Abnormal Nailfold Capillaries in Patients after Hand Transplantation
Abstract
1. Introduction
2. Experimental Section
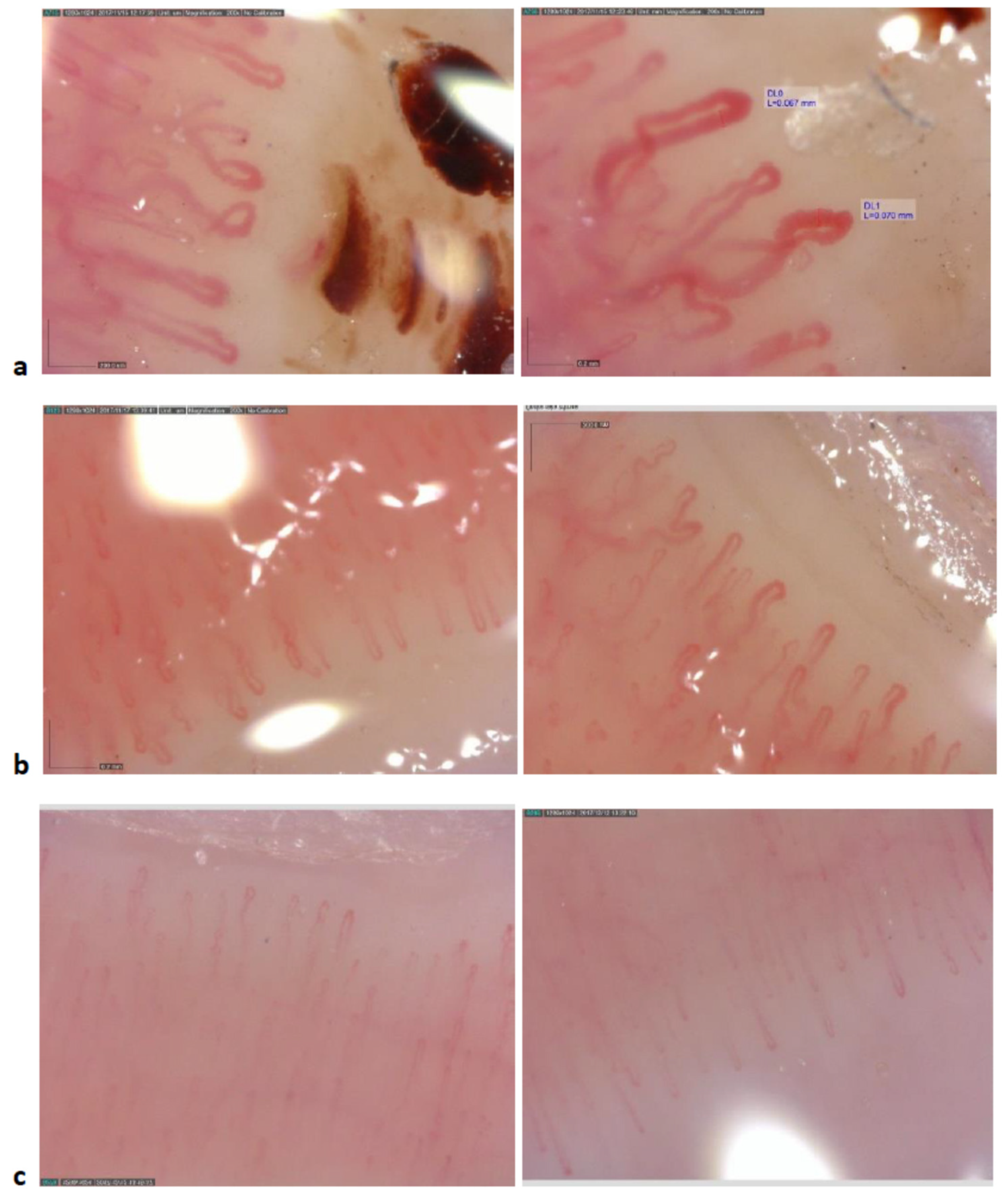
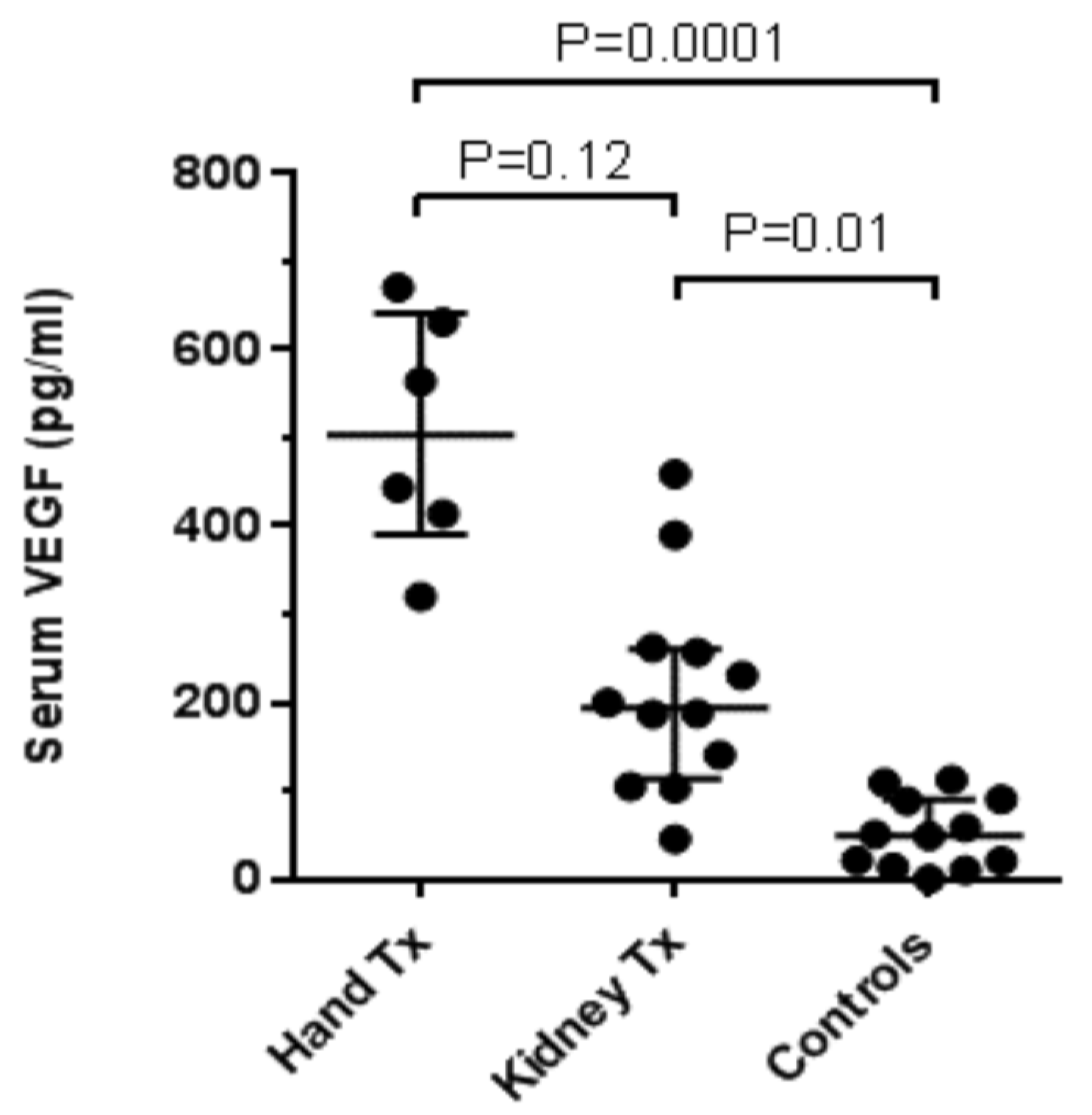
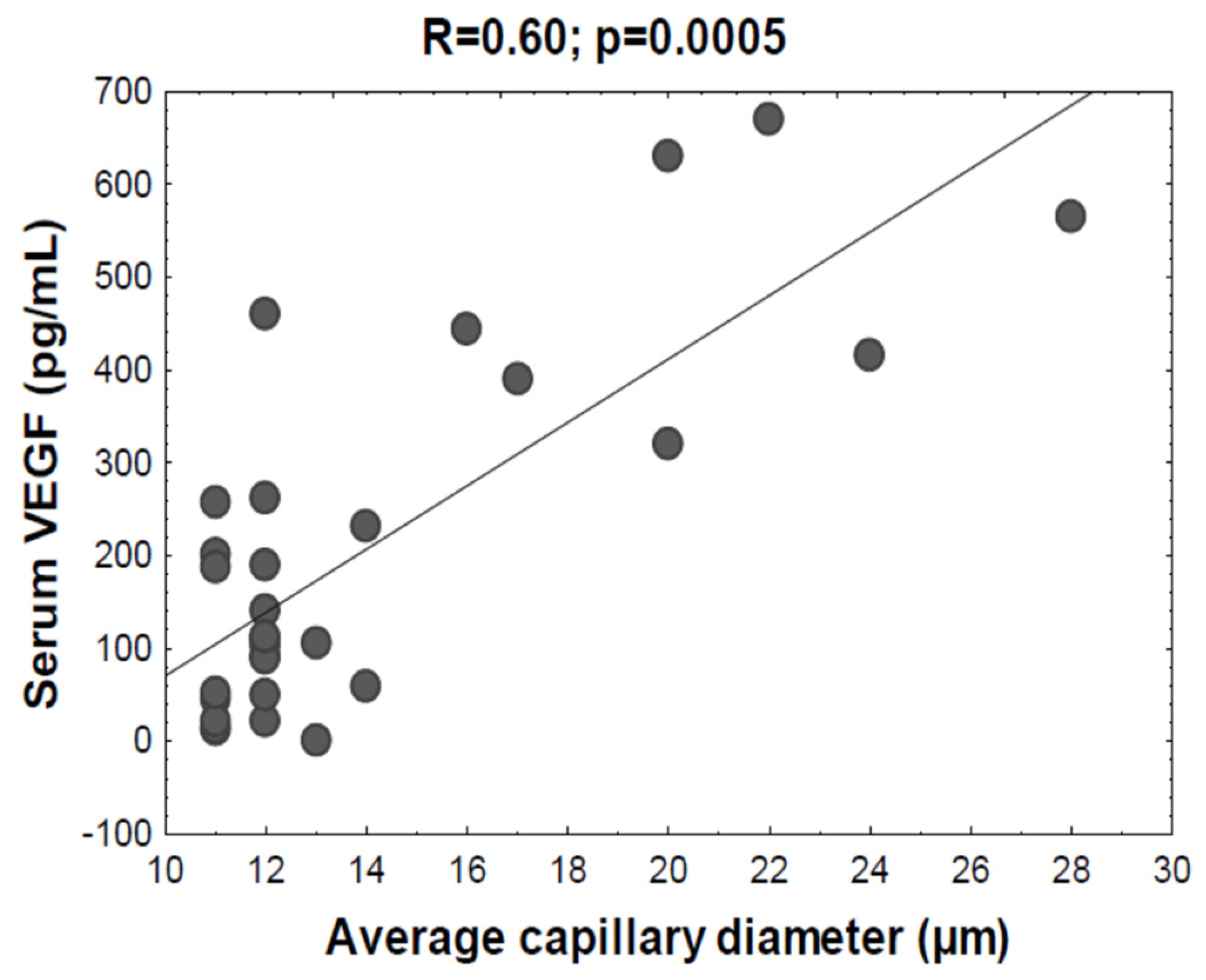
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubernard, J.M.; Owen, E.; Herzberg, G.; Lanzetta, M.; Martin, X.; Kapila, H.; Dawahra, M.; Hakim, N.S. Human hand allograft: Report on first 6 months. Lancet 1999, 353, 1315–1320. [Google Scholar] [CrossRef]
- Petruzzo, P.; Lanzetta, M.; Dubernard, J.M.; Landin, L.; Cavadas, P.; Margreiter, R.; Schneeberger, S.; Breidenbach, W.; Kaufman, C.; Jablecki, J.; et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation 2010, 90, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Malek, V.; Lee, W.P.A.; Brandacher, G. Outcomes after hand and upper extremity transplantation. J. Mater. Sci. Mater. Med. 2017, 28, 72. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J.; Petruzzo, P.; Badet, L.; Gazarian, A.; Thaunat, O.; Testelin, S.; Devauchelle, B.; Dubernard, J.M.; Morelon, E. Chronic Rejection in Human Vascularized Composite Allotransplantation (Hand and Face Recipients): An Update. Transplantation 2016, 100, 2053–2061. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Ouseph, R.; Blair, B.; Kutz, J.E.; Tsai, T.M.; Scheker, L.R.; Tien, H.Y.; Moreno, R.; Ozyurekoglu, T.; Banegas, R.; et al. Graft vasculopathy in clinical hand transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2012, 12, 1004–1016. [Google Scholar] [CrossRef]
- Boratynska, M.; Obremska, M.; Malecki, R.; Gacka, M.; Magott, M.; Kaminska, D.; Banasik, M.; Kusztal, M.; Chelmonski, A.; Jablecki, J.; et al. Impact of immunosuppressive treatment on the cardiovascular system in patients after hand transplantation. Transplant. Proc. 2014, 46, 2890–2893. [Google Scholar] [CrossRef]
- Kollar, B.; Kamat, P.; Klein, H.J.; Waldner, M.; Schweizer, R.; Plock, J.A. The Significance of Vascular Alterations in Acute and Chronic Rejection for Vascularized Composite Allotransplantation. J. Vasc. Res. 2019, 56, 163–180. [Google Scholar] [CrossRef]
