Cochlear Implantation Outcome in Children with DFNB1 locus Pathogenic Variants
Abstract
1. Introduction
2. Methods
2.1. Subjects
- HAs usage prior to CI;
- Cochlear implant received before 24 months of age;
- Completed genetic testing of the DFNB1 locus;
- Evaluated auditory development;
- Excluded environmental risk factors for HL development (e.g., severe prematurity, asphyxia, high hyperbilirubinemia, ototoxic drugs, cytomegalovirus infection);
- Excluded risk factors for auditory development (e.g., severe birth defects, deaf-mute parents).
2.2. Genetic Testing
2.3. Audiological Evaluation and Assessment of Auditory Development
2.4. Statistical Analysis
3. Results
3.1. Genetic Etiology
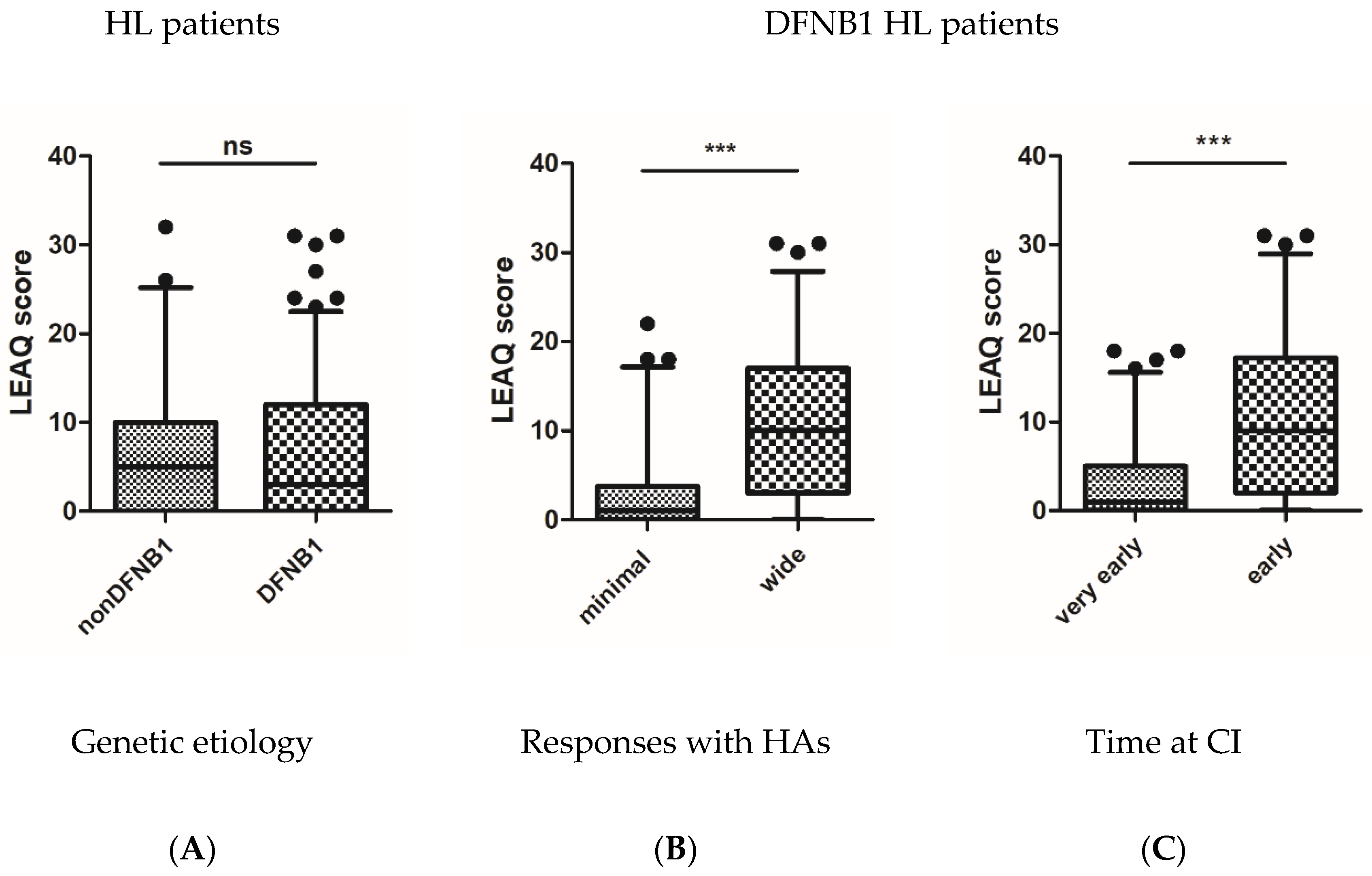
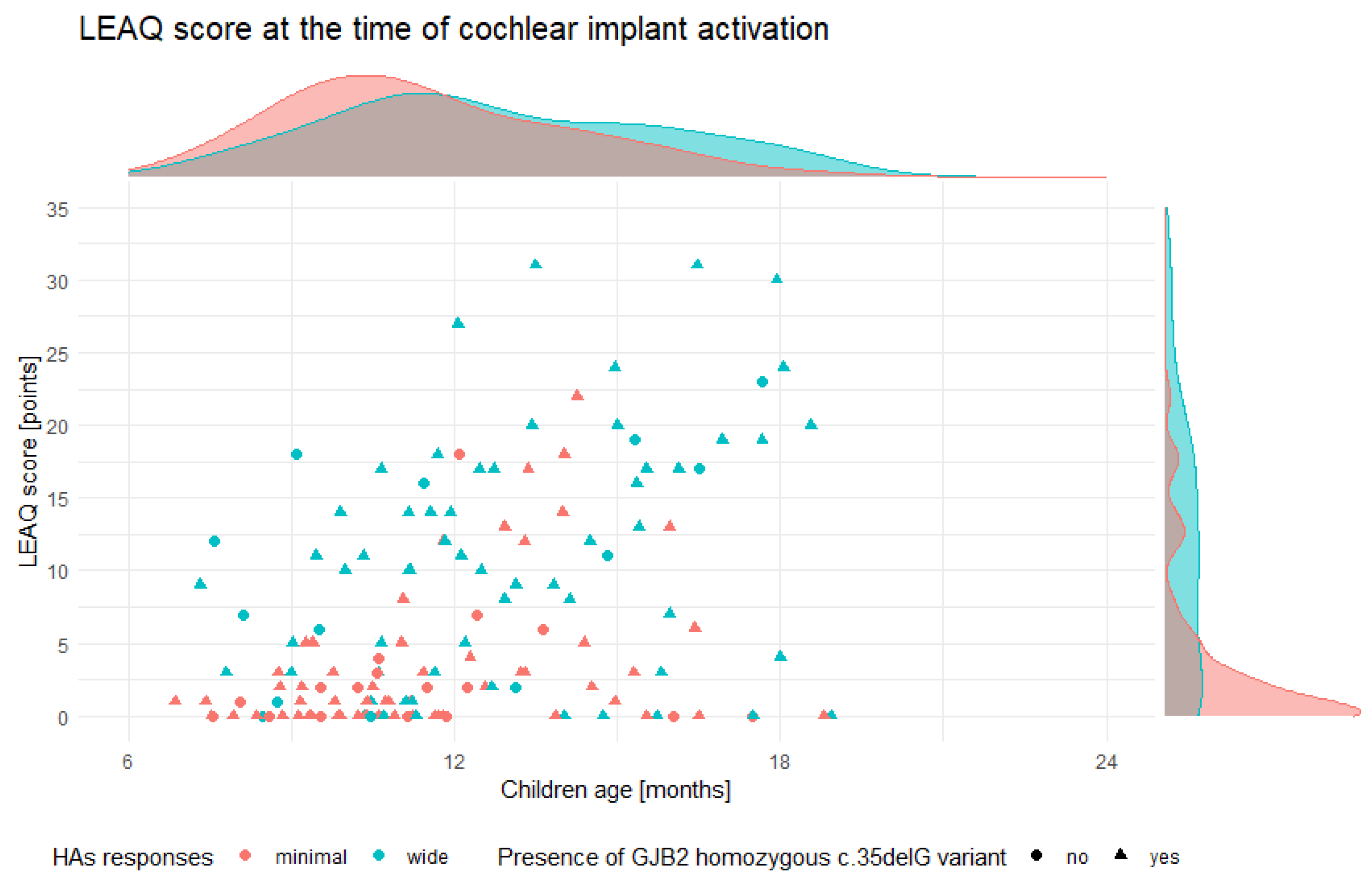
3.2. Initial Auditory Status of Tested Subjects
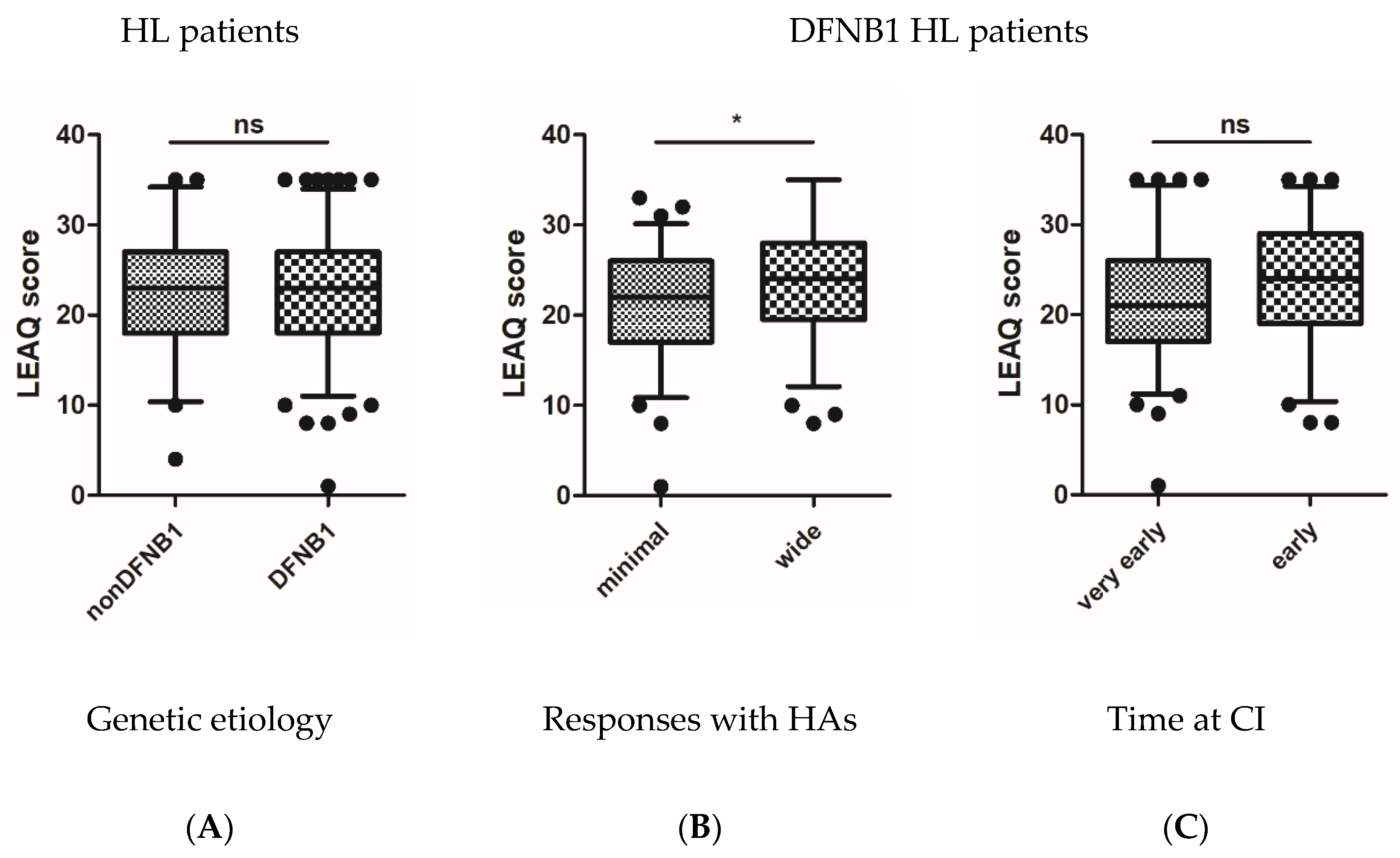
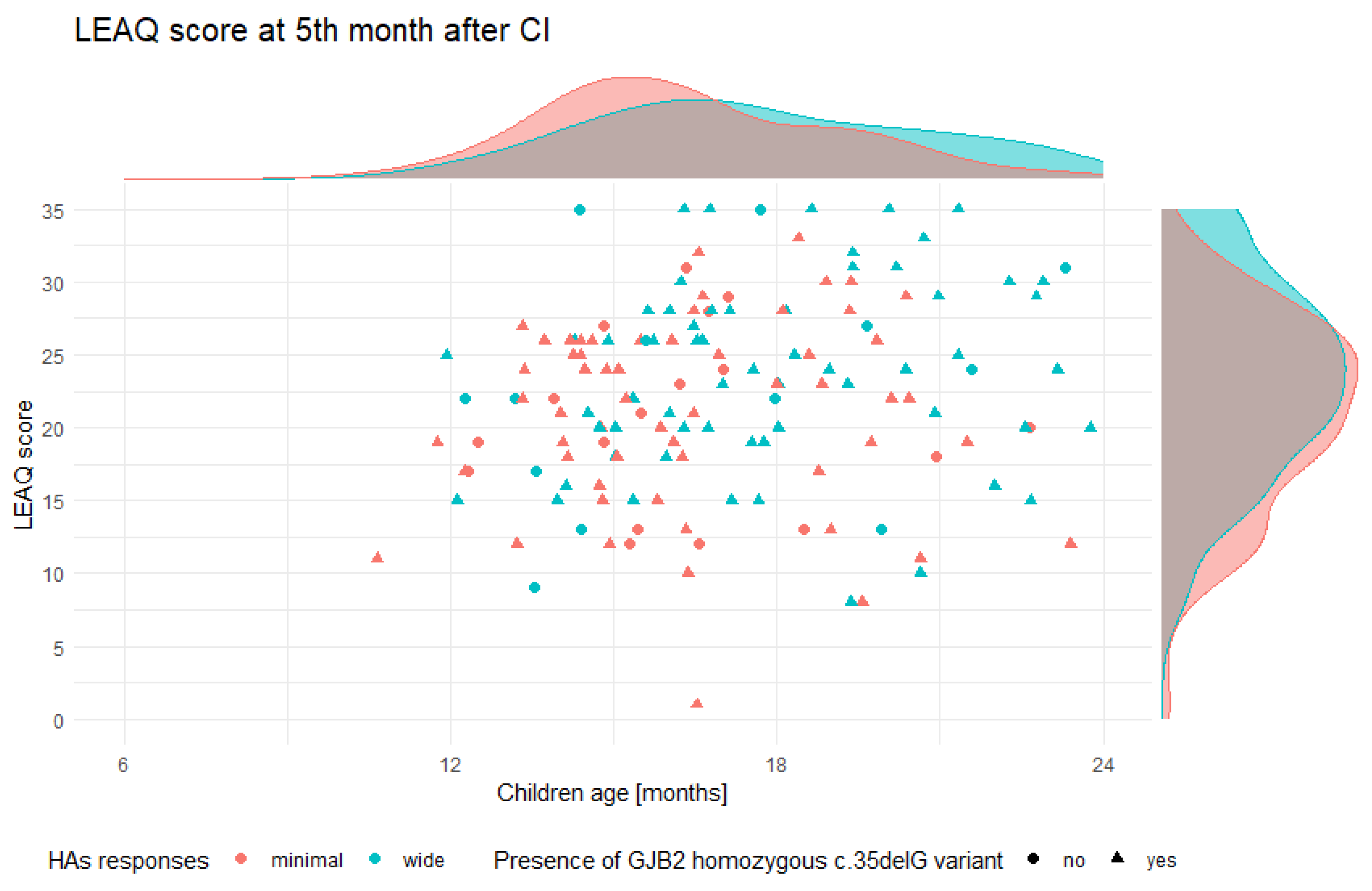
3.3. Postoperative Auditory Status of Tested Subjects
3.4. CI Outcomes
4. Discussion
4.1. Auditory Development of DFNB1 HL Children Receiving CI
4.2. Molecular Mechanism Supporting the Observed CI Outcome
4.3. Possible Explanations for Uncertain Data from the Literature
4.4. Limitations of Our Study and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tucci, D.; Merson, M.H.; Wilson, B.S. A summary of the literature on global hearing impairment: Current status and priorities for action. Otol. Neurotol. 2010, 31, 31–41. [Google Scholar] [CrossRef]
