Relationship between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae
Abstract
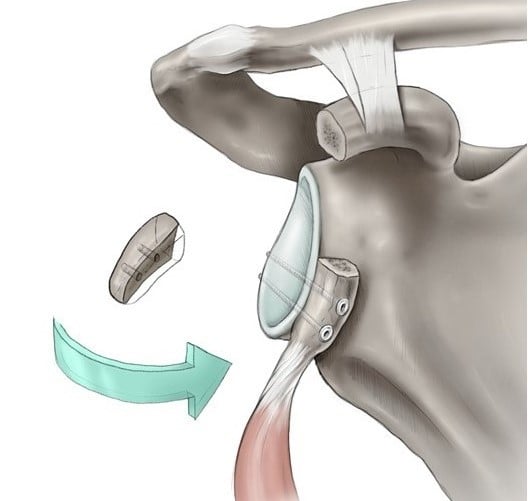
1. Introduction
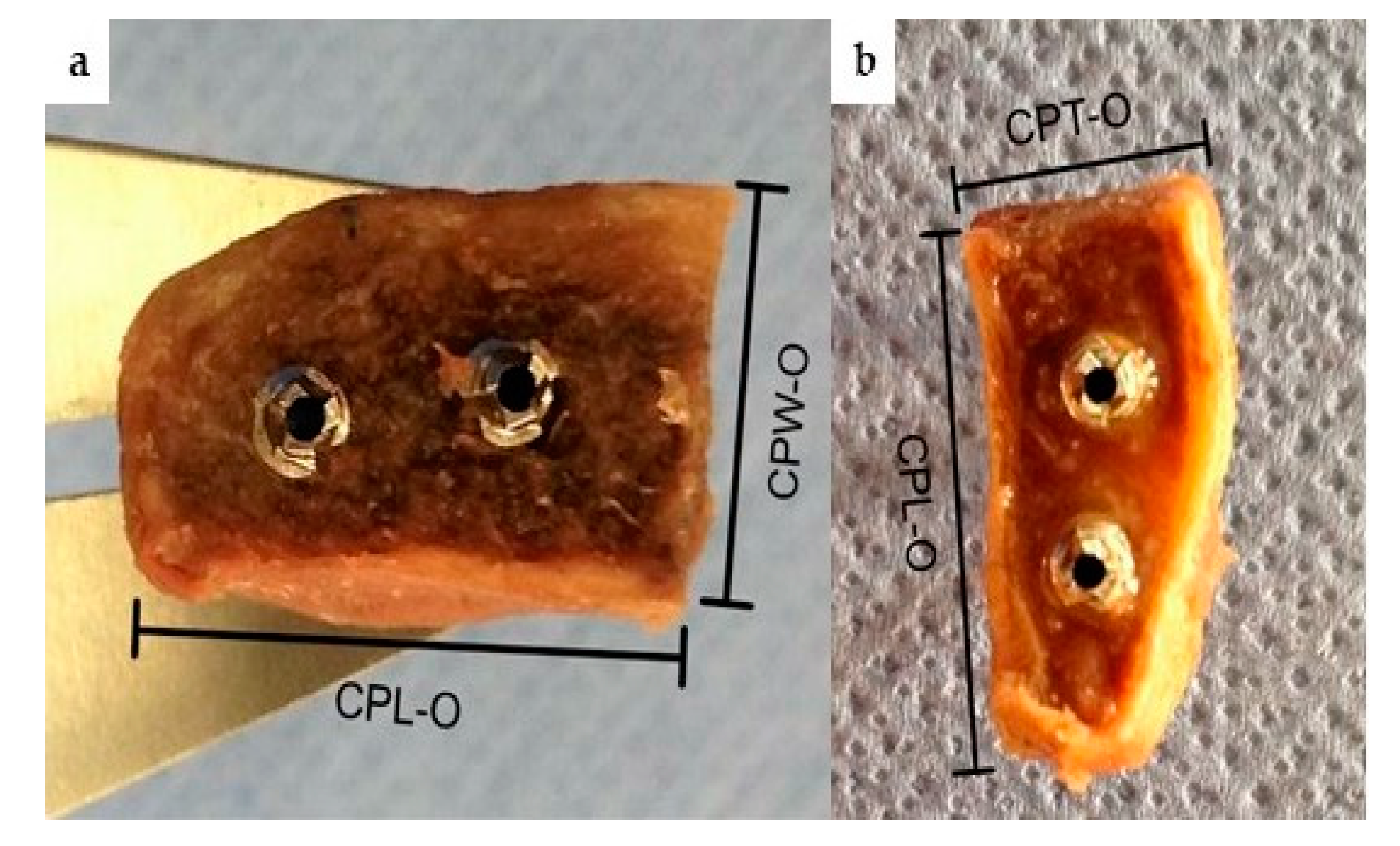
2. Material and Methods
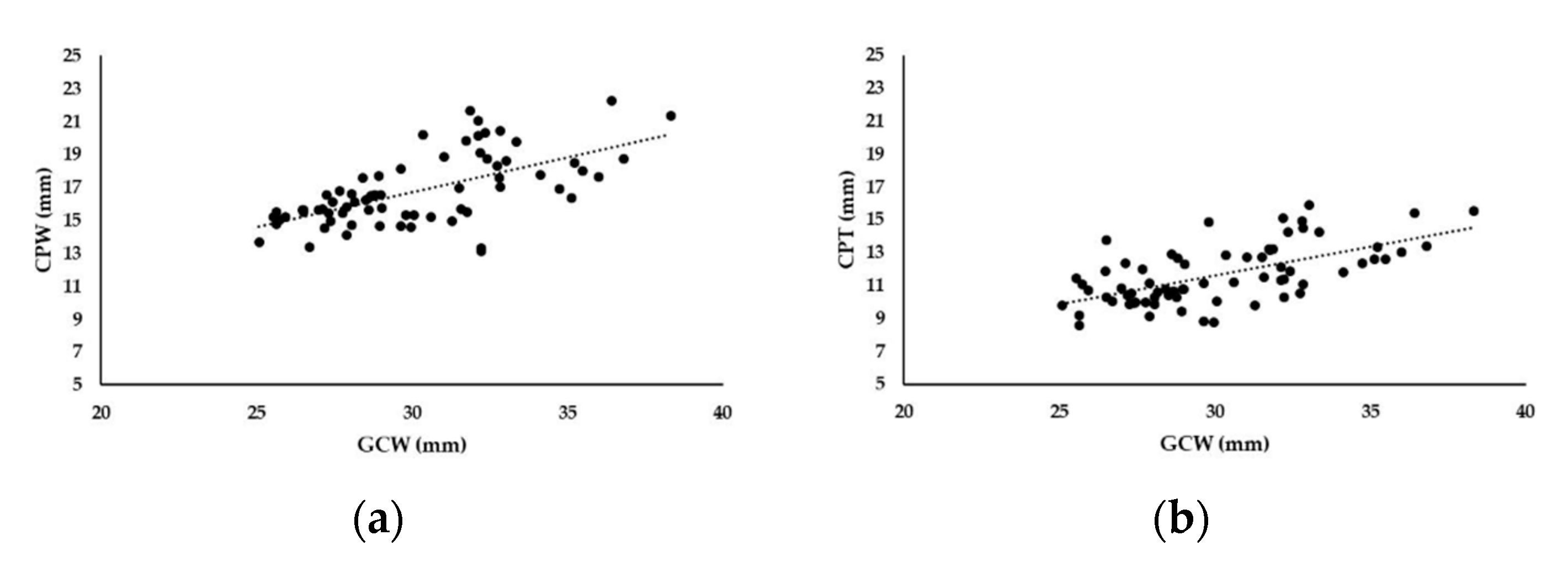
3. Results
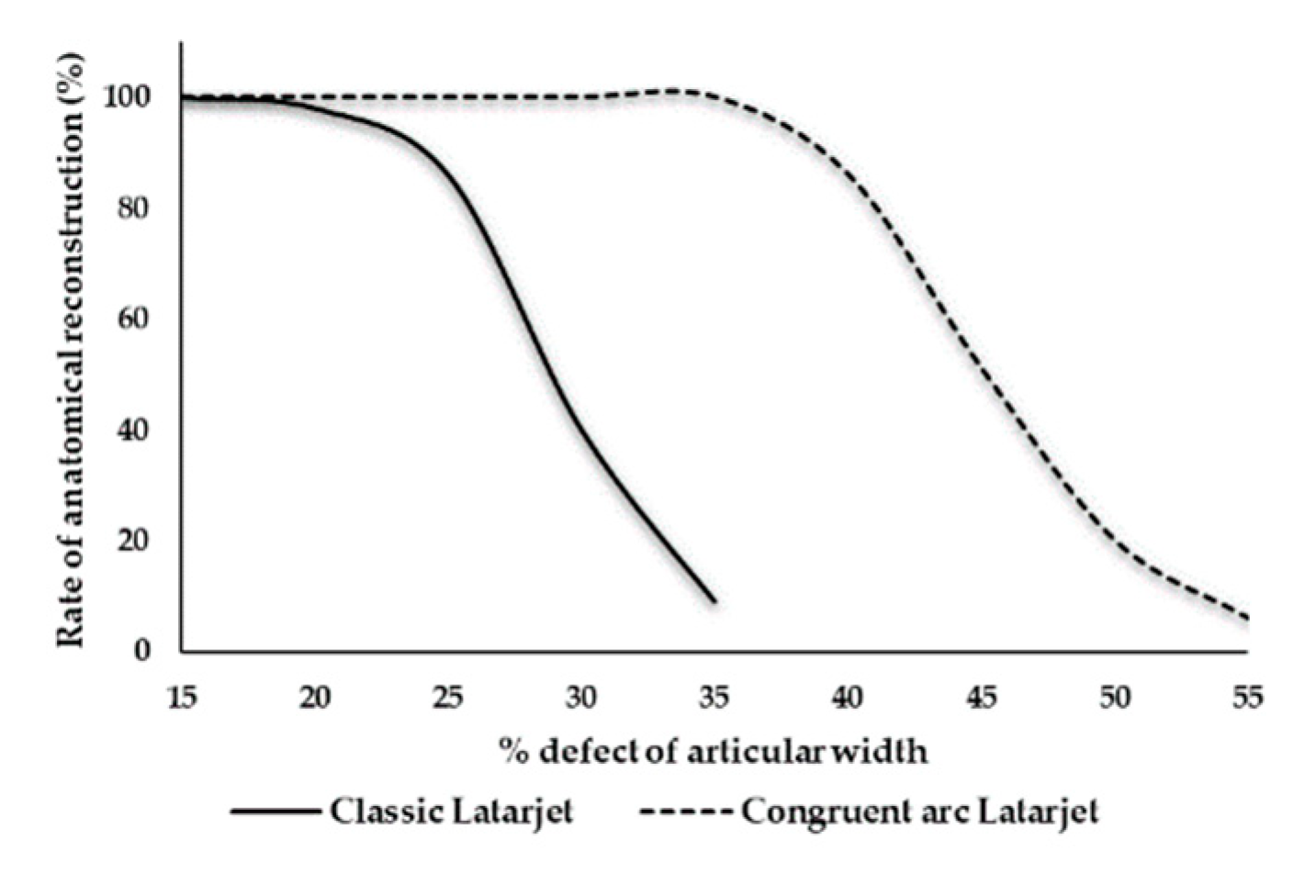
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sugaya, H.; Moriishi, J.; Dohi, M.; Kon, Y.; Tsuchiya, A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J. Bone Jt. Surg. Am. 2003, 85, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.B.; Boulahia, A.; Walch, G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy 2003, 19, 732–739. [Google Scholar] [CrossRef]
- Lo, I.K.; Parten, P.M.; Burkhart, S.S. The inverted pear glenoid: An indicator of significant glenoid bone loss. Arthroscopy 2004, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S.; De Beer, J.F.; Barth, J.R.; Cresswell, T.; Roberts, C.; Richards, D.P. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007, 23, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Villalba, M.; Héry, J.Y.; Balg, F.; Ahrens, P.; Neyton, L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J. Bone Jt. Surg. Am. 2006, 88, 1755–1763. [Google Scholar] [CrossRef]
- Shaha, S.; Cook, J.B.; Song, D.J.; Rowles, D.J.; Bottoni, C.R.; Shaha, S.H.; Tokish, J.M. Redefining “Critical” Bone Loss in Shoulder Instability: Functional Outcomes Worsen With “Subcritical” Bone Loss. Am. J. Sports Med. 2015, 43, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.C.; Donaldson, C.T.; Bishop, J.Y. Treatment of chronic glenoid defects in the setting of recurrent anterior shoulder instability: A systematic review. J. Shoulder Elbow Surg. 2010, 19, 769–780. [Google Scholar] [CrossRef]
- Bigliani, L.U.; Newton, P.M.; Steinmann, S.P.; Connor, P.M.; Mcllveen, S.J. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am. J. Sports Med. 1998, 26, 41–45. [Google Scholar] [CrossRef]