- Jablecki, J.; Kaczmarzyk, L.; Domanasiewicz, A.; Chelmonski, A.; Paruzel, M.; Elsaftawy, A.; Syrko, M.; Kaczmarzyk, J. Result of arm-level upper-limb transplantation in two recipients at 19- and 30-month follow-up. Ann. Transplant. 2012, 17, 126–132. [Google Scholar]
- Jablecki, J. World experience after more than a decade of clinical hand transplantation: Update on the Polish program. Hand Clin. 2011, 27, 433–442, viii. [Google Scholar] [CrossRef]
- Hein, R.E.; Ruch, D.S.; Klifto, C.S.; Leversedge, F.J.; Mithani, S.K.; Pidgeon, T.S.; Richard, M.J.; Cendales, L.C. Hand transplantation in the United States: A review of the Organ Procurement and Transplantation Network/United Network for Organ Sharing Database. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 20, 1417–1423. [Google Scholar] [CrossRef]
- Etra, J.W.; Raimondi, G.; Brandacher, G. Mechanisms of rejection in vascular composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Morelon, E.; Petruzzo, P.; Kanitakis, J. Chronic rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 582–591. [Google Scholar] [CrossRef]
- Borges, T.J.; O’Malley, J.T.; Wo, L.; Murakami, N.; Smith, B.; Azzi, J.; Tripathi, S.; Lane, J.D.; Bueno, E.M.; Clark, R.A.; et al. Codominant Role of Interferon-gamma- and Interleukin-17-Producing T Cells During Rejection in Full Facial Transplant Recipients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 2158–2171. [Google Scholar] [CrossRef]
- Krstic, D.; Tomic, N.; Radosavljevic, B.; Avramovic, N.; Dragutinovic, V.; Skodric, S.R.; Colovic, M. Biochemical Markers of Renal Function. Curr. Med. Chem. 2016, 23, 2018–2040. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.P.; Seifert, M.E.; Chandraker, A.; Zurakowski, D.; Nohria, A.; Givertz, M.M.; Karumanchi, S.A.; Briscoe, D.M. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 32, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Armstrong, B.; Baran, D.A.; Bridges, N.D.; Chandraker, A.; Gordon, R.; De Marco, T.; Givertz, M.M.; Heroux, A.; Ikle, D.; et al. Early immune biomarkers and intermediate-term outcomes after heart transplantation: Results of Clinical Trials in Organ Transplantation-18. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2019, 19, 1518–1528. [Google Scholar] [CrossRef]
