Angiotensin-Converting Enzyme) Inhibitors on Virus Infection, Inflammatory Status, and Clinical Outcomes in Patients with COVID-19 and Hypertension: A Single-Center Retrospective Study. Hypertension 2020, 76, 51–58. [Google Scholar] [CrossRef]
- Mackey, K.; King, V.J.; Gurley, S.; Kiefer, M.; Liederbauer, E.; Vela, K.; Sonnen, P.; Kansagara, D. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann. Intern. Med. 2020, 173, 195–203. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, Y.; Hong, Y. Decreased Mortality of COVID-19 with Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients with Hypertension. Hypertension 2020, 76, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Pan, L.-Y.; Jiang, H.-Y. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol. Res. 2020, 158, 104927. [Google Scholar] [CrossRef] [PubMed]
- Abstract title: Continuing Versus Suspending ACE Inhibitors and ARBs: Impact of Adverse Outcomes in Hospitalized Patients with COVID-19—The BRACE CORONA Trial. n.d. Available online: https://www.escardio.org/The-ESC/Press-Office/Press-releases/LOPES (accessed on 5 September 2020).
- Mehta, N.; Kalra, A.; Nowacki, A.S.; Anjewierden, S.; Han, Z.; Bhat, P.; Carmona-Rubio, A.E.; Jacob, M.; Procop, G.W.; Harrington, S.; et al. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1020. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sanga, V.; Barton, M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. eLife 2020, 9, e57278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cai, G. Bulk and Single-Cell Transcriptomics Identify Tobacco-Use Disparity in Lung Gene Expression of ACE2, the Receptor of 2019-nCov. Infectious Diseases (Except HIV/AIDS). 2020. Available online: https://doi.org/10.1101/2020.02.05.20020107 (accessed on 10 September 2020).
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Majdic, G. Could Sex/Gender Differences in ACE2 Expression in the Lungs Contribute to the Large Gender Disparity in the Morbidity and Mortality of Patients Infected With the SARS-CoV-2 Virus? Front. Cell. Infect. Microbiol. 2020, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Conover, M.M.; You, S.C.; Pratt, N.L.; Kostka, K.; Duarte-Salles, T.; Fernandez-Bertolin, S.; Aragon, M.; Duvall, S.L.; E Lynch, K.; et al. Renin-angiotensin system blockers and susceptibility to COVID-19: A multinational open science cohort study. Cardiovasc. Med. 2020. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.F.; Heide-Jørgensen, U.; Rasmussen, T.B.; Bodilsen, J.; Søgaard, O.S.; Maeng, M.; Vistisen, S.T.; Schmidt, M.; Pottegård, A.; Lund, L.C.; et al. Renin–Angiotensin System Blockers and Adverse Outcomes of Influenza and Pneumonia: A Danish Cohort Study. J. Am. Heart Assoc. 2020, 9, e017297. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Cingolani, O.; Lakatta, E.G. The Dilemma of Coronavirus Disease 2019, Aging, and Cardiovascular Disease: Insights from Cardiovascular Aging Science. JAMA Cardiol. 2020, 5, 747. [Google Scholar] [CrossRef]
- Kim, I.-C.; Kim, H.A.; Park, J.S.; Nam, C.-W. Updates of Cardiovascular Manifestations in COVID-19: Korean Experience to Broaden Worldwide Perspectives. Korean Circ. J. 2020, 50, 543–554. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Gormez, S.; Ekicibasi, E.; Degirmencioglu, A.; Paudel, A.; Erdim, R.; Gumusel, H.K.; Eroglu, E.; Tanboga, I.H.; Dagdelen, S.; Sariguzel, N.; et al. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil–lymphocyte ratio, D-Dimer and clinical severity of COVID-19 in hospitalized patients: A multicenter, observational study. J. Hum. Hypertens. 2020, 1–10. [Google Scholar] [CrossRef]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.; Leung, W.K. Association between angiotensin blockade and COVID-19 severity in Hong Kong. Can. Med. Assoc. J. 2020, 192, E635. [Google Scholar] [CrossRef]
- Johnson, K.; Khayyat-Kholghi, M.; Johnson, B.; Tereshchenko, L. The Association between the Rate of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Use and the Number of COVID-19 Confirmed Cases and Deaths in the United States: Geospatial Study. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Kreutz, R.; AlGharably, E.A.E.-H.; Azizi, M.; Dobrowolski, P.; Guzik, T.; Januszewicz, A.; Persu, A.; Prejbisz, A.; Riemer, T.G.; Wang, J.-G.; et al. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: Implications for COVID-19. Cardiovasc. Res. 2020, 116, 1688–1699. [Google Scholar] [CrossRef]
- ESC Council on Hypertension. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers 2020; ESC Council on Hypertension, 2020; Available online: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang ((accessed on 24 June 2020).
