Creatinine to Body Weight Ratio Is Associated with Incident Diabetes: Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
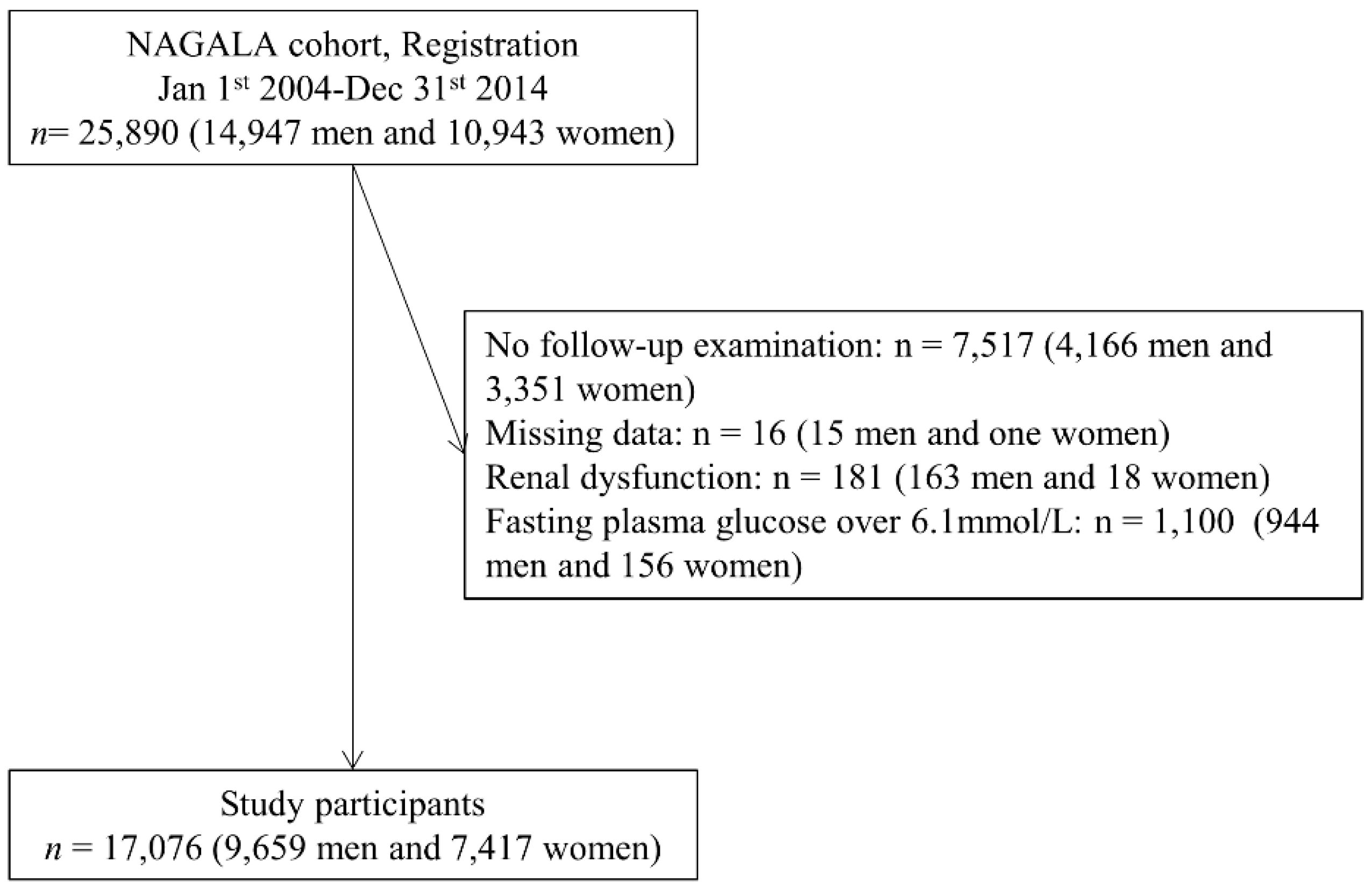
2.1. Study Patients
2.2. Data Collection and Measurements
