The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. The AktivA Program
2.3. The Physiotherapist Certification Course
2.4. The Patient Education and Exercise Program
2.5. The AktivA Quality Register
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015, 49, 1554–1557. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Hernandez-Molina, G.; Reichenbach, S. Exercise for osteoarthritis of the hip. Cochrane Database Syst. Rev. 2014, 4, CD007912. [Google Scholar] [CrossRef]
- Regnaux, J.P.; Lefevre-Colau, M.M.; Trinquart, L.; Nguyen, C.; Boutron, I.; Brosseau, L.; Ravaud, P. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst. Rev. 2015, CD010203. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Kraus, V.B.; Sprow, K.; Powell, K.E.; Buchner, D.; Bloodgood, B.; Piercy, K.; George, S.M.; Kraus, W.E.; Physical Activity Guidelines Advisory Committee. Effects of Physical Activity in Knee and Hip Osteoarthritis: A Systematic Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Hagen, K.B.; Smedslund, G.; Osteras, N.; Jamtvedt, G. Quality of Community-Based Osteoarthritis Care: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016, 68, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Basedow, M.; Runciman, W.B.; Lipworth, W.; Esterman, A. Australian general practitioner attitudes to clinical practice guidelines and some implications for translating osteoarthritis care into practice. Aust. J. Prim. Health 2016, 22, 403–408. [Google Scholar] [CrossRef]
- Eyles, J.P.; Hunter, D.J.; Bennell, K.L.; Dziedzic, K.S.; Hinman, R.S.; van der Esch, M.; Holden, M.A.; Bowden, J.L.; Joint Effort Initiative Members. Priorities for the effective implementation of osteoarthritis management programs: An OARSI international consensus exercise. Osteoarthr. Cartil. 2019, 27, 1270–1279. [Google Scholar] [CrossRef]
- Teo, P.L.; Hinman, R.S.; Egerton, T.; Dziedzic, K.S.; Bennell, K.L. Identifying and Prioritizing Clinical Guideline Recommendations Most Relevant to Physical Therapy Practice for Hip and/or Knee Osteoarthritis. J. Orthop. Sports Phys. 2019, 49, 501–512. [Google Scholar] [CrossRef]
- Brand, C.; Hunter, D.; Hinman, R.; March, L.; Osborne, R.; Bennell, K. Improving care for people with osteoarthritis of the hip and knee: How has national policy for osteoarthritis been translated into service models in Australia? Int. J. Rheum. Dis. 2011, 14, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Towler, S.C.; Speerin, R.; March, L.M. Models of care for musculoskeletal health in Australia: Now more than ever to drive evidence into health policy and practice. Aust. Health Rev. 2014, 38, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.; Risberg, M.A.; Roos, E.M.; Skou, S.T. A Pragmatic Approach to the Implementation of Osteoarthritis Guidelines Has Fewer Potential Barriers Than Recommended Implementation Frameworks. J. Orthop. Sports Phys. 2019, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zadro, J.; O’Keeffe, M.; Maher, C. Do physical therapists follow evidence-based guidelines when managing musculoskeletal conditions? Systematic review. BMJ Open 2019, 9, e032329. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Ekvall Hansson, E.; Thorstensson, C.A.; Eek, F.; Bergman, P.; Dahlberg, L.E. The effect of education and supervised exercise on physical activity, pain, quality of life and self-efficacy—An intervention study with a reference group. BMC Musculoskelet. Disord. 2018, 19, 198. [Google Scholar] [CrossRef]
- Skou, S.T.; Roos, E.M. Good Life with osteoArthritis in Denmark (GLA:D): Evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet. Disord. 2017, 18, 72. [Google Scholar] [CrossRef]
- Skou, S.T.; Bricca, A.; Roos, E.M. The impact of physical activity level on the short- and long-term pain relief from supervised exercise therapy and education: A study of 12,796 Danish patients with knee osteoarthritis. Osteoarthr. Cartil. 2018. [Google Scholar] [CrossRef]
- LaMorte, W.W. The Transtheoretical Model (Stages of Change). Available online: http://sphweb.bumc.bu.edu/otlt/MPH-Modules/SB/BehavioralChangeTheories/BehavioralChangeTheories6.html (accessed on 9 September 2019).