- HFSA/ACC/AHA. HFSA/ACC/AHA Statement: Using RAAS Antagonists in COVID-19; HFSA/ACC/AHA: 2020. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 (accessed on 24 June 2020).

| Overall Cohort | PS Cohort | |||||
|---|---|---|---|---|---|---|
| RASi N(%)/M(IQR) | RASi-Free N(%)/M(IQR) | p-Value | RASi N(%)/M(IQR) | RASi-Free N(%)/M(IQR) | p-Value | |
| N | 282 | 490 | 113 | 113 | ||
| Age (years) | 75 (66–83) | 64 (50–75) | <0.001 | 73 (64–84) | 73 (61–85) | 0.61 |
| Age ≥ 65 years old | 221 (78.4) | 243 (49.6) | <0.001 | 84 (74.3) | 80 (70.8) | 0.65 |
| Male | 170 (60.3) | 274 (55.9) | 0.27 | 68 (60.2) | 61 (54) | 0.42 |
| BMI (kg/m2) (N = 662) | 28 (25–33) | 27 (24–31) | 0.004 | 27 (24–31) | 26 (24–31) | 0.69 |
| eGFR (mL/min/1.73 m2) on admission (N = 756) | 68 (44–84) | 88 (68.5–102.5) | <0.001 | 70 (44–84) | 77 (43–93) | 0.26 |
| eGFR ≥ 90 | 45 (16) | 231 (48.6) | 22 (19.6) | 35 (30.9) | 0.07 | |
| 60 ≤ eGFR < 90 | 122 (43.4) | 143 (30.1) | 44 (39.3) | 32 (28.3) | 0.11 | |
| 30 ≤ eGFR < 60 | 76 (27) | 66 (13.9) | 32 (28.6) | 27 (23.9) | 0.51 | |
| eGFR < 30 | 38 (13.5) | 35 (7.4) | 14 (12.5) | 19 (16.8) | 0.47 | |
| Cardiovascular risk factors | ||||||
| Hypertension (N = 769) | 273 (96.8) | 158 (32.4) | <0.001 | 104 (92) | 104 (92) | 1 |
| Diabetes (N = 769) | 134 (47.5) | 85 (17.5) | <0.001 | 40 (35.4) | 34 (30.1) | 0.48 |
| Dyslipidemia (N = 769) | 166 (58.9) | 107 (21.9) | <0.001 | 60 (53.1) | 56 (49.6) | 0.69 |
| Smoking (history or current) (N = 671) | 70 (27.9) | 85 (20.2) | 0.029 | 35 (31) | 34 (30.1) | 1 |
| Obesity (N = 690) | 107 (42.1) | 134 (30.7) | 0.003 | 35 (31) | 35 (31) | 1 |
| Medical history | ||||||
| Heart disease (N = 768) | 83 (29.4) | 41 (8.4) | <0.001 | 22 (19.5) | 23 (20.3) | 1 |
| Ischemic heart disease | 65 (23) | 26 (5.3) | <0.001 | 14 (12.4) | 12 (10.6) | 0.83 |
| Chronic heart failure | 24 (8.5) | 19 (3.9) | 0.011 | 7 (6.2) | 10 (8.8) | 0.61 |
| HFrEF | 16 (5.7) | 8 (1.6) | 0.004 | 4 (3.5) | 4 (3.5) | 1 |
| Chronic kidney disease (N = 769) | 61 (21.6) | 51 (10.5) | <0.001 | 26 (23) | 31 (27.4) | 0.54 |
| Chronic respiratory disease (N = 769) | 35 (12.4) | 56 (11.5) | 0.80 | 12 (10.6) | 16 (14.2) | 0.53 |
| COPD | 22 (7.8) | 23 (4.7) | 0.11 | 8 (7.1) | 10 (8.9) | 0.79 |
| Active cancer | 19 (6.7) | 34 (6.9) | 1 | 5 (4.4) | 11 (9.7) | 0.20 |
| Cognitive impairment (N = 768) | 48 (17.1) | 47 (9.7) | 0.003 | 18 (15.9) | 20 (17.7) | 0.85 |
