Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension—An Observational Study
Abstract
1. Introduction
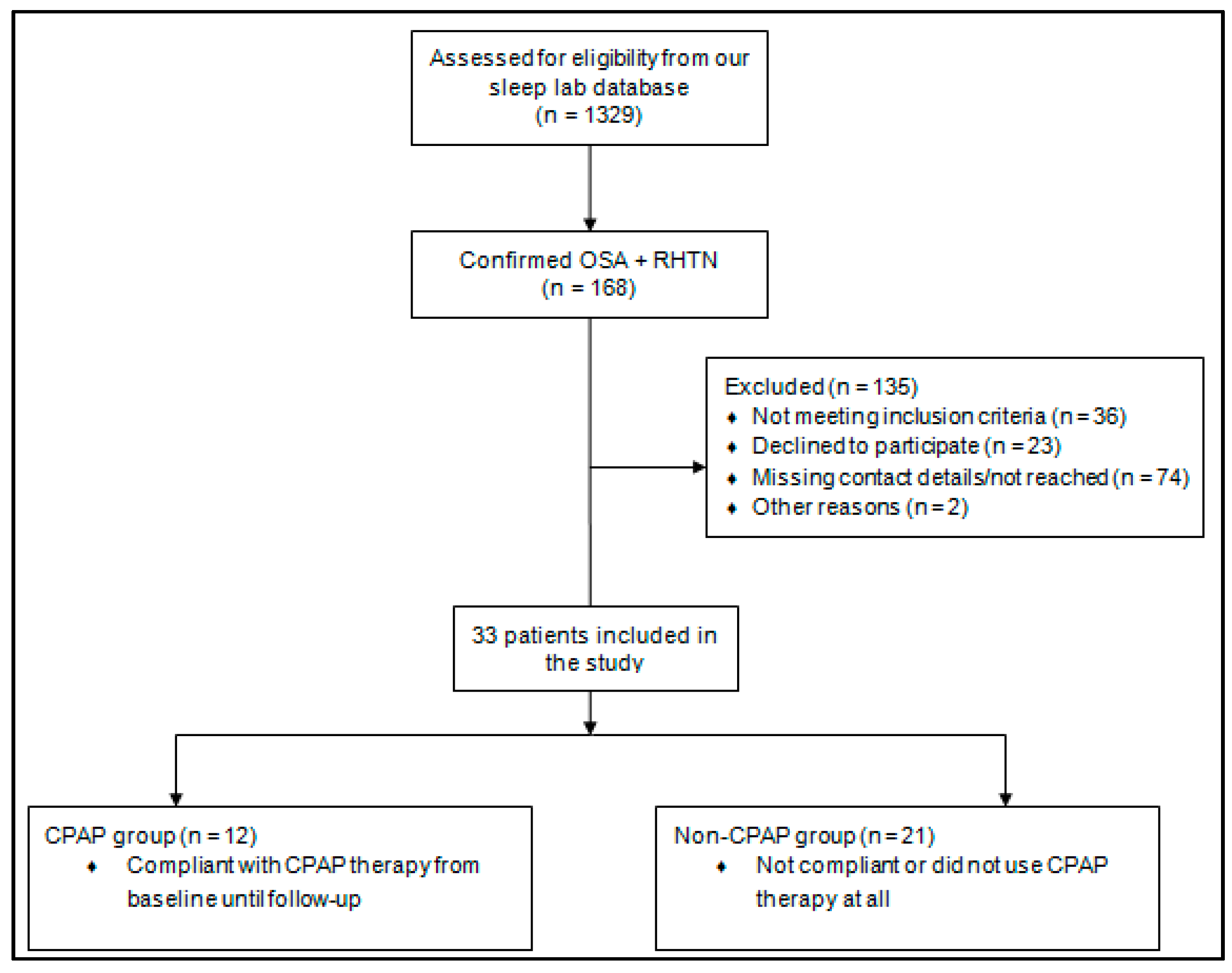
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics
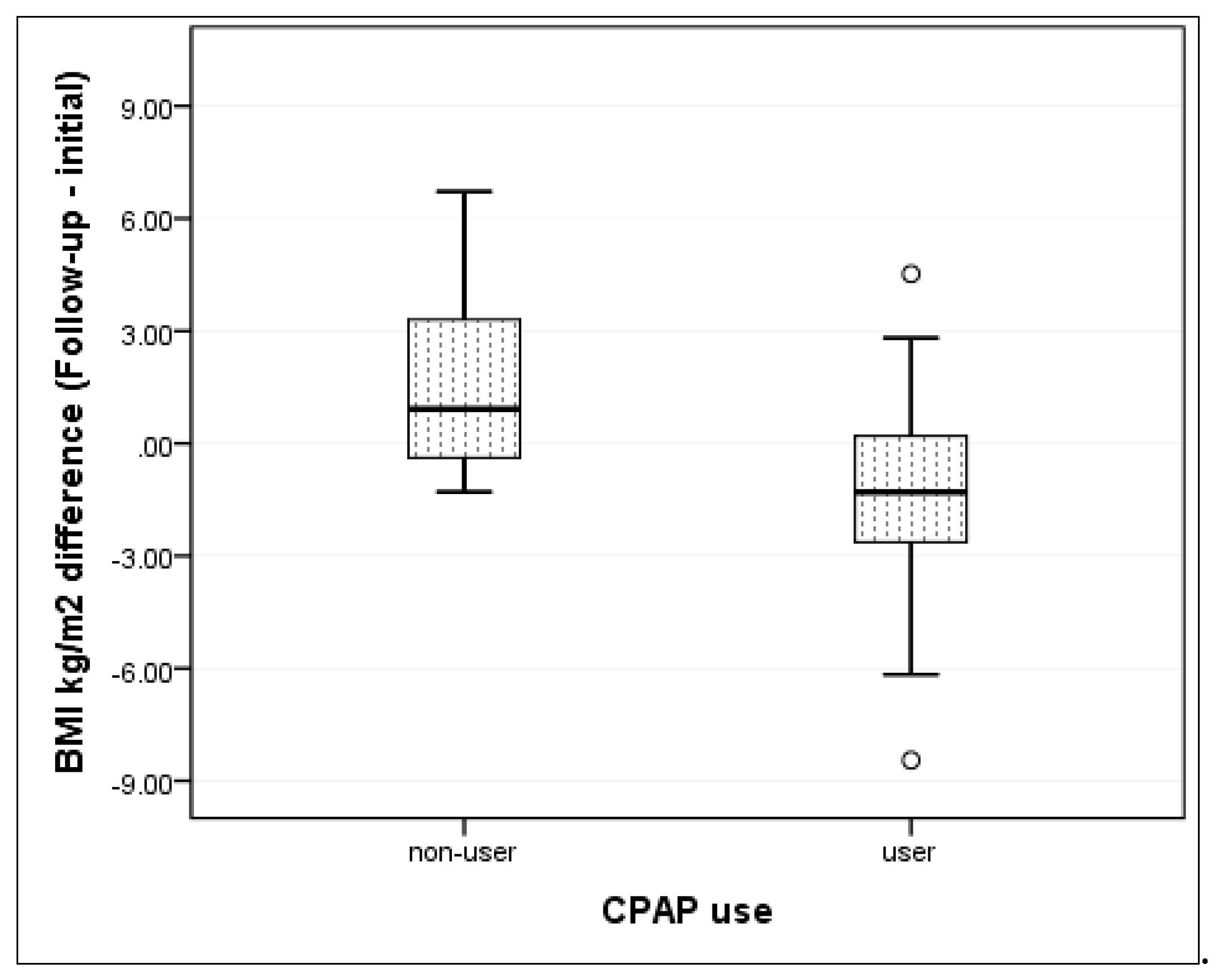
4.2. CPAP Effect on Change in BMI
4.3. CPAP Effect on HR and Prevalence of Cardiovascular Comorbidities
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant hypertension: Detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Persell, S.D. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011, 57, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Glaser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Dudenbostel, T.; Calhoun, D.A. Resistant hypertension, obstructive sleep apnoea and aldosterone. J. Hum. Hypertens. 2012, 26, 281–287. [Google Scholar] [CrossRef]
- Chiu, K.L.; Ryan, C.M.; Shiota, S.; Ruttanaumpawan, P.; Arzt, M.; Haight, J.S.; Chan, C.T.; Floras, J.S.; Bradley, T.D. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am. J. Respir. Crit. Care Med. 2006, 174, 1378–1383. [Google Scholar] [CrossRef]
- Tsioufis, C.; Kordalis, A.; Flessas, D.; Anastasopoulos, I.; Tsiachris, D.; Papademetriou, V.; Stefanadis, C. Pathophysiology of resistant hypertension: The role of sympathetic nervous system. Int. J. Hypertens. 2011, 2011, 642416. [Google Scholar] [CrossRef]
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef]
