The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover
Abstract
1. Introduction
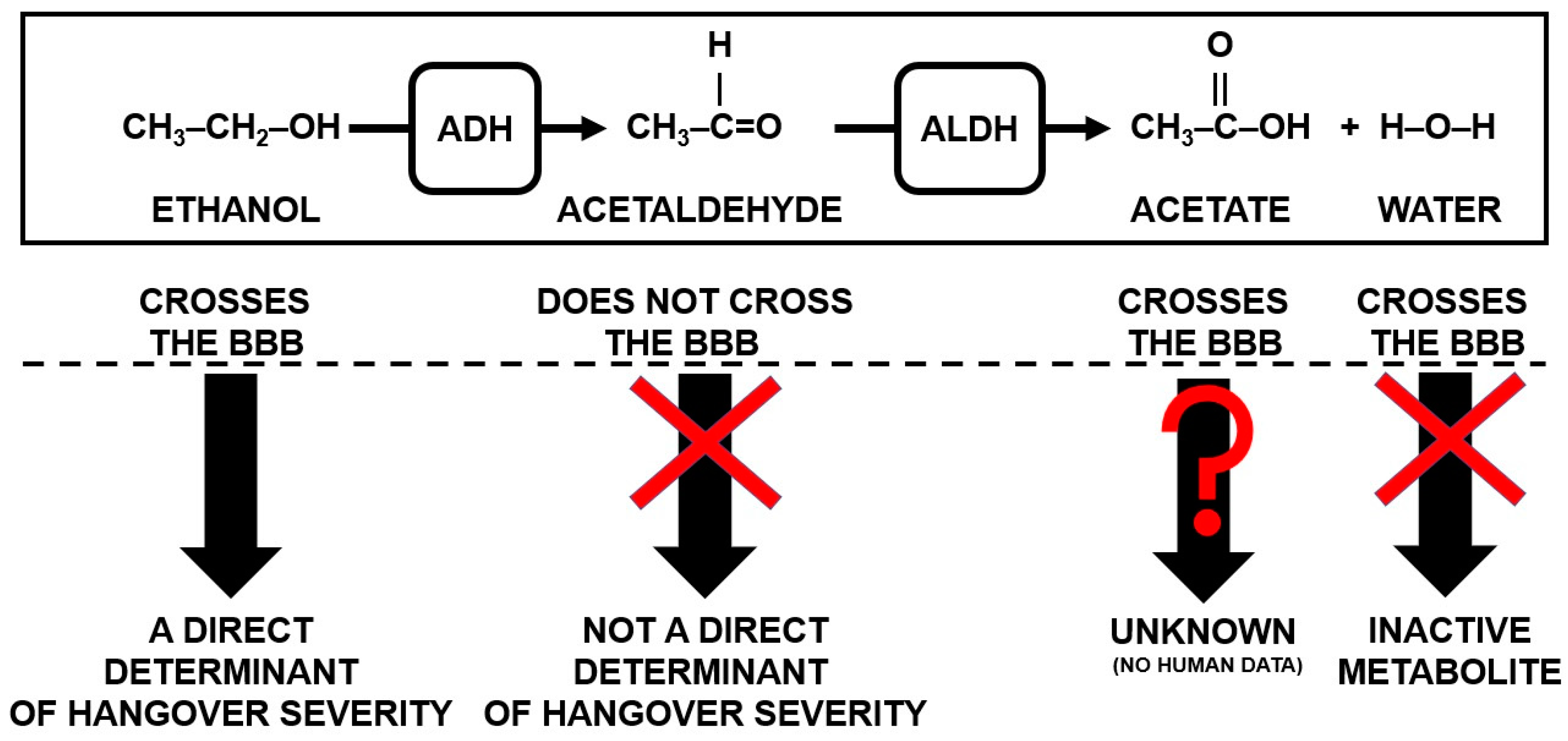
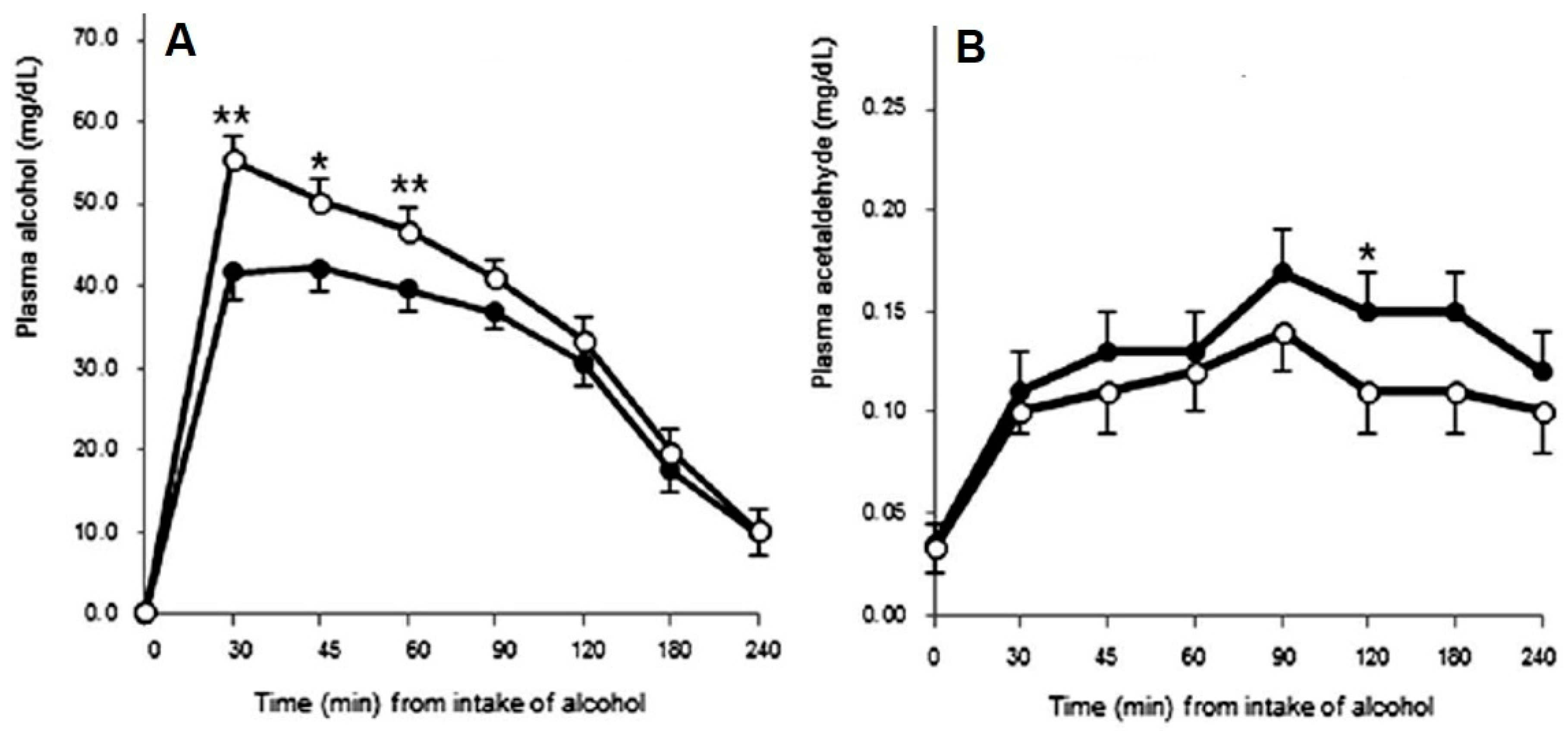
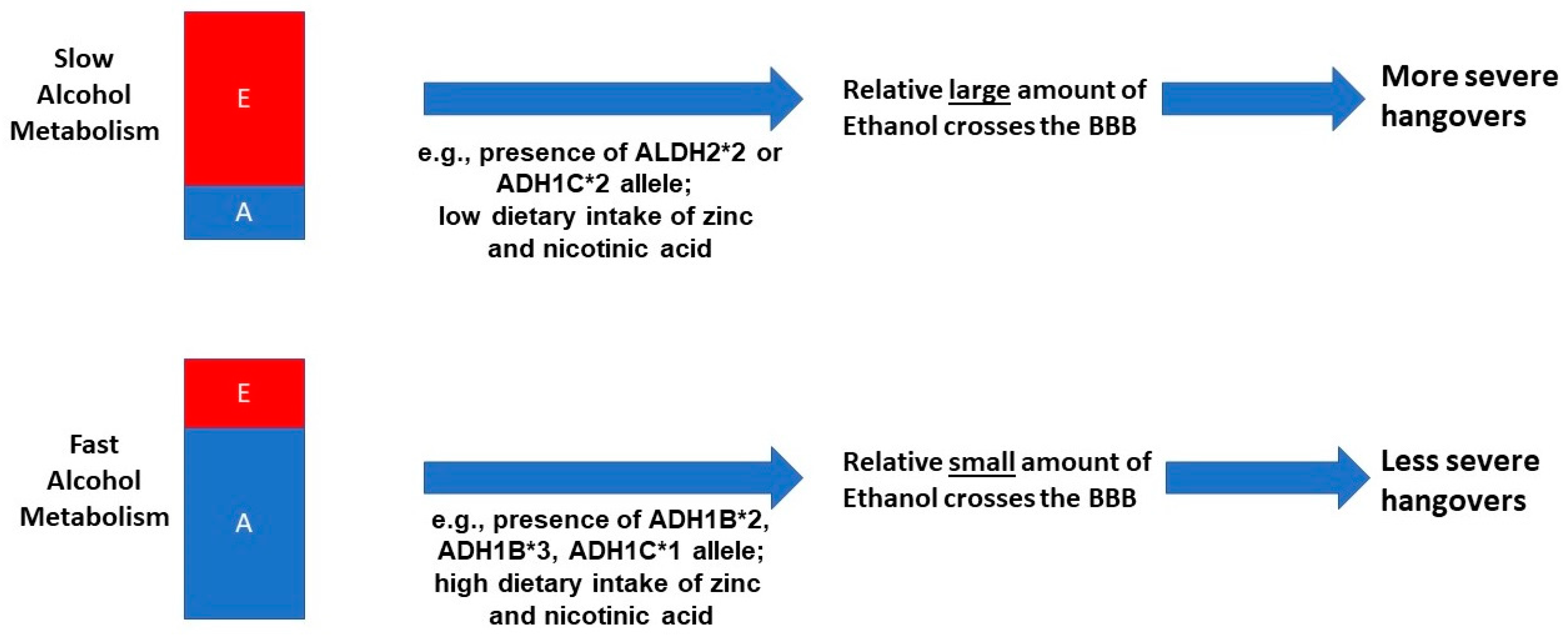
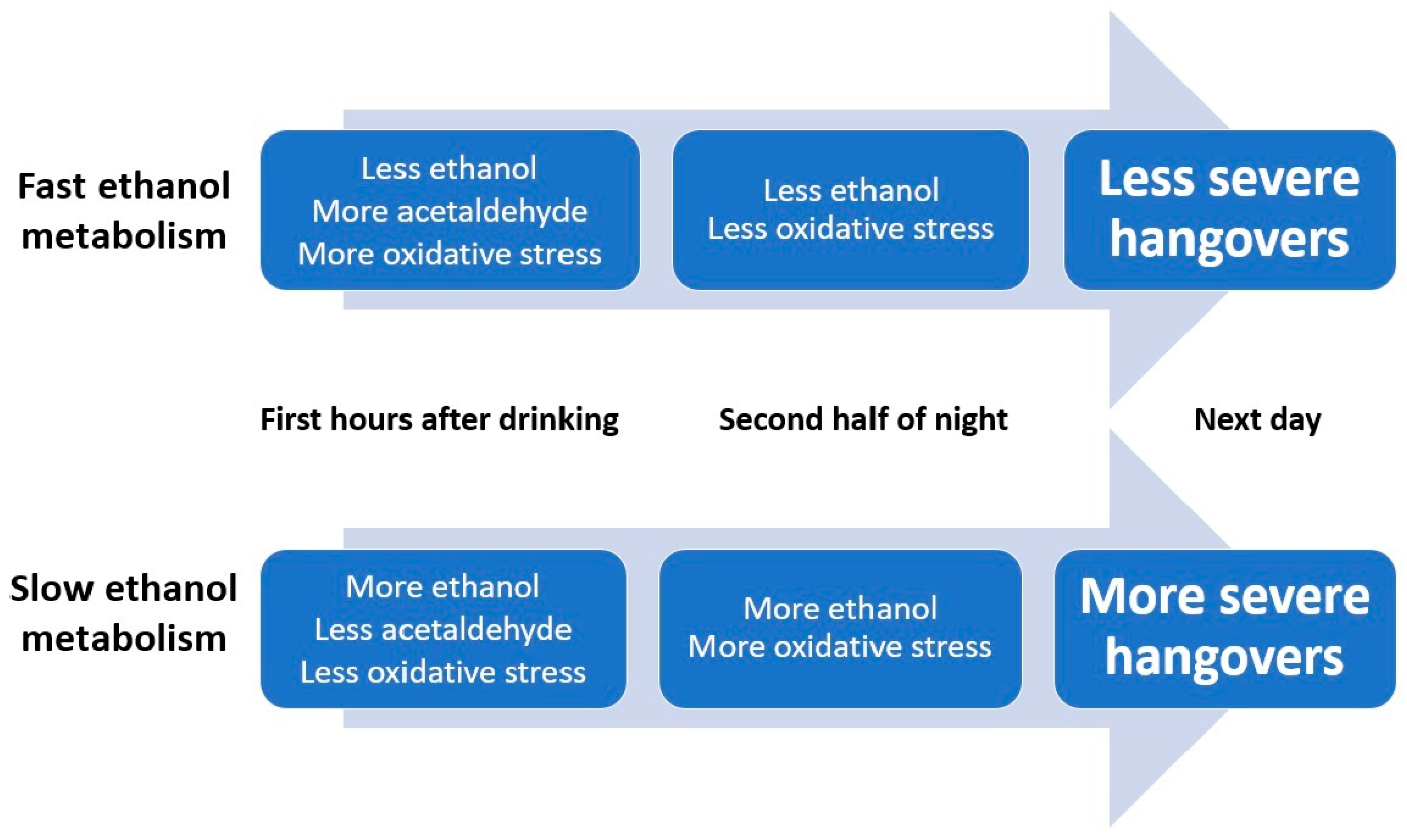
1.1. Alcohol Metabolism and Hangover Severity
1.2. Accelerating Ethanol or Acetaldehyde Breakdown in Reducing Hangover Severity
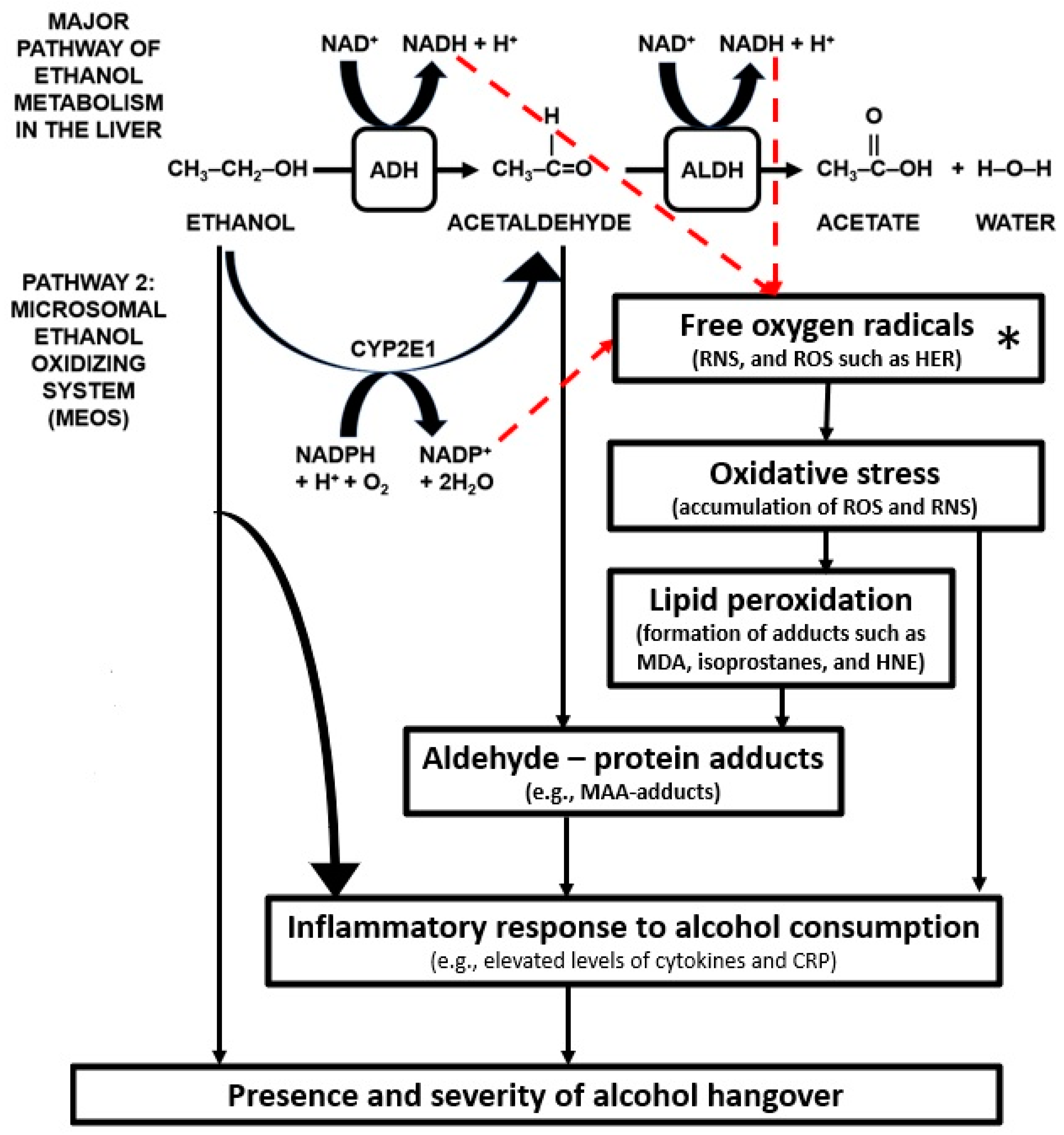
1.3. Oxidative Stress
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Schrojenstein Lantman, M.; van de Loo, A.J.; Mackus, M.; Verster, J.C. Development of a definition for the alcohol hangover: Consumer descriptions and expert consensus. Curr. Drug Abuse Rev. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Verster, J.C.; Scholey, A.; van de Loo, A.J.A.E.; Benson, S.; Stock, A.K. Updating the definition of the alcohol hangover. J. Clin. Med. 2020, 9, 823. [Google Scholar] [CrossRef]
- Prat, G.; Adan, A.; Pérez-Pàmies, M.; Sànchez-Turet, M. Neurocognitive effects of alcohol hangover. Addict. Behav. 2008, 33, 15–23. [Google Scholar] [CrossRef]
- Prat, G.; Adan, A.; Sánchez-Turet, M. Alcohol hangover: A critical review of explanatory factors. Hum. Psychopharmacol. 2009, 24, 259–267. [Google Scholar] [CrossRef]
- Gunn, C.; Mackus, M.; Griffin, C.; Munafò, M.R.; Adams, S. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction 2018, 113, 2182–2193. [Google Scholar] [CrossRef]
- Kruisselbrink, L.D. The neurocognitive effects of alcohol hangover: Patterns of impairment/nonimpairment within the neurocognitive domain of the Diagnostic and Statistical Manual of Mental Disorders. In Neuroscience of Alcohol: Mechanisms and Treatment, 5th ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 391–402. [Google Scholar]
- Verster, J.C.; Bervoets, A.C.; de Klerk, S.; Vreman, R.A.; Olivier, B.; Roth, T.; Brookhuis, K.A. Effects of alcohol hangover on simulated highway driving performance. Psychopharmacology 2014, 231, 2999–3008. [Google Scholar] [CrossRef]
- Alford, C.; Broom, C.; Carver, H.; Johnson, S.J.; Reece, R.; Lands, S.; Verster, J.C. The impact of alcohol hangover on simulated driving performance during a ‘commute to work’—Zero and residual alcohol effects compared. J. Clin. Med. 2020, 9, 1435. [Google Scholar] [CrossRef]