- Zhang, W.; Doherty, M.; Peat, G.; Bierma-Zeinstra, M.A.; Arden, N.K.; Bresnihan, B.; Herrero-Beaumont, G.; Kirschner, S.; Leeb, B.F.; Lohmander, L.S.; et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 483–489. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Stensrud, S.; Roos, E.M.; Risberg, M.A. A 12-week exercise therapy program in middle-aged patients with degenerative meniscus tears: A case series with 1-year follow-up. J. Orthop. Sports Phys. 2012, 42, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Nilsdotter, A.K.; Lohmander, L.S.; Klassbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS)—Validity and responsiveness in total hip replacement. Bmc Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. 1998, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Stratford, P.W.; Riddle, D.L. Assessing the amount of change in an outcome measure is not the same as assessing the importance of change. Physiother. Can. 2013, 65, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Moseng, T.; Tveter, A.; Holm, I.; Dagfinrud, H. Pasient-Spesifikk Funksjons Skala—Et nyttig verktøy for fysioterapeuter i primærhelsetjenesten. Fysioterapeuten 2013, 2, 20–26. [Google Scholar]
- Moholdt, T.; Wisloff, U.; Lydersen, S.; Nauman, J. Current physical activity guidelines for health are insufficient to mitigate long-term weight gain: More data in the fitness versus fatness debate (The HUNT study, Norway). Br. J. Sports Med. 2014, 48, 1489–1496. [Google Scholar] [CrossRef]
- Osteras, N.; Garratt, A.; Grotle, M.; Natvig, B.; Kjeken, I.; Kvien, T.K.; Hagen, K.B. Patient-reported quality of care for osteoarthritis: Development and testing of the osteoarthritis quality indicator questionnaire. Arthritis Care Res. 2013, 65, 1043–1051. [Google Scholar] [CrossRef]
- Brand, E.; Nyland, J.; Henzman, C.; McGinnis, M. Arthritis self-efficacy scale scores in knee osteoarthritis: A systematic review and meta-analysis comparing arthritis self-management education with or without exercise. J. Orthop. Sports Phys. 2013, 43, 895–910. [Google Scholar] [CrossRef]