- Yamamoto, N.; Itoi, E.; Abe, H.; Kikuchi, K.; Seki, N.; Minagawa, H.; Tuoheti, Y. Effect of an anterior glenoid defect on anterior shoulder stability: A cadaveric study. Am. J. Sports Med. 2009, 37, 949–954. [Google Scholar] [CrossRef]
- Provencher, M.T.; Bhatia, S.; Ghodadra, N.S.; Grumet, R.C.; Bach, B.R., Jr.; Dewing, C.B.; LeClere, L.; Romeo, A.A. Recurrent shoulder instability: Current concepts for evaluation and management of glenoid bone loss. J. Bone Jt. Surg. Am. 2010, 92 (Suppl. 2), 133–151. [Google Scholar] [CrossRef]
- Armitage, M.S.; Elkinson, I.; Giles, J.W.; Athwal, G.S. An anatomic, computed tomographic assessment of the coracoid process with special reference to the congruent-arc latarjet procedure. Arthroscopy 2011, 27, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Van Thiel, G.S.; Gupta, D.; Ghodadra, N.; Cole, B.J.; Bach, B.R., Jr.; Shewman, E.; Wang, V.M.; Romeo, A.A.; Verma, N.N.; et al. Comparison of glenohumeral contact pressures and contact areas after glenoid reconstruction with latarjet or distal tibial osteochondral allografts. Am. J. Sports Med. 2013, 41, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.J.; Gill, T.J.; O’Hollerhan, J.D.; Pathare, N.; Millett, P.J. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am. J. Sports Med. 2006, 34, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Patte, D.; Bernageau, J.; Bancel, P. The anteroinferior vulnerable point of the glenoid rim. In Surgery of the Shoulder; Bateman, J.E., Welsh, R.P., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 94–99. [Google Scholar]
- Giles, J.W.; Boons, H.W.; Elkinson, I.; Faber, K.J.; Ferreira, L.M.; Johnson, J.A.; Athwal, G.S. Does the dynamic sling effect of the Latarjet procedure improve shoulder stability? A biomechanical evaluation. J. Shoulder Elbow Surg. 2013, 22, 821–827. [Google Scholar] [CrossRef]