- Watanabe, K.; Karimpour-Fard, A.; Michael, A.; Miyamoto, S.D.; Nakano, S.J. Elevated serum vascular endothelial growth factor and development of cardiac allograft vasculopathy in children. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J.; Karayannopoulou, G.; Lanzetta, M.; Petruzzo, P. Graft vasculopathy in the skin of a human hand allograft: Implications for diagnosis of rejection of vascularized composite allografts. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 27, e118–e123. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Smith, V.; Sulli, A.; Cutolo, M. Capillaroscopy in Routine Diagnostics: Potentials and Limitations. Curr. Rheumatol. Rev. 2018, 14, 5–11. [Google Scholar] [CrossRef]
- Klein-Weigel, P.F.; Sunderkotter, C.; Sander, O. Nailfold capillaroscopy microscopy–an interdisciplinary appraisal. VASA Z. Gefasskrankh. 2016, 45, 353–364. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best practice & research. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef]
- Cutolo, M.; Smith, V. State of the art on nailfold capillaroscopy: A reliable diagnostic tool and putative biomarker in rheumatology? Rheumatology 2013, 52, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Secchi, M.E.; Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Capillaroscopy. Best practice & research. Clin. Rheumatol. 2005, 19, 437–452. [Google Scholar] [CrossRef]
- Lu, W.; Wang, D.; Liu, L.; Xiong, J.; He, Q. Nail fold capillary observation in replanted severed fingers. Microsurgery 2008, 28, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Jablecki, J.; Kaczmarzyk, L.; Patrzalek, D.; Domanasiewicz, A.; Boratynska, Z. First Polish forearm transplantation: Report after 17 months. Transplant. Proc. 2009, 41, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Sulli, A.; Smith, V.; Pizzorni, C.; Paolino, S.; Alessandri, E.; Cutolo, M. Microvascular damage evaluation in systemic sclerosis: The role of nailfold videocapillaroscopy and laser techniques. Reumatismo 2017, 69, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.; Pyrpasopoulou, A.; Simos, G.; Paraskeva, E.; Nikolaidou, C.; Venizelos, I.; Koukoulis, G.; Aslanidis, S.; Douma, S. Upregulation of VEGF expression is associated with accumulation of HIF-1alpha in the skin of naive scleroderma patients. Mod. Rheumatol. 2013, 23, 1245–1248. [Google Scholar] [CrossRef]
