Association between Obstructive Sleep Apnea and SYNTAX Score
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Polysomnography
2.3. CAG and SYNTAX Score
2.4. Other Data Collection
2.5. Statistical Analyses
3. Results
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.; Esnaola, S.; Rubio, R.; Iztueta, A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am. J. Respir. Crit. Care. Med. 2001, 163, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Mooe, T.; Rabben, T.; Wiklund, U.; Franklin, K.A.; Eriksson, P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996, 109, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Milleron, O.; Pilliere, R.; Foucher, A.; de Roquefeuil, F.; Aegerter, P.; Jondeau, G.; Raffestin, B.G.; Dubourg, O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: A long-term follow-up study. Eur. Heart J. 2004, 25, 728–734. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D. Obstructive sleep apnea and heart failure: Pathophysiologic and therapeutic implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef]
- Lindberg, E.; Theorell-Haglow, J.; Svensson, M.; Gislason, T.; Berne, C.; Janson, C. Sleep apnea and glucose metabolism: A long-term follow-up in a community-based sample. Chest 2012, 142, 935–942. [Google Scholar] [CrossRef]
- Nadeem, R.; Singh, M.; Nida, M.; Waheed, I.; Khan, A.; Ahmed, S.; Nassem, J.; Champeau, D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: A meta-regression analysis. J. Clin. Sleep Med. 2014, 10, 475–489. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, Y.J.; Yu, Y.; Wu, S.J.; Sun, Y.; Wang, Z.J.; Liu, X.L.; King, B.E.; Zhao, Y.X.; Shi, D.M.; et al. Obstructive sleep apnea is associated with severity and long-term prognosis of acute coronary syndrome. J. Geriatr. Cardiol. 2018, 15, 146–152. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Ozcan, C.; Deleskog, A.; Schjerning Olsen, A.M.; Nordahl Christensen, H.; Lock Hansen, M.; Hilmar Gislason, G. Coronary artery disease severity and long-term cardiovascular risk in patients with myocardial infarction: A Danish nationwide register-based cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 25–35. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Botker, H.E.; Sorensen, H.T.; Schmidt, M.; Pedersen, L.; Sand, N.P.; Jensen, J.M.; Steffensen, F.H.; Tilsted, H.H.; Bøttcher, M.; et al. Prognostic assessment of stable coronary artery disease as determined by coronary computed tomography angiography: A Danish multicentre cohort study. Eur. Heart J. 2017, 38, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical Specifications; Version 2.2.; American Academy of Sleep Medicine: Darien, IL, USA, 2015. [Google Scholar]
- Kasai, T.; Floras, J.S.; Bradley, T.D. Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation 2012, 126, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Kopp, A.F.; Baumbach, A.; Meisner, C.; Kuettner, A.; Georg, C.; Ohnesorge, B.; Herdeg, C.; Claussen, C.D.; Karsch, K.R. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J. Am. Coll. Cardiol. 2001, 37, 1430–1435. [Google Scholar] [CrossRef]
