Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Patients’ Characteristics
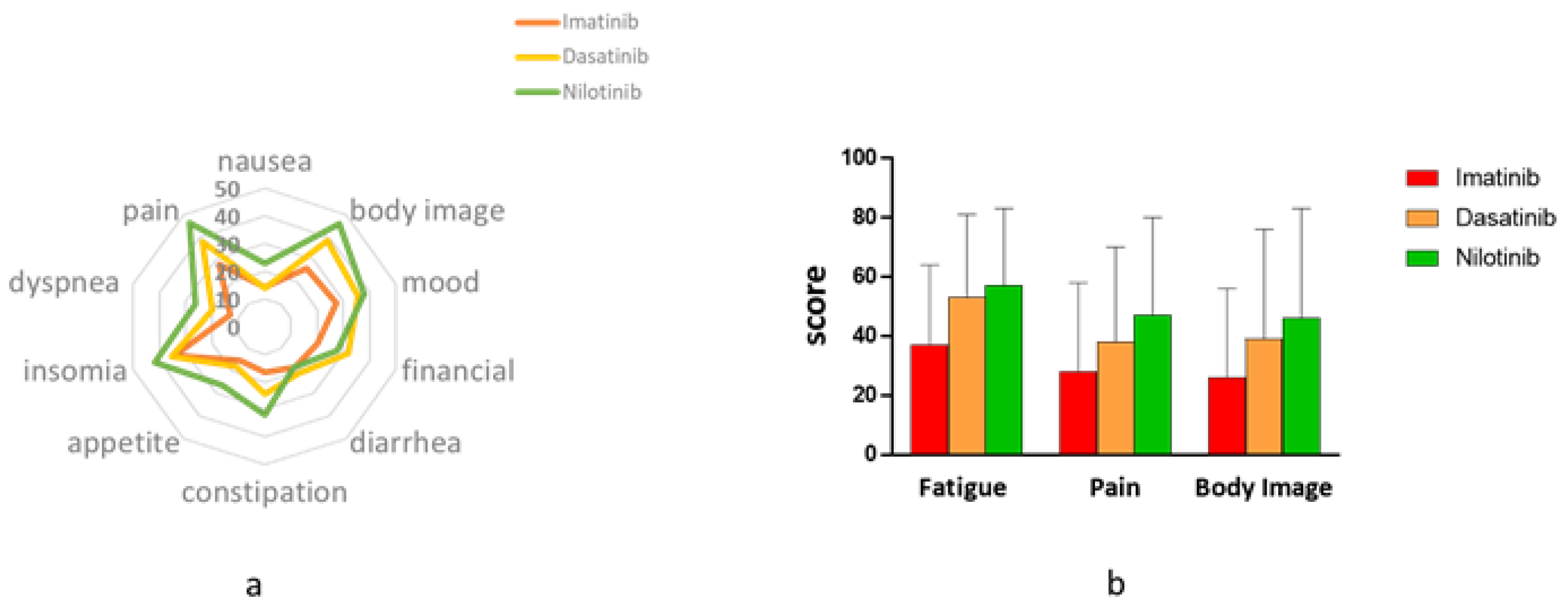
3.2. Symptom Burden-EORTC (The QLQ-30 and the QLQ-CML24)
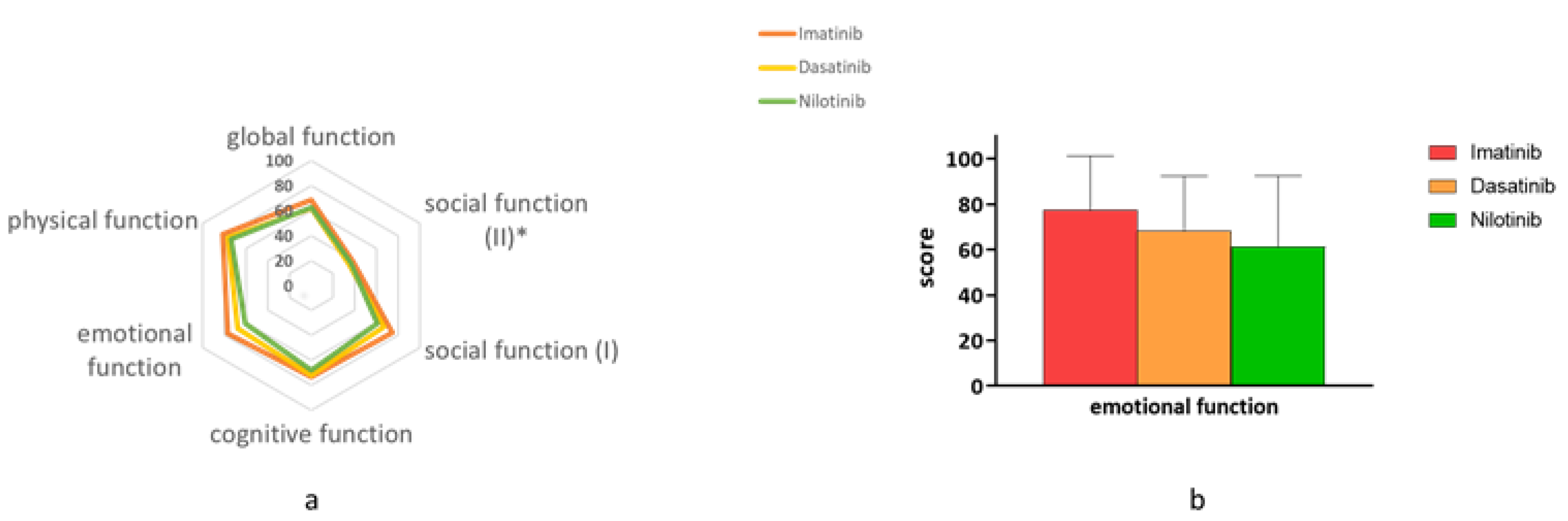
3.3. Functional Status—EORTC (The QLQ-30 and the QLQ-CML24)
3.4. Additional Health-Related (HR) Items
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, M.H.; Johnson, J.R.; Pazdur, R. U.S. Food and Drug Administration Drug Approval Summary: Conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin. Cancer Res. 2005, 11, 12–19. [Google Scholar] [PubMed]
- Hoffmann, V.S.; Baccarani, M.; Hasford, J.; Castagnetti, F.; Di Raimondo, F.; Casado, L.F.; Turkina, A.; Zackova, D.; Ossenkoppele, G.; Zaritskey, A.; et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia 2016, 31, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Cannella, L. The value of quality of life assessment in chronic myeloid leukemia patients receiving tyrosine kinase inhibitors. Hematology 2016, 2016, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.-L. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef]
- Sasaki, K.; Strom, S.S.; O’Brien, S.; Jabbour, E.; Ravandi, F.; Konopleva, M.; Borthakur, G.; Pemmaraju, N.; Daver, N.; Jain, P.; et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: Analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015, 2, e186–e193. [Google Scholar] [CrossRef]
- Phillips, K.M.; Pinilla-Ibarz, J.; Sotomayor, E.; Lee, M.R.; Jim, H.S.L.; Small, B.J.; Sokol, L.; Lancet, J.; Tinsley, S.; Sweet, K.; et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A controlled comparison. Support. Care Cancer 2012, 21, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef]
- Efficace, F.; Baccarani, M.; Breccia, M.; Saussele, S.; Abel, G.; Caocci, G.; Guilhot, F.; Cocks, K.; Naeem, A.; Sprangers, M.; et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: The EORTC QLQ-CML24. Qual. Life Res. 2013, 23, 825–836. [Google Scholar] [CrossRef]
- Fayers, P.M. Interpreting quality of life data. Eur. J. Cancer 2001, 37, 1331–1334. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef]