- Wellmann, M.; Petersen, W.; Zantop, T.; Herbort, M.; Kobbe, P.; Raschke, M.J.; Hurschler, C. Open shoulder repair of osseous glenoid defects: Biomechanical effectiveness of the Latarjet procedure versus a contoured structural bone graft. Am. J. Sports Med. 2009, 37, 87–94. [Google Scholar] [CrossRef]
- Latarjet, M. Treatment of recurrent dislocation of the shoulder. Lyon Chir. 1954, 49, 994–999. [Google Scholar]
- Allain, J.; Goutallier, D.; Glorion, C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J. Bone Jt. Surg. Am. 1998, 80, 841–852. [Google Scholar] [CrossRef]
- Shah, A.A.; Butler, R.B.; Romanowski, J.; Goel, D.; Karadagli, D.; Warner, J.J. Short-term complications of the Latarjet procedure. J. Bone Jt. Surg. Am. 2012, 94, 495–501. [Google Scholar] [CrossRef]
- Moon, S.C.; Cho, N.S.; Rhee, Y.G. Quantitative assessment of the latarjet procedure for large glenoid defects by computed tomography: A coracoid graft can sufficiently restore the glenoid arc. Am. J. Sports Med. 2015, 43, 1099–1107. [Google Scholar] [CrossRef]
- Schmid, S.L.; Farshad, M.; Catanzaro, S.; Gerber, C. The Latarjet procedure for the treatment of recurrence of anterior instability of the shoulder after operative repair: A retrospective case series of forty-nine consecutive patients. J. Bone Jt. Surg. Am. 2012, 94, e75. [Google Scholar] [CrossRef]
- Young, A.A.; Maia, R.; Berhouet, J.; Walch, G. Open Latarjet procedure for management of bone loss in anterior instability of the glenohumeral joint. J. Shoulder Elbow Surg. 2011, 20 (Suppl. 2), S61–S69. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Frank, R.M.; Ghodadra, N.S.; Hsu, A.R.; Romeo, A.A.; Bach, B.R., Jr.; Boileau, P.; Provencher, M.T. The outcomes and surgical techniques of the Latarjet procedure. Arthroscopy 2014, 30, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gordins, V.; Hovelius, L.; Sandström, B.; Rahme, H.; Bergström, U. Risk of arthropathy after the Bristow-Latarjet repair: A radiologic and clinical thirty-three to thirty-five years of follow-up of thirty-one shoulders. J. Shoulder Elbow Surg. 2015, 24, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Lafosse, L.; Boyle, S.; Gutierrez-Aramberri, M.; Shah, A.; Meller, R. Arthroscopic latarjet procedure. Orthop. Clin. N. Am. 2010, 41, 393–405. [Google Scholar] [CrossRef]