| VTE (N = 769) | 27 (9.6) | 31 (6.4) | 0.138 | 13 (11.5) | 14 (12.4) | 1 |
| Admission treatment | ||||||
| Antithrombotic treatment on admission | 159 (56.8) | 108 (22.2) | <0.001 | 55 (48.7) | 53 (46.9) | 0.89 |
| Antiplatelet (N = 766) | 107 (38.4) | 61 (12.5) | <0.001 | 40 (35.4) | 28 (24.8) | 0.11 |
| Anticoagulation (N = 765) | 61 (21.9) | 57 (11.7) | <0.001 | 16 (14.2) | 28 (24.8) | 0.07 |
| Antihypertensive drugs (N = 767) | ||||||
| Diuretics | 116 (41.4) | 60 (12.3) | <0.001 | 42 (37.2) | 41 (36.3) | 1 |
| Beta-blockers | 121 (43.4) | 94 (19.3) | <0.001 | 46 (40.7) | 52 (46) | 0.50 |
| COVID-19 diagnosis | ||||||
| Positive PCR | 282 (100) | 490 (100) | - | 113 (100) | 113 (100) | |
| Low-dose chest CT | 230 (81.6) | 388 (79.2) | 0.42 * | 91 (97.8) | 81 (94.2) | 0.38 * |
| normal | 3 (1.3) | 14 (3.6) | 2 (2.2) | 5 (5.8) | ||
| uncertain abnormalities | 5 (2.2) | 7 (1.8) | 2 (2.2) | 3 (3.5) | ||
| minimal abnormalities (<10%) | 33 (14.3) | 51 (13.1) | 14 (15) | 9 (10.5) | ||
| moderate abnormalities (10–25%) | 78 (33.9) | 128 (33) | 27 (29) | 33 (38.4) | ||
| important abnormalities (25–50%) | 54 (23.5) | 108 (27.8) | 29 (31.2) | 19 (22.1) | ||
| severe abnormalities (50–75%) | 43 (18.7) | 65 (16.8) | 16 (17.2) | 14 (16.3) | ||
| critical abnormalities (>75%) | 14 (6.1) | 15 (3.9) | 3 (3.2) | 3 (3.5) | ||
| COVID-19 infection severity indicators | ||||||
| Oxygen therapy flow rate of >5 L/min | 172 (61) | 247 (50.4) | 0.015 | 67 (59.8) | 55 (51.4) | 0.26 |
| Intubation/HNFO therapy/NIV | 82 (29.1) | 151 (30.8) | 0.67 | 38 (33.6) | 30 (26.5) | 0.31 |
| Intubation | 80 (28.4) | 140 (28.6) | 1 | 38 (33.6) | 26 (23) | 0.10 |
| HFNO therapy/NIV | 11(3.9) | 2 (0.4) | 0.14 | 0 | 4 (3.5) | 0.12 |
| CT scan extension > 25% (N = 618) | 111 (48.3) | 188 (48.5) | 1 | 0 | 4 (3.5) | 0.12 |
| CRP ≥ 100 mg/L (N = 746) | 182 (65.7) | 293 (62.5) | 0.42 | 71 (64.5) | 68 (60.2) | 0.59 |
| D-dimer count ≥ 1500 µg/L (N = 350) | 99 (76.1) | 151 (68.6) | 0.16 | 48 (82.8) | 35 (70) | 0.18 |
| Lymphopenia < 1000/µL (N = 753) | 208 (174) | 347 (73.5) | 0.95 | 85 (75.9) | 86 (77.5) | 0.90 |
| hs-cTnl ≥ 100 ng/L (N = 376) | 37 (24.8) | 40 (17.6) | 0.12 | 14 (20.9) | 12 (21.4) | 1 |
| Total N(%)/M(IQR) | RASi N(%)/M(IQR) | RASi-Free N(%)/M(IQR) | p-Value | |
|---|---|---|---|---|
| N | 772 | 282 | 490 | |