- Hendricson, W.D.; Russell, I.J.; Prihoda, T.J.; Jacobson, J.M.; Rogan, A.; Bishop, G.D.; Castillo, R. Development and initial validation of a dual-language english–spanish format for the arthritis impact measurement scales. Arthritis Rheum. 1989, 32, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Quinten, C.; Coens, C.; Ghislain, I.; Zikos, E.; Sprangers, M.A.G.; Ringash, J.; Martinelli, F.; Ediebah, D.E.; Maringwa, J.; Reeve, B.B.; et al. The effects of age on health-related quality of life in cancer populations: A pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur. J. Cancer 2015, 51, 2808–2819. [Google Scholar] [CrossRef] [PubMed]
- Bantema-Joppe, E.J.; De Bock, G.H.; Iersel, M.W.-V.; Busz, D.M.; Ranchor, A.V.; Langendijk, J.A.; Maduro, J.H.; Heuvel, E.R.V.D. The impact of age on changes in quality of life among breast cancer survivors treated with breast-conserving surgery and radiotherapy. Br. J. Cancer 2015, 112, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, R.; Taylor, A.W.; Hugo, G.; Wittert, G. Are Baby Boomers Healthier than Generation X? A Profile of Australia’s Working Generations Using National Health Survey Data. PLoS ONE 2014, 9, e93087. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Stagno, F.; Iurlo, A.; Breccia, M.; Cottone, F.; Bonifacio, M.; Abruzzese, E.; Castagnetti, F.; Caocci, G.; Crugnola, M.; et al. Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia 2019, 34, 488–498. [Google Scholar] [CrossRef]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Campiotti, L.; Suter, M.B.; Guasti, L.; Piazza, R.; Gambacorti-Passerini, C.; Grandi, A.M.; Squizzato, A. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur. J. Cancer 2017, 77, 48–56. [Google Scholar] [CrossRef]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.-W.; Issaragrisil, S.; Le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef]
- Cortes, J.; Jimenez, C.A.; Mauro, M.J.; Geyer, A.; Pinilla-Ibarz, J.; Smith, B.D. Pleural Effusion in Dasatinib-Treated Patients With Chronic Myeloid Leukemia in Chronic Phase: Identification and Management. Clin. Lymphoma Myeloma Leuk. 2017, 17, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.-W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Réa, D.; Schwarz, M.; Grille, P.; Nicolini, F.E.; Rosti, G.; Levato, L.; Giles, F.J.; Dombret, H.; Mirault, T.; et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013, 27, 1316–1321. [Google Scholar] [CrossRef]