2.3. Statistical Analysis
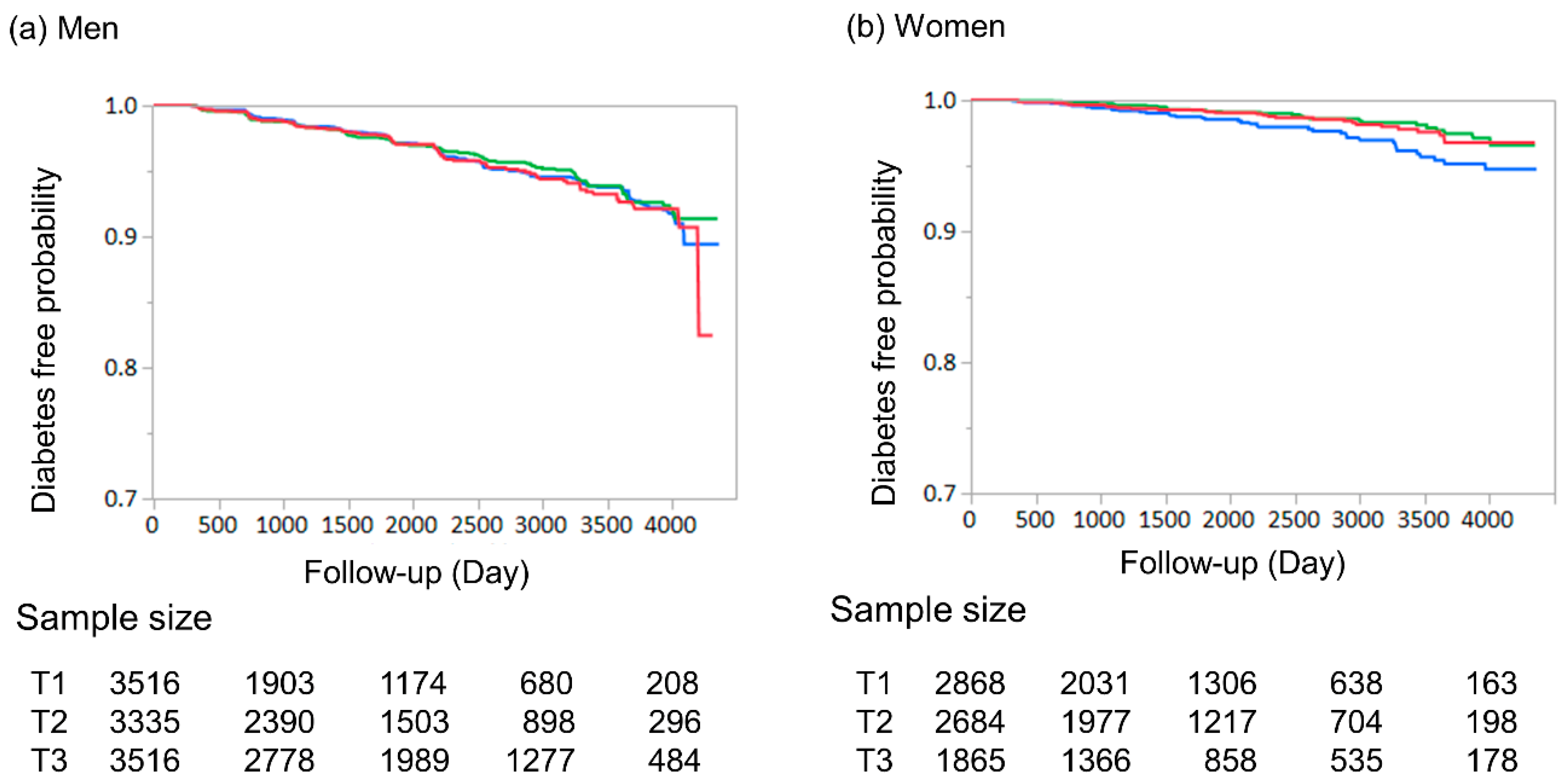
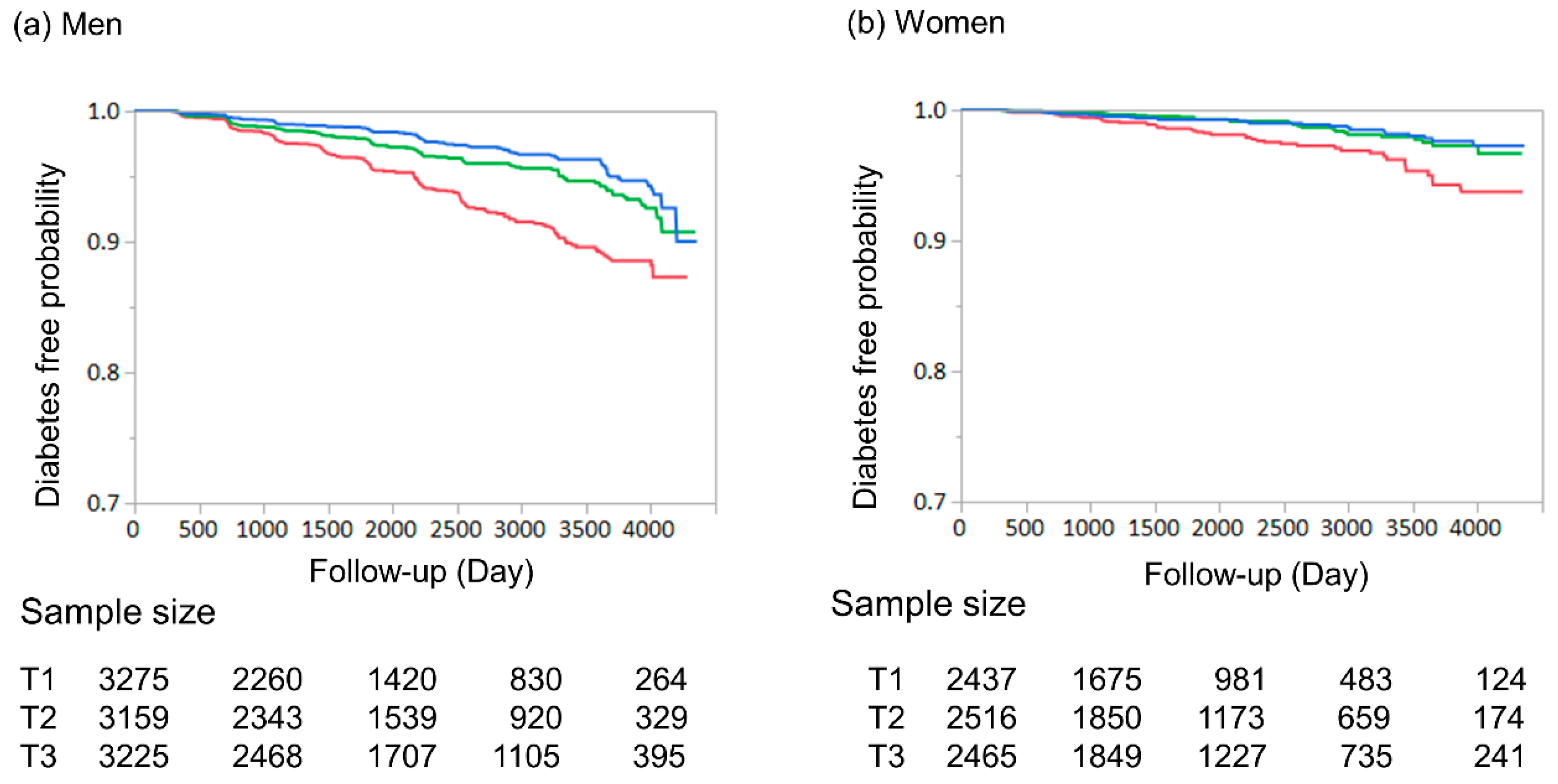
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Health, Labour and Welfare Statistics Association. Journal of Health and Welfare Statistics 2016–2017. (In Japanese) Tokyo Japan. Available online: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku.pdf2019 (accessed on 28 March 2019).