- Cowan, D.C.; Livingston, E. Obstructive sleep apnoea syndrome and weight loss: Review. Sleep Disord. 2012, 2012, 163296. [Google Scholar] [CrossRef]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Sala, C.; Grassi, G. Left-ventricular hypertrophy and obesity: A systematic review and meta-analysis of echocardiographic studies. J. Hypertens. 2014, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive sleep apnea in cardiovascular disease: A review of the literature and proposed multidisciplinary clinical management strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, H.K.; Hursel, R.; Rutters, F.; Martens, E.A.; Westerterp-Plantenga, M.S. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br. J. Nutr. 2013, 109, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; O’Keeffe, M.; Roberts, A.L.; Zammit, G.K.; RoyChoudhury, A.; St-Onge, M.P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R883–R889. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Chin, K.; Akamizu, T.; Morita, S.; Sumi, K.; Oga, T.; Matsumoto, H.; Niimi, A.; Tsuboi, T.; Fukuhara, S.; et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology 2008, 13, 810–816. [Google Scholar] [CrossRef]
- Loube, D.I.; Loube, A.A.; Erman, M.K. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J. Am. Diet. Assoc. 1997, 97, 896–897. [Google Scholar] [CrossRef]
- Garcia, J.M.; Sharafkhaneh, H.; Hirshkowitz, M.; Elkhatib, R.; Sharafkhaneh, A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir. Res. 2011, 12, 80. [Google Scholar] [CrossRef]