- Penning, R.; van Nuland, M.; Fliervoet, L.A.L.; Olivier, B.; Verster, J.C. The pathology of alcohol hangover. Curr. Drug Abuse Rev. 2010, 3, 68–75. [Google Scholar] [CrossRef]
- Tipple, C.T.; Benson, S.; Scholey, A. A Review of the Physiological Factors Associated with Alcohol Hangover. Curr. Drug Abuse Rev. 2016, 9, 93–98. [Google Scholar] [CrossRef]
- Palmer, E.; Tyacke, R.; Sastre, M.; Lingford-Hughes, A.; Nutt, D.; Ward, R.J. Alcohol Hangover: Underlying Biochemical, Inflammatory and Neurochemical Mechanisms. Alcohol Alcohol. 2019, 54, 196–203. [Google Scholar] [CrossRef]
- Bullock, C. The biochemistry of alcohol metabolism—A brief review. Biochemical Educ. 1990, 18, 62–66. [Google Scholar] [CrossRef]
- Kawai, S.; Murata, K. Structure and function of NAD kinase and NADP phosphatase: Key enzymes that regulate the intracellular balance of NAD(H) and NADP(H). Biosci. Biotechnol. Biochem. 2008, 72, 919–930. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Jones, A.W. Evidence-based survey of the elimination rates of ethanol from blood with applications in forensic casework. Forensic Sci. Int. 2010, 200, 1–20. [Google Scholar] [CrossRef]
- Heier, C.; Xie, H.; Zimmermann, R. Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB Life 2016, 68, 916–923. [Google Scholar] [CrossRef]
- Fein, G.; Meyerhoff, D.J. Ethanol in human brain by magnetic resonance spectroscopy: Correlation with blood and breath levels, relaxation, and magnetization transfer. Alcohol Clin. Exp. Res. 2000, 24, 1227–1235. [Google Scholar] [CrossRef]
- Hillbom, M.E.; Lindros, K.O.; Larsen, A. The calcium carbimide-ethanol interaction: Lack of relation between electroencephalographic response and cerebrospinal fluid acetaldehyde. Toxicol. Lett. 1981, 9, 113–119. [Google Scholar] [CrossRef]
- Pösö, A.R.; Hillbom, M.E.; Eriksson, L. Acetaldehyde penetrates the blood-liquor barrier of goats. Toxicol. Lett. 1981, 8, 57–62. [Google Scholar] [CrossRef]
- Heap, L.; Ward, R.J.; Abiaka, C.; Dexter, D.; Lawlor, M.; Pratt, O.; Thomson, A.; Shaw, K.; Peters, T.J. The influence of brain acetaldehyde on oxidative status, dopamine metabolism and visual discrimination task. Biochem Pharmacol. 1995, 50, 263–270. [Google Scholar] [CrossRef]
- Jones, A.W. Measuring and reporting the concentration of acetaldehyde in human breath. Alcohol Alcohol. 1995, 30, 271–285. [Google Scholar]