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. 2019, 43, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Hashimoto, Y.; Tanaka, M.; Toda, H.; Matsumoto, S.; Ushigome, E.; Asano, M.; Yamazaki, M.; Oda, Y.; Fukui, M. Combined effect of body mass index and waist-height ratio on incident diabetes; a population based cohort study. J. Clin. Biochem. Nutr. 2017, 61, 118–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Lien, A.S.; Hwang, J.S.; Jiang, Y.D. Diabetes related fatigue sarcopenia, frailty. J. Diabetes Investig. 2018, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Someya, Y.; Tamura, Y.; Suzuki, R.; Kaga, H.; Kadowaki, S.; Sugimoto, D.; Kakehi, S.; Funayama, T.; Furukawa, Y.; Takeno, K.; et al. Characteristics of Glucose Metabolism in Underweight Japanese Women. J. Endocr. Soc. 2018, 2, 279–289. [Google Scholar] [CrossRef]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Osaka, T.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. The The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr. J. 2016, 63, 877–884. [Google Scholar] [CrossRef]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Peng, T.C.; Wu, L.W.; Chen, W.L.; Liaw, F.Y.; Chang, Y.W.; Kao, T.W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): The importance of sarcopenia definition. Clin. Nutr. 2019, 38, 422–428. [Google Scholar] [CrossRef]
- Osaka, T.; Hashimoto, Y.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Relationship between skeletal muscle mass and hepatic fibrosis in patients with type 2 diabetes. Diabetes Metab. 2017, 43, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Viollet, L.; Gailey, S.; Thornton, D.J.; Friedman, N.R.; Flanigan, K.M.; Mahan, J.D.; Mendell, J.R. Utility of cystatin C to monitor renal function in Duchenne muscular dystrophy. Muscle Nerve 2009, 40, 438–442. [Google Scholar] [CrossRef]
- Griggs, R.C.; Forbes, G.; Moxley, R.T.; Herr, B.E. The assessment of muscle mass in progressive neuromuscular disease. Neurology 1983, 33, 158–165. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Creatinine-to-bodyweight ratio is a predictor of incident non-alcoholic fatty liver disease: A population-based longitudinal study. Hepatol. Res. 2019, in press. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef]
- Evaluation of Elevated Creatinine. Available online: https://bestpractice.bmj.com/topics/en-us/935 (accessed on 4 May 2019).