- Svirsky, M.A.; Robbins, A.M.; Kirk, K.I.; Pisoni, D.B.; Miyamoto, R.T. Language development in profoundly deaf children with cochlear implants. Psychol. Sci. 2000, 11, 153–158. [Google Scholar] [CrossRef]
- Vlastarakos, P.V. Profound deafness and the acquisition of spoken language in children. World J. Clin. Pediatr. 2012, 1, 24–28. [Google Scholar] [CrossRef]
- May-Mederake, B.; Kuehn, H.; Vogel, A.; Keilmann, A.; Bohnert, A.; Mueller, S.; Witt, G.; Neumann, K.; Hey, C.; Stroele, A.; et al. Evaluation of auditory development in infants and toddlers who received cochlear implants under the age of 24 months with the LittlEARS) Auditory Questionnaire. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 1149–1155. [Google Scholar] [CrossRef]
- Skarzynski, H. Long-term results of partial deafness treatment. Cochlear Implants Int. 2014, 15, S21–S23. [Google Scholar] [CrossRef]
- Archbold, S.M.; Nikolopoulos, T.P.; Lloyd-Richmond, H. Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use. Cochlear Implants Int. 2009, 10, 25–40. [Google Scholar] [CrossRef]
- Raine, C.H.; Summerfield, Q.; Strachan, D.R.; Martin, J.M.; Totten, C. The cost and analysis of nonuse of cochlear implants. Otol. Neurotol. 2008, 29, 221–224. [Google Scholar] [CrossRef]
- Abdurehim, Y.; Lehmann, A.; Zeitouni, A.G. Predictive Value of GJB2 Mutation Status for Hearing Outcomes of Pediatric Cochlear Implantation. Otolaryngol. Head Neck Surg. 2017, 157, 16–24. [Google Scholar] [CrossRef]