- Eriksson, C.J.P. Human acetaldehyde levels: Aspects of current interest. ICPEMP Working paper No. 15/3. Mutation Res. 1987, 186, 235–240. [Google Scholar] [CrossRef]
- Zimatkin, S.M. Histochemical study of aldehyde dehydrogenase in the rat CNS. J. Neurochem. 1991, 56, 1–11. [Google Scholar] [CrossRef]
- Deitrich, R.A.; Dunwiddie, T.V.; Harris, R.A.; Erwin, V.G. Mechanism of Action of Ethanol—Initial Central-Nervous-System Actions. Pharmacol. Rev. 1989, 41, 489–537. [Google Scholar]
- Isse, T.; Matsuno, K.; Oyama, T.; Kitagawa, K.; Kawamoto, T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin. Exp. Res. 2005, 29, 1959–1964. [Google Scholar] [CrossRef]
- Ujihara, I.; Hitomi, S.; One, K.; Kakinoki, Y.; Hashimoto, H.; Ueta, Y.; Inegana, K. The ethanol metabolite acetaldehyde induces water and salt intake via two distinct pathways in the central nervous system of rats. Neuropharmacology 2015, 99, 589–599. [Google Scholar] [CrossRef]
- Eriksson, C.J.P.; Fukunaga, T. Human blood acetaldehyde (update 1992). Alcohol Alcohol. 1993, S2, 9–25. [Google Scholar]
- Hunt, W.A. Role of acetaldehyde in the actions of ethanol on the brain—A review. Alcohol 1996, 13, 147–151. [Google Scholar] [CrossRef]
- Tabakoff, B.; Anderson, R.A.; Ritzmann, R.F. Brain acetaldehyde after ethanol administration. Biochem. Pharmacol. 1976, 25, 1305–1309. [Google Scholar] [CrossRef]
- Westcott, J.Y.; Weiner, H.; Shultz, J.; Myers, R.D. In vivo acetaldehyde in the brain of the rat treated with ethanol. Biochem. Pharmacol. 1980, 29, 411–417. [Google Scholar] [CrossRef]
- Deitrich, R. Ethanol as a prodrug: Brain metabolism of ethanol mediates its reinforcing effects--a commentary. Alcohol Clin. Exp. Res. 2011, 35, 581–583. [Google Scholar] [CrossRef]
- Hernández, J.A.; López-Sánchez, R.C.; Rendón-Ramírez, A. Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxidative Med. Cell. Longev. 2016, 2016, 1543809. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.J. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin. Exp. Res. 2001, 25, 15S–32S. [Google Scholar] [CrossRef]
- Eriksson, C.J.; Saarenmaa, T.P.; Bykov, I.L.; Heino, P.U. Acceleration of ethanol and acetaldehyde oxidation by D-glycerate in rats. Metabolism 2007, 56, 895–898. [Google Scholar] [CrossRef]