- Hashimoto, Y.; Okamura, T.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Impact of respiratory function on the progression from metabolically healthy non-overweight to metabolically abnormal phenotype. Nutr. Metab. Cardiov. Dis. 2018, 28, 922–928. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Kim, D.I.; Kim, W.S.; Suh, B.S. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin. Chem. 2007, 53, 71–77. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2012, 35 (Suppl. 1), S64–S71. [Google Scholar]
- Fukuda, Y.; Hashimoto, Y.; Hamaguchi, M.; Fukuda, T.; Nakamura, N.; Ohbora, A.; Kato, T.; Kojima, T.; Fukui, M. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 2016, 36, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, Y.; Sato, S.; Kato, T.; Nakayama, H.; Yamada, K. Surrogate index for insulin sensitivity composed of factors not using glucose and insulin in Japanese patients with diabetes. J. Diabetes Investig. 2011, 2, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Atherosclerosis Risk in Communities Study. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lee, E.J.; Chung, H.S.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, K.M. Comparisons of three different methods for defining sarcopenia: An aspect of cardiometabolic risk. Sci. Rep. 2017, 7, 6491. [Google Scholar] [CrossRef]
- Furushima, T.; Miyachi, M.; Iemitsu, M.; Murakami, H.; Kawano, H.; Gando, Y.; Kawakami, R.; Sanada, K. Comparison between clinical significance of height-adjusted and weight-adjusted appendicular skeletal muscle mass. J. Physiol. Anthropol. 2017, 36, 15. [Google Scholar] [CrossRef]
- Takamura, T.; Kita, Y.; Nakagen, M.; Sakurai, M.; Isobe, Y.; Takeshita, Y.; Kawai, K.; Urabe, T.; Kaneko, S. Weight-adjusted lean body mass and calf circumference are protective against obesity-associated insulin resistance and metabolic abnormalities. Heliyon 2017, 3, e00347. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Park, M.S.; Kim, T.N.; Ryu, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Jones, G.; Choi, K.M. Associations of Low Muscle Mass and the Metabolic Syndrome in Caucasian and Asian Middle-aged and Older Adults. J. Nutr. Health Aging 2016, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Yoon, J.S. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab. J. 2013, 37, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Hashimoto, Y.; Okamura, T.; Fukuda, T.; Yamazaki, M.; Hamaguchi, M.; Fukui, M. Reduction of Fat to Muscle Mass Ratio Is Associated with Improvement of Liver Stiffness in Diabetic Patients with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2019, 8, 2175. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Oshida, N.; Oh, S.; Okada, K.; Shoda, J. Progressive reduction in skeletal muscle mass to visceral fat area ratio is associated with a worsening of the hepatic conditions of non-alcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019, 12, 495–503. [Google Scholar] [CrossRef]