- Chatman, A.B.; Hyams, S.P.; Neel, J.M.; Binkley, J.M.; Stratford, P.W.; Schomberg, A.; Stabler, M. The Patient-Specific Functional Scale: Measurement properties in patients with knee dysfunction. Phys. Ther. 1997, 77, 820–829. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C.; The American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef]
- Osteras, N.; Tveter, A.T.; Garratt, A.M.; Svinoy, O.E.; Kjeken, I.; Natvig, B.; Grotle, M.; Hagen, K.B. Measurement properties for the revised patient-reported OsteoArthritis Quality Indicator questionnaire. Osteoarthr. Cartil. 2018, 26, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis of Behavioural Sciences; Routledge: Abingdon, UK, 1988. [Google Scholar]
- Allen, K.D. Cost-effectiveness of physical activity and exercise therapy programs for knee osteoarthritis: Making the case for health plan coverage. Osteoarthr. Cartil. 2020, 28, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Eek, F.; Dell’Isola, A.; Dahlberg, L.E.; Ekvall Hansson, E. The Better Management of Patients with Osteoarthritis Program: Outcomes after evidence-based education and exercise delivered nationwide in Sweden. PLoS ONE 2019, 14, e0222657. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Olsson, C.B.; Ekelund, J.; Degerstedt, A.; Thorstensson, C.A. Change in self-efficacy after participation in a supported self-management program for osteoarthritis—An observational study of 11 906 patients. Disabil. Rehabil. 2019, 1–8. [Google Scholar] [CrossRef]
- Thygesen, L.C.; Ersboll, A.K. When the entire population is the sample: Strengths and limitations in register-based epidemiology. Eur. J. Epidemiol. 2014, 29, 551–558. [Google Scholar] [CrossRef]


| Baseline | 3 Months | 12 Months | 24 Months | |
|---|---|---|---|---|
| Demographic variables | x | |||