- Ylikahri, R.H.; Huttunen, M.O.; Eriksson, C.J.; Nikkilä, E.A. Metabolic studies on the pathogenesis of hangover. Eur. J. Clin. Investig. 1974, 4, 93–100. [Google Scholar] [CrossRef]
- Ylikahri, R.H.; Leino, T.; Huttunen, M.O.; Pösõ, A.R.; Eriksson, C.J.P.; Nikkilä, E.A. Effects of fructose and glucose on ethanol-induced metabolic changes and on the intensity of alcohol intoxication and hangover. Eur. J. Clin. Investig. 1976, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, A.J.A.E.; Mackus, M.; Korte-Bouws, G.A.H.; Brookhuis, K.A.; Garssen, J.; Verster, J.C. Urine ethanol concentration and alcohol hangover severity. Psychopharmacology 2017, 234, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Benson, S.; Kaufman, J.; Terpstra, C.; Ayre, E.; Verster, J.C.; Allen, C.; Devilly, G. Effects of alcohol hangover on cognitive performance: A field/internet mixed methodology approach. J. Clin. Med. 2019, 8, 440. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kanegae, T.; Saito, M.; Nagoya, T.; Shimamura, M.; Tainaka, H.; Kawagughi, M. Concentrations of blood and urine ethanol, acetaldehyde, acetate and acetone during experimental hangover in volunteers. Jpn. J. Alcohol Drug Depend. 1991, 26, 500–510. [Google Scholar]
- Maxwell, C.R.; Spangenberg, R.J.; Hoek, J.B.; Silberstein, S.D.; Oshinsky, M.L. Acetate causes alcohol hangover headache in rats. PLoS ONE 2010, 5, e15963. [Google Scholar] [CrossRef]
- Pelaez, A.M.L.; Catano, C.; Quintero Yepers, E.A.; Gamba Villaroei, R.R.; de Antoni, G.L.; Giannuzzi, L. Inhibitory activity of lactic and acetic acid on Aspergillus flavus growth for food preservation. Food Control 2012, 24, 177–183. [Google Scholar] [CrossRef]
- Van de Loo, A.J.E.A.; Mackus, M.; Kwon, O.; Krishnakumar, I.M.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The inflammatory response to alcohol consumption and its role in the development of alcohol hangover. J. Clin. Med. 2020, 9, 2081. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R. Studies on the antidotal effect of red ginseng. Korean J. Ginseng Sci. 1976, 1, 59–78. [Google Scholar]
- Koo, M.W. Effects of ginseng on ethanol induced sedation in mice. Life Sci. 1999, 64, 153–160. [Google Scholar] [CrossRef]