- Angeli, S.I.; Suarez, H.; Lopez, A.; Balkany, T.J.; Liu, X.Z. Influence of DFNB1 status on expressive language in deaf children with cochlear implants. Otol. Neurotol. 2011, 32, 1437–1443. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Zhu, Y.; Liang, C.; Zhao, H.B. Deafness induced by Connexin 26 (GJB2) deficiency is not determined by endocochlear potential (EP) reduction but is associated with cochlear developmental disorders. Biochem. Biophys. Res. Commun. 2014, 448, 28–32. [Google Scholar] [CrossRef]
- Zhao, H.B. Hypothesis of K+-Recycling Defect Is Not a Primary Deafness Mechanism for Cx26 (GJB2) Deficiency. Front. Mol. Neurosci. 2017, 10, 162. [Google Scholar] [CrossRef]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss—A Common Hereditary Deafness. Front. Cell. Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef]
- Mei, L.; Chen, J.; Zong, L.; Zhu, Y.; Liang, C.; Jones, R.O.; Zhao, H.B. A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol. Dis. 2017, 108, 195–203. [Google Scholar] [CrossRef]
- Hoefsloot, L.H.; Roux, A.F.; Bitner-Glindzicz, M. EMQN Best Practice guidelines for diagnostic testing of mutations causing non-syndromic hearing impairment at the DFNB1 locus. Eur. J. Hum. Genet. 2013, 21, 1325–1329. [Google Scholar] [CrossRef]