- Redenius, R.; Murphy, C.; O’Neill, E.; Al-Hamwi, M.; Zallek, S.N. Does CPAP lead to change in BMI? J. Clin. Sleep Med. 2008, 4, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Oscullo, G.; Torres, G.; Campos-Rodriguez, F.; Posadas, T.; Reina-Gonzalez, A.; Sapina-Beltran, E.; Barbe, F.; Martinez-Garcia, M.A. Resistant/Refractory hypertension and sleep apnoea: Current knowledge and future challenges. J. Clin. Med. 2019, 8, 1872. [Google Scholar] [CrossRef] [PubMed]
- Frent, S.M.; Tudorache, V.M.; Ardelean, C.; Mihaicuta, S. Long-term effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. Pneumologia 2014, 63, 204, 207–211. [Google Scholar] [PubMed]
- Liu, L.; Cao, Q.; Guo, Z.; Dai, Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: A meta-analysis of randomized controlled trials. J. Clin. Hypertens. (Greenwich) 2016, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, O.K.; Zou, D.; Tasbakan, M.S.; Hedner, J.; Ryan, S.; Verbraecken, J.; Escourrou, P.; Antalainen, U.; Kvamme, J.A.; Bonsignore, M.R.; et al. Change in weight and central obesity by positive airway pressure treatment in obstructive sleep apnea patients: Longitudinal data from the ESADA cohort. J. Sleep Res. 2018, 27, e12705. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008, 117, e510–e526. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb) 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Lenhard, W.L.A. Calculation of Effect Sizes. Available online: https://www.psychometrica.de/effect_size.html (accessed on 28 July 2020).