- Young, A.A.; Baba, M.; Neyton, L.; Godeneche, A.; Walch, G. Coracoid graft dimensions after harvesting for the open Latarjet procedure. J. Shoulder Elbow Surg. 2013, 22, 485–488. [Google Scholar] [CrossRef]
- Bachy, M.; Lapner, P.L.; Goutallier, D.; Allain, J.; Hernigou, P.; Bénichou, J.; Zilber, S. Coracoid process x-ray investigation before Latarjet procedure: A radioanatomic study. J. Shoulder Elbow Surg. 2013, 22, e10–e14. [Google Scholar] [CrossRef]
- De Beer, J.; Burkhart, S.S.; Roberts, C.P.; van Rooyen, K.; Cresswell, T.; du Toit, D.F. The Congruent-Arc Latarjet. Tech. Shoulder Elbow Surg. 2009, 10, 62–67. [Google Scholar] [CrossRef]
- De Beer, J.F.; Roberts, C. Glenoid bone defects: Open Latarjet with congruent arc modification. Orthop. Clin. N. Am. 2010, 41, 407–415. [Google Scholar] [CrossRef]
- Boons, H.W.; Giles, J.W.; Elkinson, I.; Johnson, J.A.; Athwal, G.S. Classic versus congruent coracoid positioning during the Latarjet procedure: An in vitro biomechanical comparison. Arthroscopy 2013, 29, 309–316. [Google Scholar] [CrossRef]
- Montgomery, S.R.; Katthagen, J.C.; Mikula, J.D.; Marchetti, D.C.; Tahal, D.S.; Dornan, G.J.; Dahl, K.D.; Brady, A.W.; Turnbull, T.L.; Millett, P.J. Anatomic and Biomechanical Comparison of the Classic and Congruent-Arc Techniques of the Latarjet Procedure. Am. J. Sports Med. 2017, 45, 1252–1260. [Google Scholar] [CrossRef]
- Hantes, M.E.; Venouziou, A.; Bargiotas, K.A.; Metafratzi, Z.; Karantanas, A.; Malizos, K.N. Repair of an anteroinferior glenoid defect by the latarjet procedure: Quantitative assessment of the repair by computed tomography. Arthroscopy 2010, 26, 1021–1026. [Google Scholar] [CrossRef]