- Lee, M.H.; Kwak, J.H.; Jeon, G.; Lee, J.W.; Seo, J.H.; Lee, H.S.; Lee, J.H. Red ginseng relieves the effects of alcohol consumption and hangover symptoms in healthy men: A randomized crossover study. Food Funct. 2014, 5, 528–534. [Google Scholar] [CrossRef]
- Silva, J.; Yu, X.; Moradian, R.; Folk, C.; Spatz, M.H.; Kim, P.; Bhatti, A.A.; Davies, D.L.; Liang, J. Dihydromyricetin protects the liver via changes in lipid metabolism and enhanced ethanol metabolism. Alcohol Clin. Exp. Res. 2020, 44, 1046–1060. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.J.; Jeong, H.Y.; Kim, J.Y.; Choi, E.K.; Chae, S.W.; Kwon, O. A standardized extract of the fruit of Hovenia dulcis alleviated alcohol-induced hangover in healthy subjects with heterozygous ALDH2: A randomized, controlled, crossover trial. J. Ethnopharmacol. 2017, 209, 167–174. [Google Scholar] [CrossRef]
- Cho, M.H.; Shim, S.M.; Lee, S.R.; Mar, W.; Kim, G.H. Effect of Evodiae fructus extracts on gene expressions related with alcohol metabolism and antioxidation in ethanol-loaded mice. Food Chem. Toxicol. 2005, 43, 1365–1371. [Google Scholar] [CrossRef]
- Choi, E.J.; Kwon, H.C.; Sohn, Y.C.; Nam, C.W.; Park, H.B.; Kim, C.Y.; Yang, H.O. Four flavonoids from Echinosophora koreensis and their effects on alcohol metabolizing enzymes. Arch. Pharm. Res. 2009, 32, 851–855. [Google Scholar] [CrossRef]
- Verster, J.C.; Vermeulen, S.A.; van de Loo, A.J.A.E.; Balikji, S.; Kraneveld, A.D.; Garssen, J.; Scholey, A. Dietary nutrient intake, alcohol metabolism, and hangover severity. J. Clin. Med. 2019, 8, 1316. [Google Scholar] [CrossRef]
- Salaspuro, V. Pharmacological treatments and strategies for reducing oral and intestinal acetaldehyde. Novartis Found. Symp. 2007, 285, 145–153; discussion 153–157, 198–199. [Google Scholar] [PubMed]
- Moritani, K.; Takeshita, T.; Shibata, Y.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015, 21, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Arnoldy, L.; Ayre, E.; Benson, S.; Balikji, S.; Bruce, G.; Chen, F.; van Lawick van Pabst, A.E.; van de Loo, A.J.A.E.; O’Neill, S.; et al. Proceedings of the 11th Alcohol Hangover Research Group meeting in Nadi, Fiji. Proceedings 2020, 43, 3001. [Google Scholar] [CrossRef]