- Audrain-McGovern, J.; Benowitz, N.L. Cigarette smoking, nicotine, and body weight. Clin. Pharmacol. Ther. 2011, 90, 164–168. [Google Scholar] [CrossRef]
- Filozof, C.; Fernandez Pinilla, M.C.; Fernandez-Cruz, A. Smoking cessation and weight gain. Obes. Rev. 2004, 5, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [PubMed]
- Strauch, B.; Zelinka, T.; Hampf, M.; Bernhardt, R.; Widimsky, J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J. Hum. Hypertens. 2003, 17, 349–352. [Google Scholar] [CrossRef]
- Buffolo, F.; Li, Q.; Monticone, S.; Heinrich, D.A.; Mattei, A.; Pieroni, J.; Mei, M.; Yang, S.; Hu, Y.H.; Yang, M.C.; et al. Primary aldosteronism and obstructive sleep apnea: A cross-sectional multi-ethnic study. Hypertension 2019, 74, 1532–1540. [Google Scholar] [CrossRef]
- Ke, X.; Guo, W.; Peng, H.; Hu, C.; Zhang, H.; Peng, C.; Wang, X. Association of aldosterone excess and apnea-hypopnea index in patients with resistant hypertension. Sci. Rep. 2017, 7, 45241. [Google Scholar] [CrossRef]
- Chin, K.; Shimizu, K.; Nakamura, T.; Narai, N.; Masuzaki, H.; Ogawa, Y.; Mishima, M.; Nakamura, T.; Nakao, K.; Ohi, M. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 1999, 100, 706–712. [Google Scholar] [CrossRef]
- Harsch, I.A.; Schahin, S.P.; Radespiel-Troger, M.; Weintz, O.; Jahreiss, H.; Fuchs, F.S.; Wiest, G.H.; Hahn, E.G.; Lohmann, T.; Konturek, P.C.; et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2004, 169, 156–162. [Google Scholar] [CrossRef]
- Quan, S.F.; Budhiraja, R.; Clarke, D.P.; Goodwin, J.L.; Gottlieb, D.J.; Nichols, D.A.; Simon, R.D.; Smith, T.W.; Walsh, J.K.; Kushida, C.A. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J. Clin. Sleep Med. 2013, 9, 989–993. [Google Scholar] [CrossRef]
- Myllyla, M.; Kurki, S.; Anttalainen, U.; Saaresranta, T.; Laitinen, T. High adherence to CPAP treatment does not prevent the continuation of weight gain among severely obese OSAS patients. J. Clin. Sleep Med. 2016, 12, 519–528. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Shechter, A. Sleep disturbances, body fat distribution, food intake and/or energy expenditure: Pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 2014, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ursavas, A.; Ilcol, Y.O.; Nalci, N.; Karadag, M.; Ege, E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: Role of obesity. Ann. Thorac. Med. 2010, 5, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A. Effects of continuous positive airway pressure on energy balance regulation: A systematic review. Eur. Respir. J. 2016, 48, 1640–1657. [Google Scholar] [CrossRef]
- Drager, L.F.; Brunoni, A.R.; Jenner, R.; Lorenzi-Filho, G.; Bensenor, I.M.; Lotufo, P.A. Effects of CPAP on body weight in patients with obstructive sleep apnoea: A meta-analysis of randomised trials. Thorax 2015, 70, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Park, A.Y.; Kim, D.H.; Lee, J.G.; Park, S.; Cho, H.J. Neck circumference and lowest oxygen saturation are independently associated with high coexistence of hypertension in obstructive sleep apnea. Yonsei Med. J. 2014, 55, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.K.; Griffith, S.D.; Foldvary-Schaefer, N.; Thomas, G.; Bravo, E.L.; Moul, D.E.; Mehra, R. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016, 149, 747–755. [Google Scholar] [CrossRef]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S.; Sleep Heart Health, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef]
- Drager, L.F.; Bortolotto, L.A.; Pedrosa, R.P.; Krieger, E.M.; Lorenzi-Filho, G. Left atrial diameter is independently associated with arterial stiffness in patients with obstructive sleep apnea: Potential implications for atrial fibrillation. Int. J. Cardiol. 2010, 144, 257–259. [Google Scholar] [CrossRef]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Navarro-Soriano, C.; Reyes-Nunez, N.; Torres, G.; Caballero-Eraso, C.; Lloberes, P.; Diaz-Cambriles, T.; Somoza, M.; Masa, J.F.; Gonzalez, M.; et al. Good long-term adherence to continuous positive airway pressure therapy in patients with resistant hypertension and sleep apnea. J. Sleep Res. 2019, 28, e12805. [Google Scholar] [CrossRef]
- Soper, D.S. Post-hoc Statistical Power Calculator for a Student t-Test. Available online: http://www.danielsoper.com/statcalc (accessed on 18 August 2020).