- Distler, J.H.; Jungel, A.; Pileckyte, M.; Zwerina, J.; Michel, B.A.; Gay, R.E.; Kowal-Bielecka, O.; Matucci-Cerinic, M.; Schett, G.; Marti, H.H.; et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007, 56, 4203–4215. [Google Scholar] [CrossRef]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2007, 2, 251–275. [Google Scholar] [CrossRef]
- Tuomisto, T.T.; Rissanen, T.T.; Vajanto, I.; Korkeela, A.; Rutanen, J.; Yla-Herttuala, S. HIF-VEGF-VEGFR-2, TNF-alpha and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis 2004, 174, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, D.; Koscielska-Kasprzak, K.; Krajewska, M.; Chelmonski, A.; Jablecki, J.; Zabinska, M.; Myszka, M.; Banasik, M.; Boratynska, M.; Gomolkiewicz, A.; et al. Immune activation- and regulation-related patterns in stable hand transplant recipients. Transpl. Int. Off. J. Eur. Soc. Organ Transpl. 2017, 30, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, W.; Xu, K.; Han, D.; Jiang, C.; Mou, K.; Li, Y.; Meng, L.; Lu, S. The elevated expression of Th17-related cytokines and receptors is associated with skin lesion severity in early systemic sclerosis. Hum. Immunol. 2015, 76, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, S.A.; Hayes, E.; Farina, G.; Lemaire, R.; Farber, H.W.; Whitfield, M.L.; Lafyatis, R. Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLoS ONE 2010, 5, e12106. [Google Scholar] [CrossRef]
- Distler, O.; Distler, J.H.; Scheid, A.; Acker, T.; Hirth, A.; Rethage, J.; Michel, B.A.; Gay, R.E.; Muller-Ladner, U.; Matucci-Cerinic, M.; et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circul. Res. 2004, 95, 109–116. [Google Scholar] [CrossRef]
- Kadono, K.; Gruszynski, M.; Azari, K.; Kupiec-Weglinski, J.W. Vascularized composite allotransplantation versus solid organ transplantation: Innate-adaptive immune interphase. Curr. Opin. Organ Transplant. 2019, 24, 714–720. [Google Scholar] [CrossRef]