- del Castillo, F.J.; Rodriguez-Ballesteros, M.; Alvarez, A.; Hutchin, T.; Leonardi, E.; de Oliveira, C.A.; Azaiez, H.; Brownstein, Z.; Avenarius, M.R.; Marlin, S.; et al. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 2005, 42, 588–594. [Google Scholar] [CrossRef]
- Del Castillo, I.; Moreno-Pelayo, M.A.; Del Castillo, F.J.; Brownstein, Z.; Marlin, S.; Adina, Q.; Cockburn, D.J.; Pandya, A.; Siemering, K.R.; Chamberlin, G.P.; et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: A multicenter study. Am. J. Hum. Genet. 2003, 73, 1452–1458. [Google Scholar] [CrossRef]
- Pollak, A.; Skarzynski, H. Prevalence of DFNB1 hearing loss among cochlear implant users established with the 3-step DFNB1 approach. J. Hear. Sci. 2017, 7, 33–40. [Google Scholar]
- Obrycka, A.; Lorens, A.; Padilla Garcia, J.L.; Piotrowska, A.; Skarzynski, H. Validation of the LittlEARS Auditory Questionnaire in cochlear implanted infants and toddlers. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 107–116. [Google Scholar] [CrossRef]
- Obrycka, A.; Garcia, J.L.; Pankowska, A.; Lorens, A.; Skarzynski, H. Production and evaluation of a Polish version of the LittlEars questionnaire for the assessment of auditory development in infants. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1035–1042. [Google Scholar] [CrossRef]
- Coninx, F.; Weichbold, V.; Tsiakpini, L.; Autrique, E.; Bescond, G.; Tamas, L.; Compernol, A.; Georgescu, M.; Koroleva, I.; Le Maner-Idrissi, G.; et al. Validation of the LittlEARS((R)) Auditory Questionnaire in children with normal hearing. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1761–1768. [Google Scholar] [CrossRef]