| Baseline Demographic and Anthropometric Characteristics | Non-CPAP Group (n = 21) | CPAP Group (n = 12) | p Value | |
|---|---|---|---|---|
| Gender | Men no. (%) | 11 (52.4) | 7 (58.3) | 0.741 * |
| Women no. (%) | 10 (47.6) | 5 (41.7) | ||
| Age (years) | Mean (SD) | 54.1 (8.3) | 55.6 (6) | 0.602 ** |
| Smoking status | Never no. (%) | 8 (38.1) | 9 (75) | 0.041 * |
| Former smoker or active smoker no. (%) | 13 (61.9) | 3 (25) | ||
| Alcohol consumption | No no. (%) | 7 (33.3) | 5 (41.7) | 0.716 * |
| Yes no. (%) | 14 (66.7) | 7 (58.3) | ||
| Systolic blood pressure (mmHg) | Mean (SD) | 152.9 (17.9) | 147.9 (19.7) | 0.468 ** |
| Diastolic blood pressure (mmHg) | Mean (SD) | 95.7 (16.3) | 95.83 (13.8) | 0.983 ** |
| BMI (kg/m2) | Mean (SD) | 36.4 (5.6) | 38.2 (8.9) | 0.538 ** |
| Neck circumference (cm) | Mean (SD) | 44.8 (3.7) | 45.9 (4.9) | 0.471 ** |
| Abdominal circumference (cm) | Mean (SD) | 121.6 (9.8) | 125.9 (17.9) | 0.455 ** |
| Hip circumference (cm) | Mean (SD) | 119.6 (9.6) | 126.9 (20) | 0.251 ** |
| Epworth score | Mean (SD) | 12.6 (4.6) | 11.7 (4.8) | 0.578 ** |
| Baseline Polysomnographic Parameters | Non-CPAP Group (n = 21) | CPAP Group (n = 12) | p Value | |
|---|---|---|---|---|
| AHI, no. of events/h | Mean (SD) | 47.1 (17.9) | 65.8 (23.3) | 0.015 * |
| Desaturation index | Mean (SD) | 30.7 (21.9) | 51.2 (31.9) | 0.039 * |
| Mean apnea duration (s) | Mean (SD) | 21(4.3) | 21.8 (4.1) | 0.585 * |
| Episodes over 5 min with SpO2 under 88% | Median (IQR) | 0(0) | 0(2) | 0.326 ** |
| Min SpO2 over 2 sec | Mean (SD) | 75.3 (12.3) | 67.1 (21.8) | 0.272 * |
| Maximum duration SpO2 under 88% | Median (IQR) | 0.3 (1.1) | 0.4 (8) | 0.927 ** |
| Average Heart Rate (beats/min) | Mean (SD) | 70.7 (13.0) | 69.2 (13.5) | 0.769 * |
| Non-CPAP Group (n = 21) | CPAP Group (n = 12) | |||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
| Alpha blockers, no. (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Beta-blockers, no. (%) | 15 (71.4) | 15 (71.4) | 8 (66.7) | 7 (58.3) |
| Calcium channel blockers, no. (%) | 13 (61.9) | 15 (71.4) | 7 (58.3) | 7 (58.3) |
| Diuretics, no. (%) | 21 (100) | 20 (95) | 12 (100) | 8 (66.7) |
| ACE inhibitors, no. (%) | 13 (61.9) | 9 (42.9) | 6 (50) | 4 (33.3) |
| Angiotensin II receptor blockers, no. (%) | 10 (47.6) | 11 (52.4) | 6 (50) | 8 (66.7) |
| Vasodilators, no. (%) | 2 (9.5) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Alpha-2 Receptor Agonists, no. (%) | 0 (0) | 2 (9.5) | 0 (0) | 0 (0) |
| Measurements | CPAP-Group (n = 12) | Non-CPAP Group (n = 21) |
|---|---|---|
| Kappa (coefficient of agreement) | 0.664 | 0.467 |
| Percentage of patients which maintained the class of severity of obesity | 75% (9) | 61.9% (13) |
| Percentage of patients which increased severity of obesity | 8.3% (1) | 23.8% (5) |
| Percentage of patients which decreased severity of obesity | 16.6% (2) | 14.3% (3) |