| Characteristics | Imatinib n = 70 | Dasatinib n = 45 | Nilotinib n = 24 | Total n = 139 | p Value |
|---|---|---|---|---|---|
| Age, years, median (range) | 67 (32 to 89) | 47 (23 to 78) | 50 (26 to 85) | 58 (23 to 89) | <0.0001 |
| Gender n (%) * | |||||
| male | 43 (62) | 28 (64) | 15 (63) | 86 (63) | 0.99 |
| female | 26 (38) | 16 (36) | 9 (37) | 51 (37) | |
| Family status * | |||||
| singles, n (%) | 3 (5) | 13 (30) | 4 (17) | 20 (15) | 0.001 |
| married, n (%) | 62 (95) | 30 (70) | 20 (83) | 112 (85) | |
| number of children, median (range) | 3 (0 to 15) | 2 (0 to 10) | 2 (0 to 6) | 3 (0 to 15) | 0.06 |
| Education level, n (%) * | |||||
| elementary | 4 (6) | 3 (7) | 3 (12) | 10 (7) | 0.6 |
| high school | 24 (36) | 19 (42) | 6 (25) | 49 (36) | |
| high education | 39 (58) | 23 (51) | 15 (63) | 77 (57) | |
| Level of religiosity, n (%) * | |||||
| secular | 40 (62) | 30 (68) | 13 (57) | 83 (63) | 0.93 |
| traditional | 14 (22) | 7 (16) | 16 (26) | 27 (21) | |
| religious | 10 (15) | 6 (14) | 4 (17) | 20 (15) | |
| ultra-orthodox | 1 (1) | 1 (2) | 0 | 2 (1) |
| Characteristics | Imatinib n = 70 | Dasatinib n = 45 | Nilotinib n = 24 | Total n = 139 | p Value |
|---|---|---|---|---|---|
| Duration of disease, months, median (range) | 10 (1 to 46) | 4 (<1 to 15) | 5 (1 to 14) | 7 (<1 to 46) | <0.0001 |
| Previous TKIs, n (%) | |||||
| 0 | 41 (59) | 23 (51) | 8 (33) | 72(52) | 0.3 |
| 1 | 25 (36) | 18 (40) | 13 (54) | 56 (40) | |
| >2 | 4 (5) | 4 (9) | 3 (12) | 11 (8) | |
| Duration of treatment with current TKI, months, median (range) | 8 (<1 to 18) | 2 (<1 to 11) | 4 (<1 to 11) | 4.3 (<1 to 18) | <0.0001 |
| Additional Questions | Imatinib n = 70 | Dasatinib n = 45 | Nilotinib n = 24 | Total n = 139 | p Value |
|---|---|---|---|---|---|
| Grade your health condition during the last 3 months *; mean (S.D.) | 5 (1.2) | 4.5 (1.3) | 4.5 (1.5) | 4.8 (1.3) | 0.04 |
| Was your QOL during the last 3 months affected by CML/treatment; n (%) | |||||
| Yes | 26 (41) | 26 (61) | 17 (71) | 69 (53) | 0.02 |
| No | 38 (59) | 17 (39) | 7 (29) | 62 (47) | |