- Barbulescu, A.L.; Vreju, A.F.; Buga, A.M.; Sandu, R.E.; Criveanu, C.; Tudorascu, D.R.; Gheonea, I.A.; Ciurea, P.L. Vascular endothelial growth factor in systemic lupus erythematosus—Correlations with disease activity and nailfold capillaroscopy changes. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2015, 56, 1011–1016. [Google Scholar]
- De Santis, M.; Ceribelli, A.; Cavaciocchi, F.; Crotti, C.; Massarotti, M.; Belloli, L.; Marasini, B.; Isailovic, N.; Generali, E.; Selmi, C. Nailfold videocapillaroscopy and serum VEGF levels in scleroderma are associated with internal organ involvement. Auto Immun. Highlights 2016, 7, 5. [Google Scholar] [CrossRef][Green Version]
- Bongard, O.; Weimer, D.; Lemoine, R.; Bolle, J.F.; Leski, M.; Bounameaux, H. Cyclosporine toxicity in renal transplant recipients detected by nailfold capillaroscopy with Na-fluorescein. Kidney Int. 2000, 58, 2559–2563. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Anyfanti, P.; Triantafyllou, G.; Zabulis, X.; Aslanidis, S.; Douma, S. Impaired metabolic profile is a predictor of capillary rarefaction in a population of hypertensive and normotensive individuals. J. Am. Soc. Hypertens. JASH 2016, 10, 640–646. [Google Scholar] [CrossRef]
- Pollock, F.E., Jr.; Smith, T.L.; Koman, L.A. Microvascular response in the rabbit ear to total body cooling: A model for study of human digits. Microsurgery 1994, 15, 433–438. [Google Scholar] [CrossRef]
| Patients after Hand Transplantation (n = 6) | Patients after Kidney Transplantation (n = 12) | p-Value | |
|---|---|---|---|
| Men, n (%) | 5 (83%) | 10 (83%) | 1.000 |
| Age, years | 38 (33–45) | 47 (43–63) | 0.089 |
| Cause of transplantation, n (%) | Trauma: 6 (100%) | Primary glomerulonephritis: 6 (50%) Polycystic kidney disease: 3 (25%) Chronic tubulointerstitial nephritis: 3 (25%) | NA |
| Time after transplantation, years | 8 (3–9) | 11 (6–15) | 0.188 |
| Post-steroid diabetes mellitus, n (%) | 1 (17%) | 2 (17%) | 1.000 |
| Hypertension, n (%) | 1 (17%) | 7 (58%) | 0.093 |
| Patients after Hand Transplantation (n = 6) -Hand Allografts | Patients after Hand Transplantation (n = 6) -Healthy Hands | Patients after Kidney Transplantation(n = 12) -Both Hands | Healthy Control Group(n = 12) -Both Hands | |