- Mizuno, N.; Seko, Y.; Kataoka, S.; Okuda, K.; Furuta, M.; Takemura, M.; Taketani, H.; Hara, T.; Umemura, A.; Nishikawa, T.; et al. Increase in the skeletal muscle mass to body fat mass ratio predicts the decline in transaminase in patients with nonalcoholic fatty liver disease. J. Gastroenterol. 2019, 54, 160–170. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Nayak, B.S.; Butcher, D.M.; Bujhawan, S.; Chang, D.; Chang, S.; Cabral-Samaroo, D.; Cadan, S.; Buchoon, V.; Budhram, L.; Boyce, M.; et al. Association of low serum creatinine, abnormal lipid profile, gender, age and ethnicity with type 2 diabetes mellitus in Trinidad and Tobago. Diabetes Res. Clin. Pract. 2011, 91, 342–347. [Google Scholar] [CrossRef]
- Moon, J.S.; Lee, J.E.; Yoon, J.S. Variation in serum creatinine level is correlated to risk of type 2 diabetes. Endocrinol. Metab. 2013, 28, 207–213. [Google Scholar] [CrossRef]
- Harita, N.; Hayashi, T.; Sato, K.K.; Nakamura, Y.; Yoneda, T.; Endo, G.; Kambe, H. Lower serum creatinine is a new risk factor of type 2 diabetes: The Kansai healthcare study. Diabetes Care 2009, 32, 424–426. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Røislien, J.; Nordstrand, N.; Hofsø, D.; Hager, H.; Hartmann, A. Low serum creatinine is associated with type 2 diabetes in morbidly obese women and men: A cross-sectional study. BMC Endocr. Disord. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Shi, H.; Wang, H.; Sun, S.; et al. Low serum creatinine predicts risk for type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3011. [Google Scholar] [CrossRef] [PubMed]
- Kashima, S.; Inoue, K.; Matsumoto, M.; Akimoto, K. Low serum creatinine is a type 2 diabetes risk factor in men and women: The Yuport Health Checkup Center cohort study. Diabetes Metab. 2017, 43, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Imano, H.; Muraki, I.; Shimizu, Y.; Hayama-Terada, M.; Kitamura, A.; Okada, T.; Kiyama, M.; Iso, H. Serum creatinine levels and risk of incident type 2 diabetes mellitus or dysglycemia in middle-aged Japanese men: A retrospective cohort study. BMJ Open Diabetes Res. Care 2018, 6, e000492. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Nakagawa, T.; Honda, T.; Yamamoto, S.; Okazaki, H.; Yamamoto, M.; Miyamoto, T.; Eguchi, M.; Kochi, T.; Shimizu, M.; et al. Japan Epidemiology Collaboration on Occupational Health Study Group. Low serum creatinine and risk of diabetes: The Japan Epidemiology Collaboration on Occupational Health Study. J. Diabetes Investig. 2019, 10, 1209–1214. [Google Scholar] [CrossRef]
- Goto, A.; Goto, M.; Noda, M.; Tsugane, S. Incidence of Type 2 Diabetes in Japan: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e74699. [Google Scholar] [CrossRef]
| Men | ALL, n = 9659 | Tertile 1 (Cre/BW Ratio < 0.01275), n = 3176 | Tertile 2 (0.01275 ≤ Cre/BW Ratio < 0.0148), n = 3258 | Tertile 3 (Cre/BW Ratio ≥ 0.0148), n = 3225 | p |
| Age (year) | 45.3 (9.3) | 43.5 (8.5) | 45.4 (9.1) * | 47.1 (9.8) *,† | <0.001 |
| Body weight (kg) | 67.6 (10.0) | 75.2 (9.9) | 67.0 (7.0) * | 60.6 (6.7) *,† | <0.001 |
| Height (cm) | 170.6 (6.0) | 172.9 (5.7) | 170.6 (5.6) * | 168.1 (5.6) *,† | <0.001 |
| Body mass index (kg/m2) | 23.2 (3.0) | 25.1 (3.1) | 23.0 (2.3) * | 21.5 (2.3) *,† | <0.001 |