- Dolan, C.M.; Hariri, S.; Hart, N.D.; McAdams, T.R. An anatomic study of the coracoid process as it relates to bone transfer procedures. J. Shoulder Elbow Surg. 2011, 20, 497–501. [Google Scholar] [CrossRef]
- Bueno, R.S.; Ikemoto, R.Y.; Nascimento, L.G.; Almeida, L.H.; Strose, E.; Murachovsky, J. Correlation of coracoid thickness and glenoid width: An anatomic morphometric analysis. Am. J. Sports Med. 2012, 40, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, G.M.; Paul, J.; Sandmann, G.H.; Imhoff, A.B.; Schöttle, P.B. The coracoidal insertion of the coracoclavicular ligaments: An anatomic study. Am. J. Sports Med. 2008, 36, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S.; De Beer, J.F. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000, 16, 677–694. [Google Scholar] [CrossRef]
- Kraus, T.M.; Martetschläger, F.; Graveleau, N.; Klouche, S.; Freude, T.; Stöckle, U.; Hardy, P. CT-based quantitative assessment of the surface size and en-face position of the coracoid block post-Latarjet procedure. Arch. Orthop. Trauma Surg. 2013, 133, 1543–1548. [Google Scholar] [CrossRef]
- Ji, J.H.; Kwak, D.S.; Yang, P.S.; Kwon, M.J.; Han, S.H.; Jeong, J.J. Comparisons of glenoid bony defects between normal cadaveric specimens and patients with recurrent shoulder dislocation: An anatomic study. J. Shoulder Elbow Surg. 2012, 21, 822–827. [Google Scholar] [CrossRef]
- Saito, H.; Itoi, E.; Sugaya, H.; Minagawa, H.; Yamamoto, N.; Tuoheti, Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am. J. Sports Med. 2005, 33, 889–893. [Google Scholar] [CrossRef]
- Ghodadra, N.; Gupta, A.; Romeo, A.A.; Bach, B.R., Jr.; Verma, N.; Shewman, E.; Goldstein, J.; Provencher, M.T. Normalization of glenohumeral articular contact pressures after Latarjet or iliac crest bone-grafting. J. Bone Jt. Surg. Am. 2010, 92, 1478–1489. [Google Scholar] [CrossRef]

| Measure | ICC * | p-Value |
|---|---|---|
| GCW | 0.907 | <0.001 |
| CPL | 0.866 | <0.001 |