- Sones, F.M., Jr.; Shirey, E.K. Cine coronary arteriography. Mod. Conc. Cardiovasc. Dis. 1962, 31, 735–738. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvallm, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Tanaka, A.; Oda, N.; Okada, S.; Ohta, S.; Naito, H.; Adachi, M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 625–630. [Google Scholar] [CrossRef]
- Sorajja, D.; Gami, A.S.; Somers, V.K.; Behrenbeck, T.R.; Garcia-Touchard, A.; Lopez-Jimenezm, F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest 2008, 133, 927–933. [Google Scholar] [CrossRef]
- Kent, B.D.; Garvey, J.F.; Ryan, S.; Nolan, G.; Dodd, J.D.; McNicholas, W.T. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: A coronary computed tomographic angiography study. Eur. Respir. J. 2013, 42, 1263–1270. [Google Scholar] [CrossRef]
- Tan, A.; Hau, W.; Ho, H.H.; Ghaem Maralani, H.; Loo, G.; Khoo, S.M.; Tai, B.C.; Richards, A.M.; Ong, P.; Lee, C.H. OSA and coronary plaque characteristics. Chest 2014, 145, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M.; Phillips 3rd, H.R.; Hindman, M.C.; Mark, D.B.; Lee, K.L.; Behar, V.S.; Johnsnon, R.A.; Pryor, D.B.; Rosati, R.A.; Wagner, G.S.; et al. Prognostic value of a coronary artery jeopardy score. J. Am. Coll. Cardiol. 1985, 5, 1055–1063. [Google Scholar] [CrossRef]
- De Silva, K.; Morton, G.; Sicard, P.; Chong, E.; Indermuehle, A.; Clapp, B.; Thomas, M.; Redwood, S.; Perera, D. Prognostic utility of BCIS myocardial jeopardy score for classification of coronary disease burden and completeness of revascularization. Am. J. Cardiol. 2013, 111, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Mollayeva, T.; Gershon, A.S.; Leung, R.S.; Hawker, G.; Tomlinson, G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: A systematic review. Sleep Med. Rev. 2014, 18, 49–59. [Google Scholar] [CrossRef]
- Gensini, G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Uchikawa, S.; Imamura, H.; Kubo, K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest 2003, 124, 936–941. [Google Scholar] [CrossRef][Green Version]
- Rivera-Perez, S.J.; Martinez, D.; Araujo, G.N.; Goncalves, S.C.; Lazzaretti, L.K.; Wainstein, R.V.; Wainstein, M.V.; Ribeiro, J.P. Severity of obstructive sleep apnea and extension of coronary artery disease. Sleep Breath. 2019, 23, 747–752. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Dyck, N.V.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Head, S.J.; Davierwala, P.M.; Serruys, P.W.; Redwood, S.R.; Colombo, A.; Mack, M.J.; Morice, M.C.; Holmes, D.R., Jr.; Feldman, T.E.; Ståhle, E.; et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: Final five-year follow-up of the SYNTAX trial. Eur. Heart J. 2014, 35, 2821–2830. [Google Scholar] [CrossRef]