| Death | 173 (21.5) | 82 (29.1) | 91 (18.6) | <0.001 |
| Death/intubation | 322 (41.7) | 133 (47.2) | 189 (38.6) | 0.019 |
| Death/intubation/HFNO therapy/NIV | 332 (43) | 133 (47.2) | 199 (40.6) | 0.077 |
| Death/intubation/HFNO therapy/NIV/oxygen flow rate of ≥5 L | 438 (56.7) | 179 (63.5) | 259 (52.9) | 0.004 |
| Death/intubation/HFNO therapy/NIV/oxygen flow rate of ≥10 L | 358 (46.4) | 144 (51) | 214 (43.7) | 0.047 |
| Acute renal impairment (N = 767) | 212 (27.6) | 105 (37.5) | 107 (22) | <0.001 |
| Severe sepsis or septic shock (N = 698) | 132 (18.9) | 55 (22.2) | 77 (17.1) | 0.103 |
| Pulmonary bacterial infection (N = 747) | 60 (8) | 25 (9.2) | 35 (7.4) | 0.36 |
| Multiple organ deficiency (N = 767) | 36 (4.5) | 16 (5.7) | 20 (4.1) | 0.31 |
| VTE | 61 (7.9) | 21 (7.4) | 40 (8.2) | 0.72 |
| AF (N = 748) | 37 (4.9) | 14 (5.2) | 23 (4.8) | 0.83 |
| Stroke | 20 (2.6) | 10 (3.5) | 10 (0.2) | 0.21 |
| Major bleeding | 37 (4.8) | 14 (5) | 23 (4.7) | 0.87 |
| Encephalitis | 15 (1.9) | 6 (2.1) | 9 (1.8) | 0.86 |
| Liver injury (N = 748) | 18 (1.6) | 5 (1.8) | 13 (2.7) | 0.60 |
| Hospital length of stay | 11 (6–21) | 12 (7–24) | 10 (6–20) | 0.45 |
| Hospital length of stay of ≥30 days | 128 (16.6) | 51 (18.1) | 77 (15.7) | 0.21 |
| Total N (%)/m ± sd/ M(Q1–Q3) | Nonsurvivors N (%)/m ± sd/ M(Q1–Q3) | Survivors N (%)/m ± sd/ M(Q1–Q3) | p-Value | |
|---|---|---|---|---|
| N | 772 | 173 | 599 | |
| Age (years) | 68 (56–79) | 79 (71–85) | 65 (53–76) | <0.001 |
| Age ≥ 65 years old | 464 (60.1) | 156 (90.2) | 308 (51.4) | <0.001 |
| Male | 444 (57.5) | 108 (62.4) | 336 (56.1) | 0.16 |
| BMI (kg/m2) N = 662 | 28 (24–31) | 27 (24–31) | 28 (24–31) | 0.10 |
| Cardiovascular risk factors | ||||
| Hypertension N = 769 | 431 (56) | 129 (74.6) | 302 (50.4) | <0.001 |
| Diabetes N = 769 | 219 (28.5) | 62 (35.8) | 157 (26.2) | 0.012 |
| Dyslipidemia N = 769 | 273 (35.5) | 80 (46.5) | 193 (32.3) | <0.001 |
| Smoking (history or current) N = 671 | 155 (23.1) | 49 (31.4) | 106 (20.4) | 0.0042 |
| Obesity N = 690 | 241 (34.9) | 50 (31.6) | 191 (35.9) | 0.32 |
| Medical history | ||||
| Heart disease N = 768 | 124 (16.1) | 37 (21.5) | 87 (14.6) | 0.029 |
| Ischemic heart disease | 91 (11.8) | 58 (19.1) | 33 (9.7) | <0.001 |
| Chronic heart failure N = 769 | 43 (5.6) | 21 (12.2) | 22 (3.7) | <0.001 |
| HFrEF | 24 (3.1) | 12 (6.9) | 12 (2) | <0.001 |