- Miyagawa, M.; Nishio, S.Y.; Usami, S. A Comprehensive Study on the Etiology of Patients Receiving Cochlear Implantation with Special Emphasis on Genetic Epidemiology. Otol. Neurotol. 2016, 37, e126–e134. [Google Scholar] [CrossRef]
- Oldak, M.; Lechowicz, U.; Pollak, A.; Ozieblo, D.; Skarzynski, H. Overinterpretation of high throughput sequencing data in medical genetics: First evidence against TMPRSS3/GJB2 digenic inheritance of hearing loss. J. Transl. Med. 2019, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Eppsteiner, R.W.; Shearer, A.E.; Hildebrand, M.S.; Deluca, A.P.; Ji, H.; Dunn, C.C.; Black-Ziegelbein, E.A.; Casavant, T.L.; Braun, T.A.; Scheetz, T.E.; et al. Prediction of cochlear implant performance by genetic mutation: The spiral ganglion hypothesis. Hear. Res. 2012, 292, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, Y.H.; Liu, T.C.; Lin, K.N.; Yang, W.S.; Hsu, C.J.; Chen, P.L.; Wu, C.M. Identifying Children with Poor Cochlear Implantation Outcomes Using Massively Parallel Sequencing. Medicine 2015, 94, e1073. [Google Scholar] [CrossRef]
- Shearer, A.E.; Eppsteiner, R.W.; Frees, K.; Tejani, V.; Sloan-Heggen, C.M.; Brown, C.; Abbas, P.; Dunn, C.; Hansen, M.R.; Gantz, B.J.; et al. Genetic variants in the peripheral auditory system significantly affect adult cochlear implant performance. Hear. Res. 2017, 348, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Shearer, A.E.; Hansen, M.R. Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig. Otolaryngol. 2019, 4, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bostrom, M.; Kinnefors, A.; Rask-Andersen, H. Unique expression of connexins in the human cochlea. Hear. Res. 2009, 250, 55–62. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yu, N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J. Comp. Neurol. 2006, 499, 506–518. [Google Scholar] [CrossRef]
- Lautermann, J.; ten Cate, W.J.; Altenhoff, P.; Grummer, R.; Traub, O.; Frank, H.; Jahnke, K.; Winterhager, E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998, 294, 415–420. [Google Scholar] [CrossRef]
- Nishio, S.Y.; Usami, S.I. Outcomes of cochlear implantation for the patients with specific genetic etiologies: A systematic literature review. Acta Otolaryngol. 2017, 137, 730–742. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, A.R.; Han, J.H.; Kim, S.D.; Kim, S.H.; Koo, J.W.; Oh, S.H.; Choi, B.Y. Outcome of Cochlear Implantation in Prelingually Deafened Children According to Molecular Genetic Etiology. Ear Hear. 2017, 38, e316–e324. [Google Scholar] [CrossRef] [PubMed]
- Karamert, R.; Bayazit, Y.A.; Altinyay, S.; Yilmaz, A.; Menevse, A.; Gokdogan, O.; Gokdogan, C.; Ant, A. Association of GJB2 gene mutation with cochlear implant performance in genetic non-syndromic hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.D.; Raghunandhan, S.; Kameswaran, M.; Ranjith, R. A clinical study of cortical auditory evoked potentials in cochlear implantees. Indian J. Otolaryngol. Head Neck Surg. 2013, 65, 587–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, L.A.; Couto, M.I.; Magliaro, F.C.; Tsuji, R.K.; Bento, R.F.; de Carvalho, A.C.; Matas, C.G. Cortical maturation in children with cochlear implants: Correlation between electrophysiological and behavioral measurement. PLoS ONE 2017, 12, e0171177. [Google Scholar] [CrossRef]
| Number of Cases | Allele 1 | Allele 2 |
|---|---|---|
| 119 | GJB2 c.35delG | GJB2 c.35delG |
| 10 | GJB2 c.35delG | GJB2 c.313_326del |
| 5 | GJB2 c.35delG | GJB2 c.167delT |
| 3 | GJB2 c.35delG | GJB2 c.-23+1G>A |
| 3 | GJB2 c.35delG | GJB6 del(D13S1830) |
| 1 | GJB2 c.35delG | GJB2 c.102G>A |
| 1 | GJB2 c.35delG | GJB2 c.109G>A |
| 1 | GJB2 c.35delG | GJB2 c.235delC |
| 1 | GJB2 c.35delG | GJB2 c.290insA |
| 1 | GJB2 c.313_326del | GJB2 c.167delT |
| 1 | GJB2 c.313_326del | GJB2 c.235delC |
| 1 | GJB2 c.313_326del | GJB2 c.139G>T |
| 1 | GJB2 c.551G>C | GJB2 c.551G>C |
| 1 | GJB2 c.-23+1G>A | GJB6 del(D13S1830) |
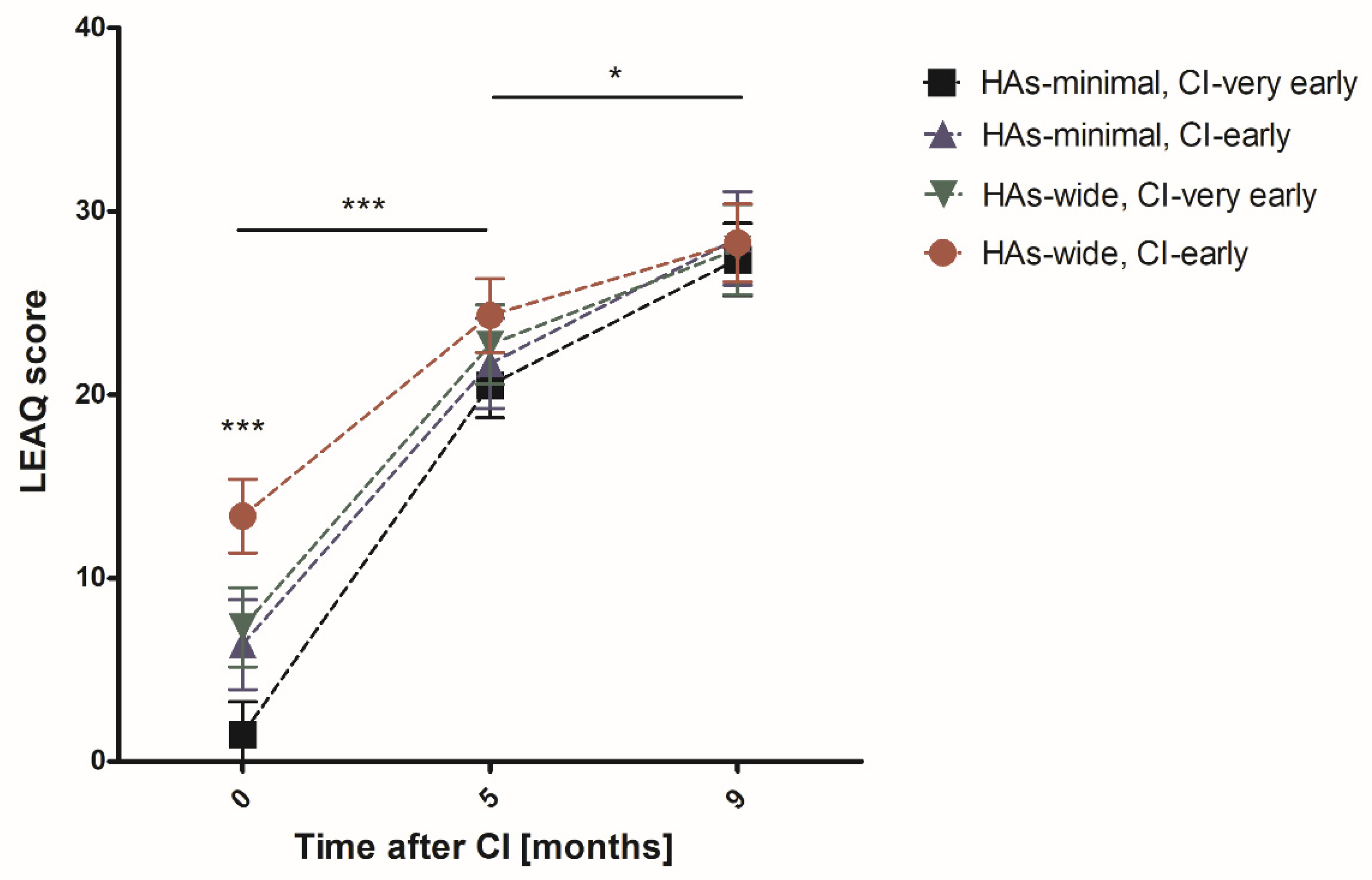
| Analyzed Group | N 0–5 | CI Activation | Five Months after CI | N 9 | Nine Months after CI |
|---|---|---|---|---|---|
| Very early CI | 83 | 3.87 ± 0.57 | 21.43 ± 0.71 | 67 | 27.60 ± 0.59 |
| Early CI | 66 | 10.52 ± 1.11 | 23.26 ± 0.85 | 60 | 28.38 ± 0.76 |