- Edenberg, H.J.; Xuei, X.; Chen, H.-J.; Tian, H.; Flury Wetherill, L.; Dick, D.M.; Almasy, L.; Bierut, L.; Bucholz, K.K.; Goate, A.; et al. Association of alcohol dehydrogenase genes with alcohol dependence: A comprehensive analysis. Hum. Mol. Genet. 2006, 15, 1539–1549. [Google Scholar] [CrossRef]
- Hurley, T.D.; Edenberg, H.J. Genes Encoding Enzymes Involved in Ethanol Metabolism. Alcohol Res. 2012, 34, 339–344. [Google Scholar]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Yokoyama, M.; Yokoyama, A.; Yokoyama, T.; Funazu, K.; Hamana, G.; Kondo, S.; Yamashita, T.; Nakamura, H. Hangover susceptibility in relation to aldehyde dehydrogenase-2 genotype, alcohol flushing, and mean corpuscular volume in Japanese workers. Alcohol Clin. Exp. Res. 2005, 29, 1165–1171. [Google Scholar] [CrossRef]
- Wall, T.L.; Horn, S.M.; Johnson, M.L.; Smith, T.L.; Carr, L.G. Hangover symptoms in Asian Americans with variations in the aldehyde dehydrogenase (ALDH2) gene. J. Stud. Alcohol 2000, 61, 13–17. [Google Scholar] [CrossRef]
- Slutske, W.S.; Piasecki, T.M.; Nathanson, L.; Statham, D.J.; Martin, N.G. Genetic influences on alcohol-related hangover. Addiction 2014, 109, 2027–2034. [Google Scholar] [CrossRef]
- Wu, S.H.; Guo, Q.; Viken, R.J.; Reed, T.; Dai, J. Heritability of usual alcohol intoxication and hangover in male twins: The NAS-NRC Twin Registry. Alcohol Clin. Exp. Res. 2014, 38, 2307–2313. [Google Scholar] [CrossRef]
- Mackus, M.; Van de Loo, A.J.A.E.; Garssen, J.; Kraneveld, A.D.; Verster, J.C. The association between ethanol elimination rate and hangover severity. Int. J. Environ. Res. Publ. Health 2020, 17, 4324. [Google Scholar] [CrossRef] [PubMed]
- Mackus, M.; van Schrojenstein Lantman, M.; van de Loo, A.J.A.E.; Brookhuis, K.A.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Alcohol metabolism in hangover sensitive versus hangover resistant social drinkers. Drug Alcohol Depend. 2018, 185, 351–355. [Google Scholar] [CrossRef]
- Hogewoning, A.; van de Loo, A.J.A.E.; Mackus, M.; Raasveld, S.J.; de Zeeuw, R.; Bosma, E.R.; Bouwmeester, N.H.; Brookhuis, K.A.; Garssen, J.; Verster, J.C. Characteristics of social drinkers with and without a hangover after heavy alcohol consumption. Subst. Abuse Rehab. 2016, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; van de Loo, A.J.A.E.; Benson, S.; Scholey, A.; Stock, A.-K. The assessment of overall hangover severity. J. Clin. Med. 2020, 9, 786. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Niemelä, O. Acetaldehyde adducts in circulation. Novartis Found. Symp. 2007, 285, 183–192; discussion 193–197. [Google Scholar] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Tuma, D.J. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic. Biol. Med. 2002, 32, 302–308. [Google Scholar]
- Niemela, O. Aldehyde-protein adducts in the liver as a result of ethanol-induced oxidative stress. Front. Biosci. 1999, 4, d506–d513. [Google Scholar] [CrossRef]
- Thiele, G.M.; Worrall, S.; Tuma, D.J.; Klassen, L.W.; Wyatt, T.A.; Nagata, N. The chemistry and biological effects of malondialdehyde-acetaldehyde adducts. Alcohol Clin. Exp. Res. 2001, 25, 218S–224S. [Google Scholar] [CrossRef] [PubMed]
- Tuma, D.J.; Casey, C.A. Dangerous byproducts of alcohol breakdown—Focus on adducts. Alcohol Res. Health 2003, 27, 285–290. [Google Scholar] [PubMed]
- Salaspuro, M. Acetaldehyde and gastric cancer. J. Dig. Dis. 2011, 12, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Salaspuro, V.; Hietala, J.; Kaihovaara, P.; Pihlajarinne, L.; Marvola, M.; Salaspuro, M. Removal of acetaldehyde from saliva by a slow-release buccal tablet of L-cysteine. Int. J. Cancer 2002, 97, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Song, J.; Kim, T.M.; Joo, S.S.; Park, D.; Jeon, J.H.; Shin, S.; Park, H.K.; Lee, W.K.; Ly, S.Y.; et al. Effects of a preparation of combined glutathione-enriched yeast and rice embryo/soybean extracts on ethanol hangover. J. Med. Food 2009, 12, 1359–1367. [Google Scholar] [CrossRef]
- Park, S.K.; Qi, X.F.; Song, S.B.; Kim, D.H.; Teng, Y.C.; Yoon, Y.S.; Kim, K.Y.; Li, J.H.; Jin, D.; Lee, K.J. Electrolyzed-reduced water inhibits acute ethanol-induced hangovers in Sprague-Dawley rats. Biomed. Res. 2009, 30, 263–269. [Google Scholar] [CrossRef]
- Pittler, M.H.; Verster, J.C.; Ernst, E. Interventions for preventing or treating alcohol hangover: Systematic review of randomized trials. Br. Med. J. 2005, 331, 1515–1518. [Google Scholar] [CrossRef]
- Verster, J.C.; Penning, R. Treatment and prevention of alcohol hangover. Curr. Drug Abuse Rev. 2010, 3, 103–109. [Google Scholar] [CrossRef]