| Chronic kidney disease N = 769 | 112 (14.6) | 55 (31.9) | 57 (9.5) | <0.001 |
| Chronic respiratory disease N = 769 | 91 (11.8) | 26 (15.1) | 65 (10.8) | 0.13 |
| COPD | 45 (5.9) | 14 (8.1) | 31 (5.2) | 0.14 |
| Active cancer | 53 (6.9) | 26 (15) | 27 (4.5) | <0.001 |
| Cognitive impairment N = 768 | 95 (12.4) | 46 (36.4) | 49 (8.2) | <0.001 |
| VTE N = 769 | 58 (7.5) | 19 (11) | 39 (6.5) | 0.048 |
| Admission treatment | ||||
| Antithrombotic treatment | 267 (34.8) | 99 (57.9) | 168 (28.2) | <0.001 |
| Antiplatelet N = 767 | 168 (21.9) | 57 (33.3) | 111 (18.6) | <0.001 |
| Anticoagulation N = 766 | 118 (15.4) | 52 (30.5) | 66 (11.1) | <0.001 |
| Antihypertensive drugs | ||||
| ACE or ARBs | 282 (36.5) | 82 (47.4) | 200 (33.4) | 0.001 |
| Diuretics | 176 (22.9) | 69 (40.6) | 107 (17.9) | <0.001 |
| Beta-blockers | 215 (28.1) | 70 (41.2) | 145 (24.3) | <0.001 |
| COVID-19 diagnosis | ||||
| Positive PCR | 772 (100) | 173 (100) | 599 (100) | |
| Low-dose CT | 618 (80) | 118 (68.2) | 500 (83.5) | <0.001 * |
| normal | 17 (2.8) | 2 (1.1) | 15 (2.5) | 0.67 § |
| uncertain abnormalities | 12 (1.9) | 6 (3.5) | 6 (1) | |
| minimal (<10%) | 84 (13.6) | 9 (5.2) | 75 (12.5) | |
| moderate (10–25%) | 206 (33.3) | 22 (12.7) | 184 (30.7) | |
| important (25–50%) | 162 (26.2) | 36 (20.8) | 126 (21) | |
| severe (50–75%) | 108 (17.5) | 33 (19.1) | 75 (12.5) | |
| critical (>75%) | 29 (4.7) | 10 (5.8) | 19 (3.2) | |
| COVID-19 infection severity indicators | ||||
| Oxygen flow rate of ≥5 L/min | 378 (48.9) | 135 (78) | 243 (40.6) | <0.001 |
| HFNO therapy/NIV/OTI | 233 (30.2) | 74 (42.8) | 159 (26.5) | <0.001 |
| OTI | 220 (28.5) | 71 (41) | 149 (24.9) | <0.001 |
| HFNO therapy/NIV | 13 (1.7) | 3 (1.7) | 10 (1.7) | 1 |
| CRP ≥ 100 mg/L (N = 746) | 476 (63.7) | 133 (80.6) | 343 (59) | <0.001 |
| Ddimer count ≥ 1500 µg/L (N = 350) | 250 (71.4) | 72 (91.1) | 178 (65.7) | <0.001 |
| Lymphopenia < 1000/µL (N = 753) | 555 (73.7) | 147 (86.5) | 408 (70) | <0.001 |
| hs-cTnl ≥ 100 ng/L (N = 376) | 77 (20.5) | 37 (40.6) | 40 (14) | <0.001 |
| In-hospital treatment | ||||
| Anticoagulant N = 769 | 625 (81.3) | 141 (82.4) | 484 (80.9) | 0.73 |
| preventive | 479 (62.2) | 84 (49.1) | 395 (66) | |
| therapeutic | 145 (19) | 56 (32.7) | 89 (14.9) | |
| ACEIs/ARBs | 125 (16.2) | 28 (16.2) | 97 (16.2) | 1 |
| Antiviral | 286 (37) | 55 (31.8) | 231 (38.6) | 0.65 |