| Minimal HAs | 76 | 3.21 ± 0.58 | 20.95 ± 0.73 | 65 | 27.82 ± 0.64 |
| Wide HAs | 73 | 10.56 ± 0.99 | 23.59 ± 0.79 | 62 | 28.13 ± 0.70 |
| Very early CI; minimal HAs | 49 | 1.47 ± 0.33 | 20.53 ± 0.88 | 40 | 27.38 ± 0.81 |
| Very early CI; wide HAs | 34 | 7.32 ± 1.05 | 22.74 ± 1.15 | 27 | 27.93 ± 0.84 |
| Early CI; minimal HAs | 27 | 6.37 ± 1.31 | 21.70 ± 1.32 | 25 | 28.52 ± 1.07 |
| Early CI; wide HAs | 39 | 13.38 ±1.49 | 24.33 ± 1.09 | 35 | 28.29 ± 1.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oziębło, D.; Obrycka, A.; Lorens, A.; Skarżyński, H.; Ołdak, M. Cochlear Implantation Outcome in Children with DFNB1 locus Pathogenic Variants. J. Clin. Med. 2020, 9, 228. https://doi.org/10.3390/jcm9010228
Oziębło D, Obrycka A, Lorens A, Skarżyński H, Ołdak M. Cochlear Implantation Outcome in Children with DFNB1 locus Pathogenic Variants. Journal of Clinical Medicine. 2020; 9(1):228. https://doi.org/10.3390/jcm9010228
Chicago/Turabian StyleOziębło, Dominika, Anita Obrycka, Artur Lorens, Henryk Skarżyński, and Monika Ołdak. 2020. "Cochlear Implantation Outcome in Children with DFNB1 locus Pathogenic Variants" Journal of Clinical Medicine 9, no. 1: 228. https://doi.org/10.3390/jcm9010228
APA StyleOziębło, D., Obrycka, A., Lorens, A., Skarżyński, H., & Ołdak, M. (2020). Cochlear Implantation Outcome in Children with DFNB1 locus Pathogenic Variants. Journal of Clinical Medicine, 9(1), 228. https://doi.org/10.3390/jcm9010228