| Difference between Initial and Follow-Up Evaluation in Anthropometrical Parameters | Non-CPAP Group (n = 21) | CPAP Group (n = 12) | p Value |
|---|---|---|---|
| BMI (kg/m2) mean ± SD | −1.6 (±2.5) | 1.4 (±3.5) | 0.006 * |
| Neck circumference (cm) mean ± SD | −0.1 (±2.5) | 2.0 (±4.1) | 0.078 * |
| Abdominal circumference (cm) mean ± SD | 0.5 (±11.9) | 7.3 (±8.6) | 0.095 * |
| Hip circumference (cm) mean ± SD | −0.1 (±26.3) | 7.0 (±11.5) | 0.385 * |
| Difference between Initial and Follow-up Evaluation in Polysomnographic Parameters | Non-CPAP Group (n = 21) | CPAP Group (n = 12) | p Value |
|---|---|---|---|
| Mean apnea duration (s) (mean ± SD) | −0.6 (±4.8) | 2.2 (±5.0) | 0.127 * |
| Episodes over 5 min with SpO2 under 88% (mean ± SD) | −1.3 (±3.4) | 1.8 (±4.9) | 0.020 * |
| Min SpO2 over 2 sec (mean ± SD) | −3.4 (±13.5) | −13.3 (±20.7) | 0.138 * |
| Maximum duration SpO2 under 88% (mean ± SD) | −5.5 (±13.5) | 1.7 (±16.7) | 0.596 * |
| Desaturation index (mean ± SD) | −1.6 (±23.0) | 30.2 (±31.0) | 0.003 * |
| Mean desaturation (mean ± SD) | 1.4 (±2.7) | −3.0 (±7.4) | 0.027 * |
| AHI, no. of events/h (mean ± SD) | 5.6 (±24.2) | 39.5 (±27.9) | 0.001 * |
| Epworth scale (mean ± SD) | 0.4 (±5.1) | 4.3 (±6.2) | 0.056 * |
| Baseline | Follow-Up | |||
|---|---|---|---|---|
| CPAP-Group (n = 12) | Non-CPAP Group (n = 21) | CPAP-Group (n = 12) | Non-CPAP Group (n = 21) | |
| Average HR, beats/min mean (SD) | 69.2 (13.5) | 70.7 (13.0) | 58.6 (9.5) | 67.8 (7.8) |
| Arrhythmias (no./%) | 6 (50.0) | 6 (28.6) | 3 (25) | 9 (42.9) |
| HF (no./%) | 4 (33.3) | 8 (38.1) | 4 (33.3) | 11 (52.4) |
| Stroke (no./%) | 2 (16.7) | 0 (0) | 2 (16.7) | 1 (4.8) |
| CAD (no./%) | 8 (66.7) | 13 (61.9) | 8 (66.7) | 18 (85.7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleava, R.; Mihaicuta, S.; Serban, C.L.; Ardelean, C.; Marincu, I.; Gaita, D.; Frent, S. Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension—An Observational Study. J. Clin. Med. 2020, 9, 2802. https://doi.org/10.3390/jcm9092802
Pleava R, Mihaicuta S, Serban CL, Ardelean C, Marincu I, Gaita D, Frent S. Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension—An Observational Study. Journal of Clinical Medicine. 2020; 9(9):2802. https://doi.org/10.3390/jcm9092802
Chicago/Turabian StylePleava, Roxana, Stefan Mihaicuta, Costela Lacrimioara Serban, Carmen Ardelean, Iosif Marincu, Dan Gaita, and Stefan Frent. 2020. "Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension—An Observational Study" Journal of Clinical Medicine 9, no. 9: 2802. https://doi.org/10.3390/jcm9092802
APA StylePleava, R., Mihaicuta, S., Serban, C. L., Ardelean, C., Marincu, I., Gaita, D., & Frent, S. (2020). Long-Term Effects of Continuous Positive Airway Pressure (CPAP) Therapy on Obesity and Cardiovascular Comorbidities in Patients with Obstructive Sleep Apnea and Resistant Hypertension—An Observational Study. Journal of Clinical Medicine, 9(9), 2802. https://doi.org/10.3390/jcm9092802