| Waist circumference (cm) | 81.1 (8.0) | 86.0 (7.8) | 80.7 (6.3) * | 76.3 (6.5) *,† | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.3 (0.4) | 5.4 (0.4) | 5.3 (0.4) * | 5.3 (0.4) *,† | <0.001 |
| Hemoglobin A1c (%) | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) * | 5.1 (0.3) *,† | <0.001 |
| Hemoglobin A1c (mmol/L) | 32.9 (3.6) | 33.4 (3.6) | 32.8 (3.5) * | 32.5 (3.6) *,† | <0.001 |
| Creatinine (mg/dL) | 0.92 (0.12) | 0.84 (0.10) | 0.92 (0.10) * | 1.01 (0.11) *,† | <0.001 |
| Creatinine (µmol/L) | 81.6 (10.5) | 74.6 (8.5) | 81.1 (8.4) * | 88.9 (9.3) *,† | <0.001 |
| Triglycerides (mmol/L) | 1.2 (0.8) | 1.3 (0.8) | 1.2 (0.8) * | 1.1 (0.7) *,† | <0.001 |
| HDL cholesterol (mmol/L) | 1.3 (0.3) | 1.2 (0.3) | 1.3 (0.3) * | 1.4 (0.4) *,† | <0.001 |
| Systolic blood pressure (mmHg) | 120.2 (14.7) | 123.4 (14.3) | 120.0 (14.4) * | 117.3 (14.8) *,† | <0.001 |
| Diastolic blood pressure (mmHg) | 76.0 (10.2) | 77.8 (10.1) | 75.9 (10.0) * | 74.3 (10.1) *,† | <0.001 |
| Exercise (-/+) | 7822/1837 | 2646/530 | 2650/608 | 2526/699 | <0.001 |
| Smoking (Non/Past/Current) | 3039/3074/3546 | 995/946/1235 | 984/1064/1210 | 1060/1064/1101 | <0.001 |
| Alcohol intake (Non-min/light/moderate/heavy) | 5727/1505/1317/1110 | 1937/465/398/376 | 1900/515/470/373 | 1890/525/449/361 | 0.111 |
| Cre/BW ratio | 0.014 (0.002) | 0.011 (0.001) | 0.014 (0.001) | 0.016 (0.002) | <0.001 |
| Incident diabetes | 362 | 169 | 107 | 86 | <0.001 |
| Women | ALL, n = 7417 | Tertile 1 (Cre/BW Ratio < 0.0118), n = 2437 | Tertile 2 (0.0118≤ Cre/BW Ratio < 0.014), n = 2515 | Tertile 3 (Cre/BW Ratio ≥ 0.014), n = 2465 | p |
| Age (year) | 44.2 (9.2) | 43.8 (8.7) | 44.1 (9.2) | 44.6 (9.5) *,† | 0.004 |
| Body weight (kg) | 52.9 (8.0) | 58.6 (8.8) | 52.1 (5.9) * | 48.0 (5.2) *,† | <0.001 |
| Height (cm) | 158.1 (5.4) | 159.5 (5.3) | 158.1 (5.3) * | 156.7 (5.3) *,† | <0.001 |
| Body mass index (kg/m2) | 21.2 (3.0) | 23.1 (3.5) | 20.9 (2.3) * | 19.6 (2.0) *,† | <0.001 |
| Waist circumference (cm) | 72.1 (8.4) | 77.5 (8.8) | 71.4 (6.5) * | 67.4 (6.3) *,† | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.0 (0.4) | 5.1 (0.4) | 5.0 (0.4) * | 4.9 (0.4) *,† | <0.001 |
| Hemoglobin A1c (%) | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) * | 5.1 (0.3) * | <0.001 |
| Hemoglobin A1c (mmol/L) | 33.2 (3.6) | 33.7 (3.7) | 33.0 (3.6) * | 32.8 (3.7) * | <0.001 |
| Creatinine (mg/dL) | 0.68 (0.10) | 0.60 (0.08) | 0.67 (0.08) * | 0.76 (0.09) *,† | <0.001 |
| Creatinine (µmol/L) | 59.8 (9.0) | 53.3 (6.7) | 59.2 (6.8) * | 66.8 (7.8)*, † | <0.001 |
| Triglycerides (mmol/L) | 0.7 (0.4) | 0.7 (0.5) | 0.7 (0.4) * | 0.7 (0.4) * | <0.001 |
| HDL cholesterol (mmol/L) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) * | 1.7 (0.4) *,† | <0.001 |
| Systolic blood pressure (mmHg) | 110.6 (15.2) | 114.4 (15.6) | 109.9 (14.7) * | 107.7 (14.5) *,† | <0.001 |
| Diastolic blood pressure (mmHg) | 68.5 (10.2) | 70.5 (10.6) | 68.0 (10.0) * | 67.1 (9.7) *,† | <0.001 |
| Exercise (−/+) | 6188/1229 | 2099/338 | 2108/407 | 1981/484 | <0.001 |
| Smoking (Non/Past/Current) | 6396/503/518 | 2072/192/173 | 2178/166/171 | 2146/145/175 | 0.084 |
| Alcohol intake (Non-min/light/moderate/heavy) | 6705/416/211/85 | 2235/120/56/26 | 2252/156/78/29 | 2218/140/77/30 | 0.209 |