- Jayawardena, R.; Thejani, T.; Ranasinghe, P.; Fernando, D.; Verster, J.C. Interventions for treatment and/or prevention of alcohol hangover: Systematic review. Hum. Psychopharmacol. 2017, 32, e2600. [Google Scholar] [CrossRef]
- Mackus, M.; van Schrojenstein Lantman, M.; van de Loo, A.J.A.E.; Nutt, D.J.; Verster, J.C. An effective hangover treatment: Friend or foe? Drug Sci. Policy Law 2017. [Google Scholar] [CrossRef]
- Scholey, A.; Ayre, E.; Stock, A.-K.; Verster, J.C.; Benson, S. The effects of Rapid Recovery on alcohol hangover severity: A double-blind, placebo-controlled, randomized and crossover trial. J. Clin. Med. 2020, 9, 2175. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.J.P.; Metsälä, M.; Möykkynen, T.; Mäkisalo, H.; Kärkkäinen, O.; Palmén, M.; Salminen, J.E.; Kauhanen, J. L-Cysteine containing vitamin supplement which prevents or alleviates alcohol-related hangover symptoms: Nausea, headache, stress and anxiety. Alcohol Alcohol. 2020. [Google Scholar] [CrossRef]
- Benson, S.; Scholey, A.; Verster, J.C. L-cysteine and the treatment of alcohol hangover: A commentary on Eriksson et al. Submitted for publication.
- Mammen, R.R.; Natinga Mulakal, J.; Mohanan, R.; Maliakel, B.; Krishnakumar, I.M. Clove bud polyphenols alleviate alterations in inflammation and oxidative stress markers associated with binge drinking: A randomized double-blinded placebo-controlled crossover study. J. Med. Food 2018, 21, 1188–1196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackus, M.; Loo, A.J.v.d.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. J. Clin. Med. 2020, 9, 3421. https://doi.org/10.3390/jcm9113421
Mackus M, Loo AJvd, Garssen J, Kraneveld AD, Scholey A, Verster JC. The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. Journal of Clinical Medicine. 2020; 9(11):3421. https://doi.org/10.3390/jcm9113421
Chicago/Turabian StyleMackus, Marlou, Aurora JAE van de Loo, Johan Garssen, Aletta D. Kraneveld, Andrew Scholey, and Joris C. Verster. 2020. "The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover" Journal of Clinical Medicine 9, no. 11: 3421. https://doi.org/10.3390/jcm9113421
APA StyleMackus, M., Loo, A. J. v. d., Garssen, J., Kraneveld, A. D., Scholey, A., & Verster, J. C. (2020). The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. Journal of Clinical Medicine, 9(11), 3421. https://doi.org/10.3390/jcm9113421