| Symptoms since the current drug started | |||||
| Was your work function impaired?; n (%) | |||||
| Yes | 22 (33) | 24 (56) | 12 (52) | 58 (44) | 0.04 |
| No | 45 (67) | 19 (44) | 11 (48) | 75 (56) | |
| Was your home function impaired?; n (%) | |||||
| Yes | 16 (24) | 21 (47) | 11 (46) | 48 (35) | 0.023 |
| No | 51 (76) | 24 (53) | 13 (54) | 88 (65) | |
| Was your sexual function impaired?; n (%) | |||||
| Yes | 25 (39) | 17 (42) | 9 (41) | 51 (40) | 0.94 |
| No | 39 (61) | 23 (58) | 13 (59) | 75 (60) | |
| Were you followed at the cardiology/vascular/pulmonology/neurology clinic?; n (%) | |||||
| Yes | 40 (61) | 25 (57) | 12 (52) | 77 (58) | 0.77 |
| No | 26 (39) | 19 (43) | 11 (48) | 56 (42) | |
| Did you have difficulties in breathing due to fluids accumulation in your lungs?; n (%) | |||||
| Yes | 8 (13) | 9 (21) | 4 (18) | 21 (16) | 0.5 |
| No | 36 (87) | 34 (79) | 18 (82) | 108 (84) | |
| Were you admitted to the hospital in the last 6 months?; n (%) | |||||
| Yes | 34 (49) | 17 (38) | 8 (23) | 59 (42) | 0.32 |
| No | 36 (51) | 28 (62) | 16 (67) | 80 (58) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shacham Abulafia, A.; Shemesh, S.; Rosenmann, L.; Berger, T.; Leader, A.; Sharf, G.; Raanani, P.; Rozovski, U. Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors. J. Clin. Med. 2020, 9, 3417. https://doi.org/10.3390/jcm9113417
Shacham Abulafia A, Shemesh S, Rosenmann L, Berger T, Leader A, Sharf G, Raanani P, Rozovski U. Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors. Journal of Clinical Medicine. 2020; 9(11):3417. https://doi.org/10.3390/jcm9113417
Chicago/Turabian StyleShacham Abulafia, Adi, Sivan Shemesh, Lena Rosenmann, Tamar Berger, Avi Leader, Giora Sharf, Pia Raanani, and Uri Rozovski. 2020. "Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors" Journal of Clinical Medicine 9, no. 11: 3417. https://doi.org/10.3390/jcm9113417
APA StyleShacham Abulafia, A., Shemesh, S., Rosenmann, L., Berger, T., Leader, A., Sharf, G., Raanani, P., & Rozovski, U. (2020). Health-Related Quality of Life in Patients with Chronic Myeloid Leukemia Treated with First- Versus Second-Generation Tyrosine Kinase Inhibitors. Journal of Clinical Medicine, 9(11), 3417. https://doi.org/10.3390/jcm9113417