| Height/weight | x | x | x | x |
| Pain (NRS) and complaints | x | x | x | x |
| HOOS/KOOS sports/recreational activities (SP) and disease-specific quality of life (QoL) [23,24] | x | x | x | x |
| Quality of life (EuroQol, EQ5D) | x | x | x | x |
| Patient-specific functional scale (PSFS) [25,26] | x | x | x | x |
| Physical activity (from HUNT questionnaire) [27] | x | x | x | x |
| Fear of physical activity | x | x | x | x |
| Osteoarthritis quality indicator questionnaire (OA-QI) [28] | x | x | x | x |
| Arthritis Self-efficacy scale (ASES) [29] | x | x | x | x |
| Total Population N = 6245 | Knee OA N = 3880 | Hip OA N = 1863 | |
|---|---|---|---|
| Age | 63.5 ± 9.7 | 63 ± 9.7 | 64 ± 9.8 |
| women/men, % | 75/25 | 74/26 | 77/23 |
| Body weight | 82.7 ± 17.7 | 83.7 ± 16.6 | 78.5 ± 15.2 |
| Body mass index | 28 ± 4.9 | 28.6 ± 5 | 27 ± 4.6 |
| Normal weight/overweight/obese, % | 28/42/30 | 24/43/33 | 35/42/23 |
| Pain intensity last month (Lickert scale, 0–10) | 5.1 ± 1.9 | 5.2 ± 1.8 | 5.2 ± 1.8 |
| Pain every day or always, % | 81 | 80 | 81 |
| On sick leave yes/no, % | 17/83 | 18/82 | 16/84 |
| ASES 1 pain | 58 ± 19 | 60 ± 19 | 56 ± 19 |
| ASES symptoms | 60 ± 18 | 61± 17 | 59 ± 18 |
| Physical activity level, low/moderate/high, % | 57/12/31 | 56/13/31 | 56/12/32 |
| Fear of physical activity, yes/no, % | 20/80 | 22/78 | 16/84 |
| Outcome | T0 Mean (SD 1) | T3 Mean (SD) | Mean Difference T0–T3 (CI 2) | Effect Size 8 | T12 Mean (SD) | Mean Difference T0–T12 (CI) | Effect Size | T24 Mean (SD) | Mean Difference T0–T24 (CI) | Effect Size |
|---|---|---|---|---|---|---|---|---|---|---|
| Pain (NRS 3 1–10) | 5.2 (1.8) | 4.5 (2.0) | −0.6 (−0.7–−0.6) | 0.33 | 4.3 (2.1) | −0.8 (−0.9–−0.7) | 0.37 | 4.1 (2.2) | −0.9 (−1.0–−0.8) | 0.39 |
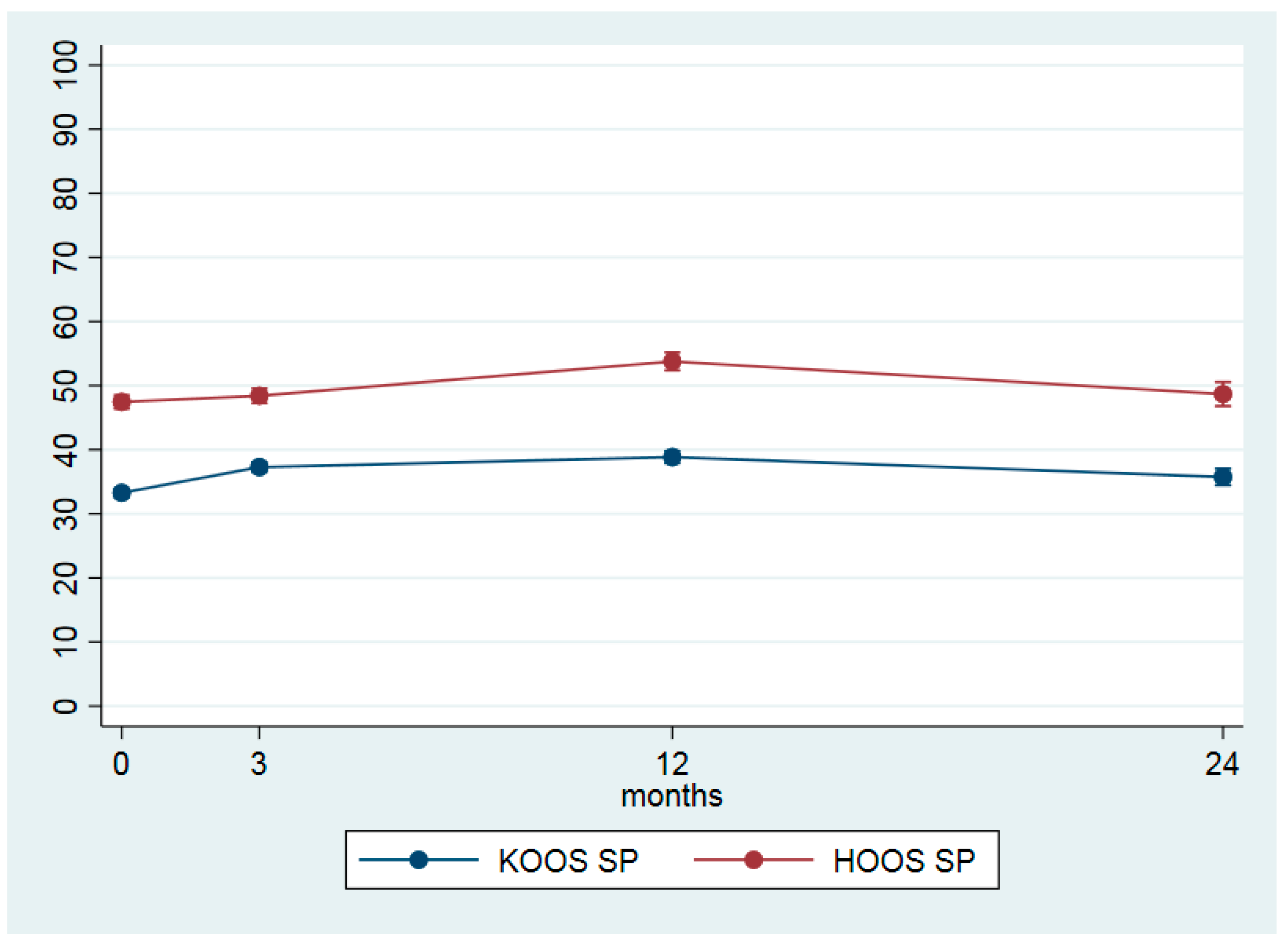
| KOOS 4 sport (0–100) | 33.3 (21.4) | 37.4 (22.4) | 4.0 (3.0–5.0) | 0.21 | 39.2 (24.5) | 5.6 (4.4–6.8) | 0.26 | 36.3 (26.6) | 2.5 (0.9–4.2) | 0.11 |