| CPW | 0.659 | <0.001 |
| CPT | 0.910 | <0.001 |
| CPL-O | 0.953 | <0.001 |
| CPW-O | 0.927 | <0.001 |
| CPT-O | 0.943 | <0.001 |
| Measure | Classic Latarjet (n = 35) | Congruent arc Latarjet (n = 35) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Med ⸷ | SD * | Min + | Max ° | Mean | Med ⸷ | SD * | Min + | Max ° | |
| GCW | 29.8 | 29.6 | 3.1 | 25.1 | 36.4 | 30.5 | 29.6 | 3.2 | 25.9 | 38.3 |
| CPL | 44.5 | 44.4 | 4.0 | 35.5 | 52.3 | 47.1 | 46.4 | 4.4 | 38.8 | 55.5 |
| CPW | 16.4 | 16.1 | 2.3 | 13.1 | 22.3 | 17.1 | 16.5 | 2.0 | 14.1 | 21.7 |
| CPT | 11.5 | 11.1 | 1.8 | 8.6 | 15.4 | 11.8 | 11.3 | 1.8 | 8.8 | 15.9 |
| CPL-O | 22.0 | 22.1 | 2.6 | 15.8 | 26.3 | 23.0 | 23.6 | 2.5 | 16.6 | 27.4 |
| CPW-O | 16.0 | 15.4 | 2.0 | 12.9 | 21.3 | 13.9 | 13.6 | 2.2 | 10.4 | 18.2 |
| CPT-O | 8.4 | 8.3 | 1.6 | 5.8 | 12.7 | 11.3 | 10.9 | 1.8 | 8.3 | 17.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregori, M.; Eichelberger, L.; Gahleitner, C.; Hajdu, S.; Pretterklieber, M. Relationship between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae. J. Clin. Med. 2020, 9, 207. https://doi.org/10.3390/jcm9010207
Gregori M, Eichelberger L, Gahleitner C, Hajdu S, Pretterklieber M. Relationship between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae. Journal of Clinical Medicine. 2020; 9(1):207. https://doi.org/10.3390/jcm9010207
Chicago/Turabian StyleGregori, Markus, Lukas Eichelberger, Claudia Gahleitner, Stefan Hajdu, and Michael Pretterklieber. 2020. "Relationship between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae" Journal of Clinical Medicine 9, no. 1: 207. https://doi.org/10.3390/jcm9010207
APA StyleGregori, M., Eichelberger, L., Gahleitner, C., Hajdu, S., & Pretterklieber, M. (2020). Relationship between the Thickness of the Coracoid Process and Latarjet Graft Positioning—An Anatomical Study on 70 Embalmed Scapulae. Journal of Clinical Medicine, 9(1), 207. https://doi.org/10.3390/jcm9010207