| lopinavir/ritonavir | 153 (19.8) | 29 (16.7) | 124 (20.7) | |
| remdesivir | 7 (0.9) | 0 | 7 (1.2) | |
| oseltamivir | 2 (0.5) | 1 (0.6) | 1 (0.2) | |
| hydoxychroloquine | 140(18.1) | 30 (17.3) | 110 (18.4) | |
| IFN | 4 (0.5) | 0 | 4 (0.7) | |
| Antibiotics | 634 (82.1) | 141 (81.5) | 493(82.3) | 0.32 |
| amoxicillin/clavulanic acid | 239 (31) | 31 (17.9) | 208 (34.7) | |
| 3GC | 414 (53.6) | 109 (63) | 305 (50.9) | |
| macrolide | 321 (41.6) | 74 (42.8) | 247 (41.2) | |
| quinolone | 13 (1.7) | 5 (2.9) | 8 (1.3) | |
| other | 3 (0.4) | 2 (1.2) | 1 (0.2) |
| Outcome | ACEIs N (%) n = 129 | ARBs N (%) n = 152 | Unadjusted OR ACEIs vs. ARBs (95%CI) | p-Value |
|---|---|---|---|---|
| Death | 44 (34.1) | 37 (24.3) | 1.60 (0.95–2.71) | 0.072 |
| Severe pneumonia | 86 (66.6) | 92 (60.5) | 1.30 (0.80–2.13) | 0.288 |
| Acute renal insufficiency | 42 (32.6) | 64 (42.1) | 0.66 (0.40–1.08) | 0.100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeanu, E.-M.; Jambert, L.; Severac, F.; Lambach, H.; Tousch, J.; Heitz, M.; Mirea, C.; Hamadé, A.; Younes, W.; Frantz, A.-S.; et al. Outcomes of COVID-19 Hospitalized Patients Previously Treated with Renin-Angiotensin System Inhibitors. J. Clin. Med. 2020, 9, 3472. https://doi.org/10.3390/jcm9113472
Cordeanu E-M, Jambert L, Severac F, Lambach H, Tousch J, Heitz M, Mirea C, Hamadé A, Younes W, Frantz A-S, et al. Outcomes of COVID-19 Hospitalized Patients Previously Treated with Renin-Angiotensin System Inhibitors. Journal of Clinical Medicine. 2020; 9(11):3472. https://doi.org/10.3390/jcm9113472
Chicago/Turabian StyleCordeanu, Elena-Mihaela, Lucas Jambert, Francois Severac, Hélène Lambach, Jonathan Tousch, Marie Heitz, Corina Mirea, Amer Hamadé, Waël Younes, Anne-Sophie Frantz, and et al. 2020. "Outcomes of COVID-19 Hospitalized Patients Previously Treated with Renin-Angiotensin System Inhibitors" Journal of Clinical Medicine 9, no. 11: 3472. https://doi.org/10.3390/jcm9113472
APA StyleCordeanu, E.-M., Jambert, L., Severac, F., Lambach, H., Tousch, J., Heitz, M., Mirea, C., Hamadé, A., Younes, W., Frantz, A.-S., Merdji, H., Schini-Kerth, V., Bilbault, P., Meziani, F., Ohlmann, P., Andres, E., & Stephan, D. (2020). Outcomes of COVID-19 Hospitalized Patients Previously Treated with Renin-Angiotensin System Inhibitors. Journal of Clinical Medicine, 9(11), 3472. https://doi.org/10.3390/jcm9113472