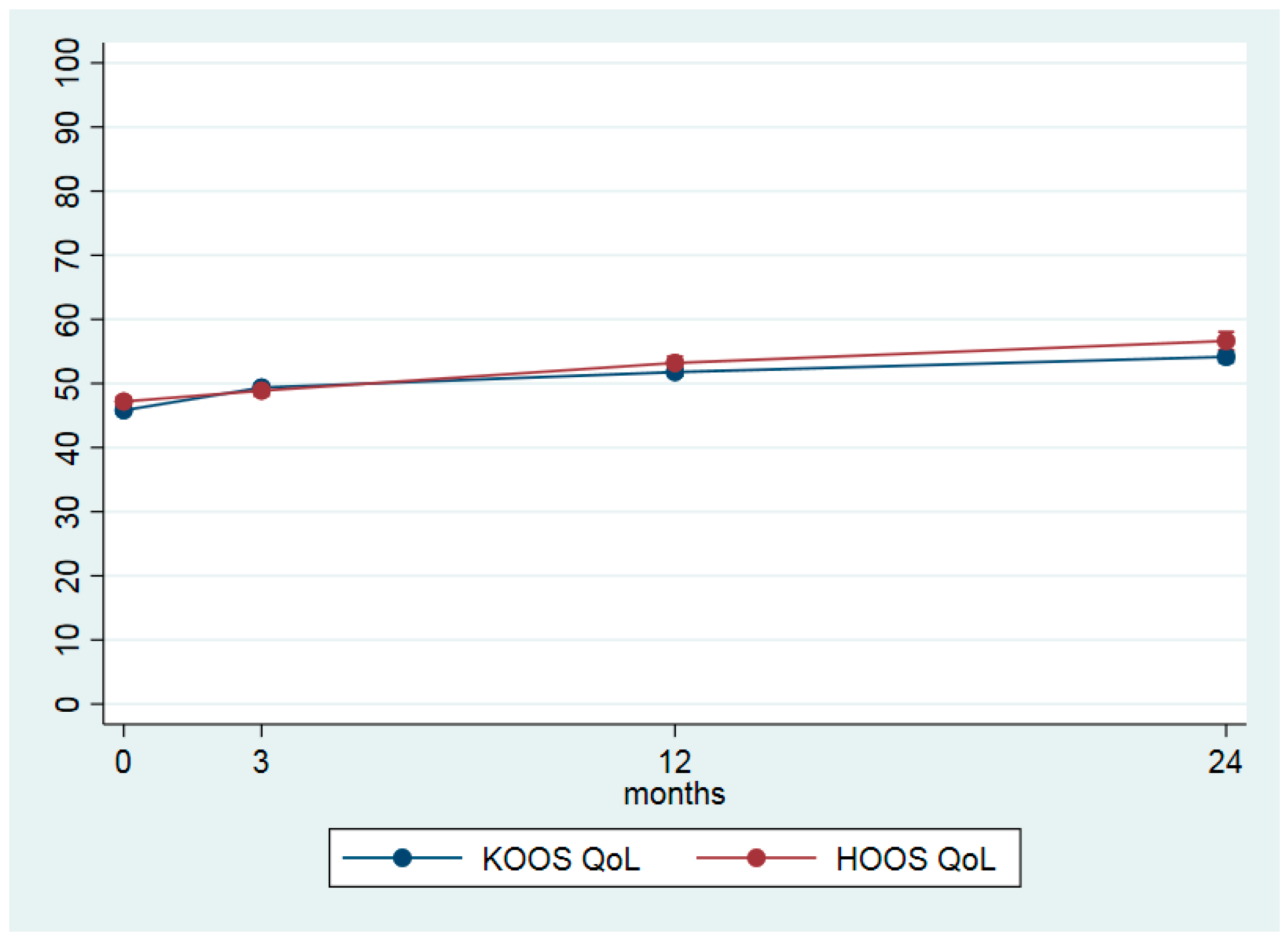
| KOOS QoL (0–100) | 45.8 (14.5) | 49.6 (15.4) | 3.5 (2.9–4.2) | 0.27 | 52.3 (17.0) | 6.0 (5.1–6.8) | 0.38 | 55.3 (18.3) | 8.3 (7.2–9.5) | 0.49 |
| HOOS 5 sport (0–100) | 47.5 (21.9) | 48.7 (22.6) | 1.0 (−0.5–2.4) | 0.04 | 54.8 (25.2) | 6.3 (4.5–8.1) | 0.25 | 51.0 (26.5) | 1.2 (−1.2–3.7) | 0.02 |
| HOOS QoL (0–100) | 47.2 (14.8) | 49.0 (16.0) | 1.7 (0.6–2.8) | 0.12 | 53.9 (17.7) | 6.0 (4.6–7.4) | 0.32 | 58.0 (19.9) | 9.4 (7.5–11.4) | 0.41 |
| PSFS 6 1 (0–10) | 3.3 (2.4) | 4.1 (2.9) | 0.7 (0.6–0.8) | 0.28 | 4.5 (3.1) | 1.1 (0.9–1.2) | 0.37 | 4.5 (3.1) | 1.1 (1.0–1.3) | 0.37 |
| EQ-5D 7 health (0–100) | 64.1 (19.5) | 66.5 (19.5) | 2.2 (1.5–3.0) | 0.10 | 68.2 (18.7) | 3.5 (2.5–4.4) | 0.14 | 70.7 (17.6) | 6.0 (4.7–7.3) | 0.28 |
| EQ-5D utility index (0–1) | 0.721 (0.111) | 0.746 (0.115) | 0.025 (0.019–0.029) | 0.16 | 0.759 (0.120) | 0.036 (0.029–0.043) | 0.27 | 0.773 (0.117) | 0.048 (0.039–0.057) | 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holm, I.; Pripp, A.H.; Risberg, M.A. The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up. J. Clin. Med. 2020, 9, 3112. https://doi.org/10.3390/jcm9103112
Holm I, Pripp AH, Risberg MA. The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up. Journal of Clinical Medicine. 2020; 9(10):3112. https://doi.org/10.3390/jcm9103112
Chicago/Turabian StyleHolm, Inger, Are Hugo Pripp, and May Arna Risberg. 2020. "The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up" Journal of Clinical Medicine 9, no. 10: 3112. https://doi.org/10.3390/jcm9103112
APA StyleHolm, I., Pripp, A. H., & Risberg, M. A. (2020). The Active with OsteoArthritis (AktivA) Physiotherapy Implementation Model: A Patient Education, Supervised Exercise and Self-Management Program for Patients with Mild to Moderate Osteoarthritis of the Knee or Hip Joint. A National Register Study with a Two-Year Follow-Up. Journal of Clinical Medicine, 9(10), 3112. https://doi.org/10.3390/jcm9103112