- Hein, T.; Loo, G.; Ng, W.Y.; Tai, B.C.; Kajiya, T.; Tan, A.; Khoo, S.M.; Chan, M.; Low, A.F.; Chia, B.L.; et al. Relationship between apnoea-hypopnoea index and angiographiccoronary disease phenotypes in patients presenting with acutemyocardial infarction. Acute Card. Care 2013, 15, 26–33. [Google Scholar] [CrossRef]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R., Jr.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Campos, C.M.; van Klaveren, D.; Iqbal, J.; Onuma, Y.; Zhang, Y.J.; Garcia-Garcia, H.M.; Morel, M.A.; Farooq, V.; Shiomi, H.; Furukawa, Y.; et al. Predictive Performance of SYNTAX Score II in Patients with Left Main and Multivessel Coronary Artery Disease-analysis of CREDO-Kyoto registry. Circ. J. 2014, 78, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Sotomi, Y.; Cavalcante, R.; van Klaveren, D.; Ahn, J.M.; Lee, C.W.; de Winter, R.J.; Wykrzykowska, J.J.; Onuma, Y.; Steyerberg, E.W.; Park, S.J.; et al. Individual Long-Term Mortality Prediction Following Either Coronary Stenting or Bypass Surgery in Patients with Multivessel and/or Unprotected Left Main Disease: An External Validation of the SYNTAX Score II Model in the 1,480 Patients of the BEST and PRECOMBAT Randomized Controlled Trials. JACC Cardiovasc. Interv. 2016, 9, 1564–1572. [Google Scholar] [PubMed]
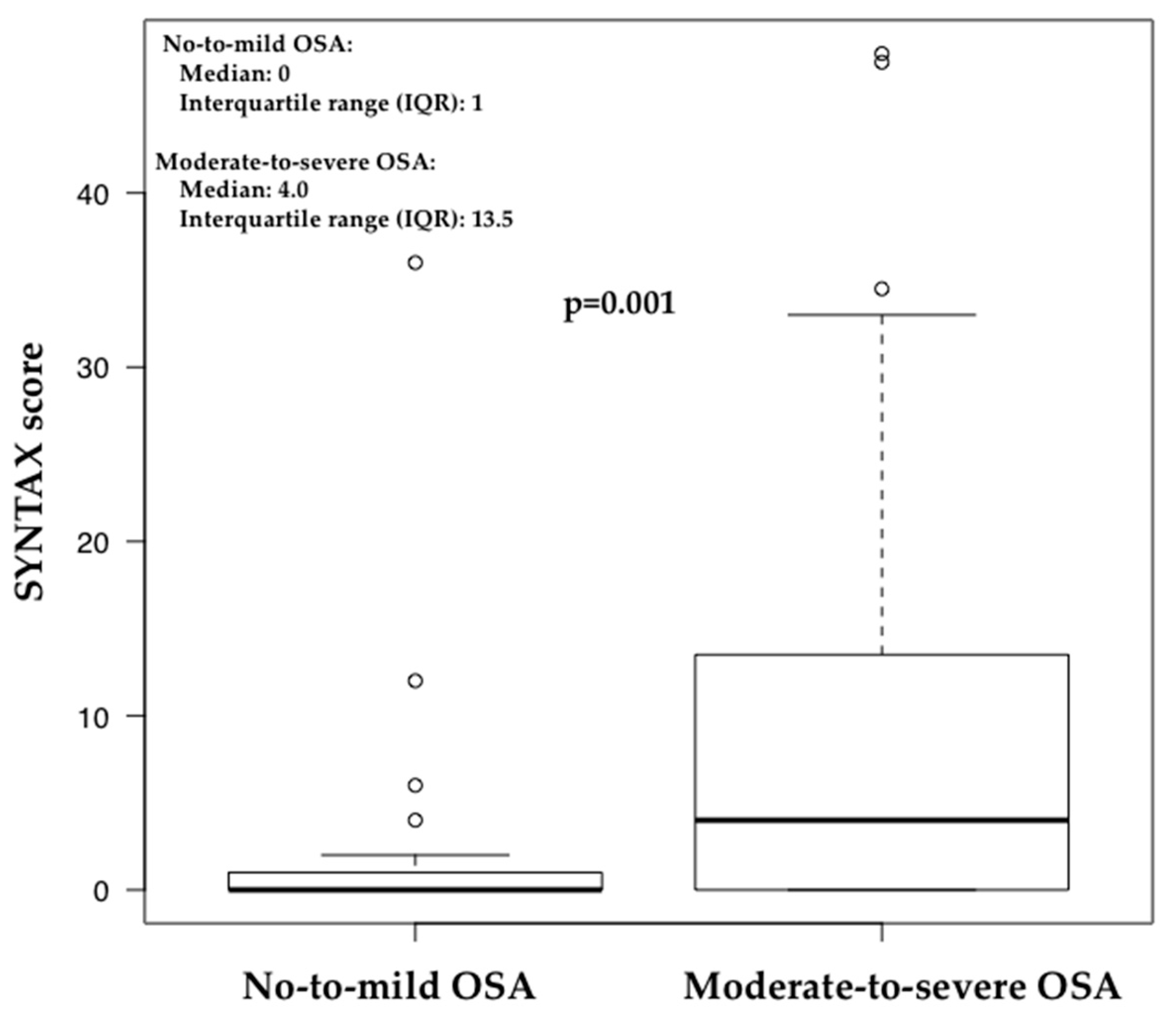
| No-to-Mild OSA (n = 23) | Moderate-to-Severe OSA (n = 75) | p Value | |
|---|---|---|---|
| Age, years | 56 ± 14 | 59 ± 12 | 0.232 |
| Male sex, n (%) | 22 (96%) | 68 (91%) | 0.672 |
| BMI, kg/m2 | 25.1 ± 2.9 | 26.4 ± 5.1 | 0.317 |
| Diabetes mellitus, n (%) | 5 (25%) | 26 (39.4%) | 0.295 |
| Family history, n (%) | 5 (25%) | 8 (12.3%) | 0.175 |
| Hyperlipidemia, n (%) | 9 (45%) | 37 (55.2%) | 0.454 |
| Hypertension, n (%) | 12 (52.2%) | 35 (47.3%) | 0.812 |
| Current smoking, n (%) | 6 (26.1%) | 17 (23.3%) | 0.784 |
| Obesity, n (%) | 9 (39.1%) | 43 (57.3%) | 0.155 |
| TST, min | 356.6 ± 56.6 | 309.8 ± 104.5 | 0.063 |
| REM, %TST | 13.9 ± 7.1 | 10.9 ± 6.0 | 0.068 |
| Stage N3, %TST | 9.9 ± 7.3 | 9.3 ± 9.5 | 0.796 |
| T90, % | 0.3 (1.25) | 10.9 (23.2) | <0.001 |
| Arousal index,/h | 25.3 ± 8.1 | 43.5 ± 19.7 | <0.001 |
| AHI,/h | 8.7 ± 4.0 | 41.0 ± 20.0 | <0.001 |
| OAHI,/h | 7.3 ± 4.3 | 36.2 ± 19.2 | <0.001 |
| Partial Correlations | p-Value | |
|---|---|---|
| Age, years | 0.29 | 0.011 |
| Male sex | 0.09 | 0.423 |
| Obesity (BMI ≥ 25 kg/m2) | 0.13 | 0.245 |
| Hypertension | 0.08 | 0.474 |
| Hyperlipidemia | 0.32 | 0.005 |
| Diabetes mellitus | 0.19 | 0.103 |
| Current smoking | 0.07 | 0.568 |
| Family history of CAD | 0.10 | 0.387 |
| Moderate-to-severe OSA | 0.24 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiwata, S.; Tomita, Y.; Ishiwata, S.; Narui, K.; Daida, H.; Kasai, T. Association between Obstructive Sleep Apnea and SYNTAX Score. J. Clin. Med. 2020, 9, 3314. https://doi.org/10.3390/jcm9103314
Ishiwata S, Tomita Y, Ishiwata S, Narui K, Daida H, Kasai T. Association between Obstructive Sleep Apnea and SYNTAX Score. Journal of Clinical Medicine. 2020; 9(10):3314. https://doi.org/10.3390/jcm9103314
Chicago/Turabian StyleIshiwata, Sayaki, Yasuhiro Tomita, Sugao Ishiwata, Koji Narui, Hiroyuki Daida, and Takatoshi Kasai. 2020. "Association between Obstructive Sleep Apnea and SYNTAX Score" Journal of Clinical Medicine 9, no. 10: 3314. https://doi.org/10.3390/jcm9103314
APA StyleIshiwata, S., Tomita, Y., Ishiwata, S., Narui, K., Daida, H., & Kasai, T. (2020). Association between Obstructive Sleep Apnea and SYNTAX Score. Journal of Clinical Medicine, 9(10), 3314. https://doi.org/10.3390/jcm9103314