|---|---|---|---|---|
| capillary disorganization, n (%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) |
| avascular areas, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| capillary density (per mm) | 6 (5–6) | 7 (7–8) | 8 (8–9) | 8 (8–9) |
| hemorrhages, n (%) | 3 (50%) | 1 (17%) | 1 (8%) | 0 (0%) |
| tortuous capillaries, n (%) | 6 (100%) | 6 (100%) | 7 (58%) | 5 (42%) |
| branching capillaries, n (%) | 4 (67%) | 3 (50%) | 1 (8%) | 0 (0%) |
| ectasic capillaries, n (%) | 6 (100%) | 6 (100%) | 5 (42%) | 4 (33%) |
| giant capillaries, n (%) | 3 (50%) | 0 (0%) | 0 (0%) | 0 (0%) |
| maximal diameter of capillaries (µm) | 48 (42–56) | 30 (27–36) | 20 (18–26) | 19 (19–25) |
| average diameter of capillaries (µm) | 29 (26–32) | 21 (20–24) | 12 (11–12) | 12 (11–13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, D.; Samborski, W.; Kamińska, D.; Kusztal, M.; Jabłecki, J.; Nijakowski, K.; Oko, A.; Karczewski, M.; Korybalska, K.; Witowski, J. Abnormal Nailfold Capillaries in Patients after Hand Transplantation. J. Clin. Med. 2020, 9, 3422. https://doi.org/10.3390/jcm9113422
Sikorska D, Samborski W, Kamińska D, Kusztal M, Jabłecki J, Nijakowski K, Oko A, Karczewski M, Korybalska K, Witowski J. Abnormal Nailfold Capillaries in Patients after Hand Transplantation. Journal of Clinical Medicine. 2020; 9(11):3422. https://doi.org/10.3390/jcm9113422
Chicago/Turabian StyleSikorska, Dorota, Włodzimierz Samborski, Dorota Kamińska, Mariusz Kusztal, Jerzy Jabłecki, Kacper Nijakowski, Andrzej Oko, Marek Karczewski, Katarzyna Korybalska, and Janusz Witowski. 2020. "Abnormal Nailfold Capillaries in Patients after Hand Transplantation" Journal of Clinical Medicine 9, no. 11: 3422. https://doi.org/10.3390/jcm9113422
APA StyleSikorska, D., Samborski, W., Kamińska, D., Kusztal, M., Jabłecki, J., Nijakowski, K., Oko, A., Karczewski, M., Korybalska, K., & Witowski, J. (2020). Abnormal Nailfold Capillaries in Patients after Hand Transplantation. Journal of Clinical Medicine, 9(11), 3422. https://doi.org/10.3390/jcm9113422