| Cre/BW ratio | 0.013 (0.003) | 0.010 (0.001) | 0.013 (0.001) * | 0.016 (0.002) *,† | <0.001 |
| Incident diabetes | 101 | 47 | 27 | 27 | 0.013 |
| Men | Model 1 | Model 2 | ||
| HR with 95% CI | p Value | HR with 95% CI | p Value | |
| Age, years | 1.05 (1.03–1.06) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| Fasting plasma glucose, 0.1 mmol/L | 1.32 (1.28–1.37) | <0.001 | 1.32 (1.27–1.36) | <0.001 |
| Light drinker | 0.65 (0.47–0.90) | 0.001 | 0.66 (0.48–0.91) | 0.009 |
| Moderate drinker | 0.54 (0.38–0.77) | <0.001 | 0.55 (0.38–0.77) | <0.001 |
| Heavy drinker | 0.92 (0.68–1.24) | 0.586 | 0.95 (0.70–1.26) | 0.716 |
| Regular exerciser | 0.73 (0.53–0.99) | 0.046 | 0.73 (0.52–0.99) | 0.039 |
| Ex-smoker | 0.85 (0.63–1.13) | 0.263 | 0.84 (0.62–1.12) | 0.231 |
| Current smoker | 1.58 (1.22–2.05) | <0.001 | 1.55 (1.19–2.02) | 0.010 |
| Cre/BW ratio tertiles | ||||
| T1 | Reference | - | - | - |
| T2 | 0.56 (0.44–0.71) | <0.001 | - | - |
| T3 | 0.42 (0.32–0.54) | <0.001 | - | - |
| Cre/BW ratio, 0.001 incremental | - | - | 0.84 (0.80–0.88) | <0.001 |
| Women | Model 1 | Model 2 | ||
| HR with 95% CI | p Value | HR with 95% CI | p Value | |
| Age, years | 1.05 (1.02–1.07) | <0.001 | 1.05 (1.02–1.07) | <0.001 |
| Fasting plasma glucose, 0.1 mmol/L | 1.40 (1.33–1.48) | <0.001 | 1.40 (1.32–1.48) | <0.001 |
| Light drinker | 0.35 (0.09–0.94) | 0.035 | 0.34 (0.08–0.91) | 0.030 |
| Moderate drinker | 0.81 (0.20–2.24) | 0.720 | 0.78 (0.19–2.14) | 0.667 |
| Heavy drinker | 0.64 (0.10–2.12) | 0.512 | 0.69 (0.11–2.26) | 0.587 |
| Regular exerciser | 0.84 (0.46–1.42) | 0.527 | 0.86 (0.50–1.50) | 0.605 |
| Ex-smoker | 1.50 (0.62–3.08) | 0.341 | 1.53 (0.63–3.12) | 0.319 |
| Current smoker | 3.49 (1.85–6.09) | <0.001 | 3.57 (1.91–6.23) | <0.001 |
| Cre/BW ratio tertiles | ||||
| T1 | Reference | - | - | - |
| T2 | 0.61 (0.37–0.98) | 0.042 | - | - |
| T3 | 0.53 (0.32–0.85) | 0.008 | - | - |
| Cre/BW ratio, Δ0.001 incremental | - | - | 0.88 (0.81–0.96) | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Okamura, T.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Creatinine to Body Weight Ratio Is Associated with Incident Diabetes: Population-Based Cohort Study. J. Clin. Med. 2020, 9, 227. https://doi.org/10.3390/jcm9010227
Hashimoto Y, Okamura T, Hamaguchi M, Obora A, Kojima T, Fukui M. Creatinine to Body Weight Ratio Is Associated with Incident Diabetes: Population-Based Cohort Study. Journal of Clinical Medicine. 2020; 9(1):227. https://doi.org/10.3390/jcm9010227
Chicago/Turabian StyleHashimoto, Yoshitaka, Takuro Okamura, Masahide Hamaguchi, Akihiro Obora, Takao Kojima, and Michiaki Fukui. 2020. "Creatinine to Body Weight Ratio Is Associated with Incident Diabetes: Population-Based Cohort Study" Journal of Clinical Medicine 9, no. 1: 227. https://doi.org/10.3390/jcm9010227
APA StyleHashimoto, Y., Okamura, T., Hamaguchi, M., Obora, A., Kojima, T., & Fukui, M. (2020). Creatinine to Body Weight Ratio Is Associated with Incident Diabetes: Population-Based Cohort Study. Journal of Clinical Medicine, 9(1), 227. https://doi.org/10.3390/jcm9010227








