Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Sample and Data Collection
2.3. Quantification of sCOMP
2.4. Statistical Analysis
3. Results
3.1. Associations between sCOMP and Other Laboratory Parameters at Baseline—With and without eGFR Adjustment
3.2. Associations between sCOMP and Clinical Characteristics of OA Patients at Baseline—With and without eGFR Adjustment
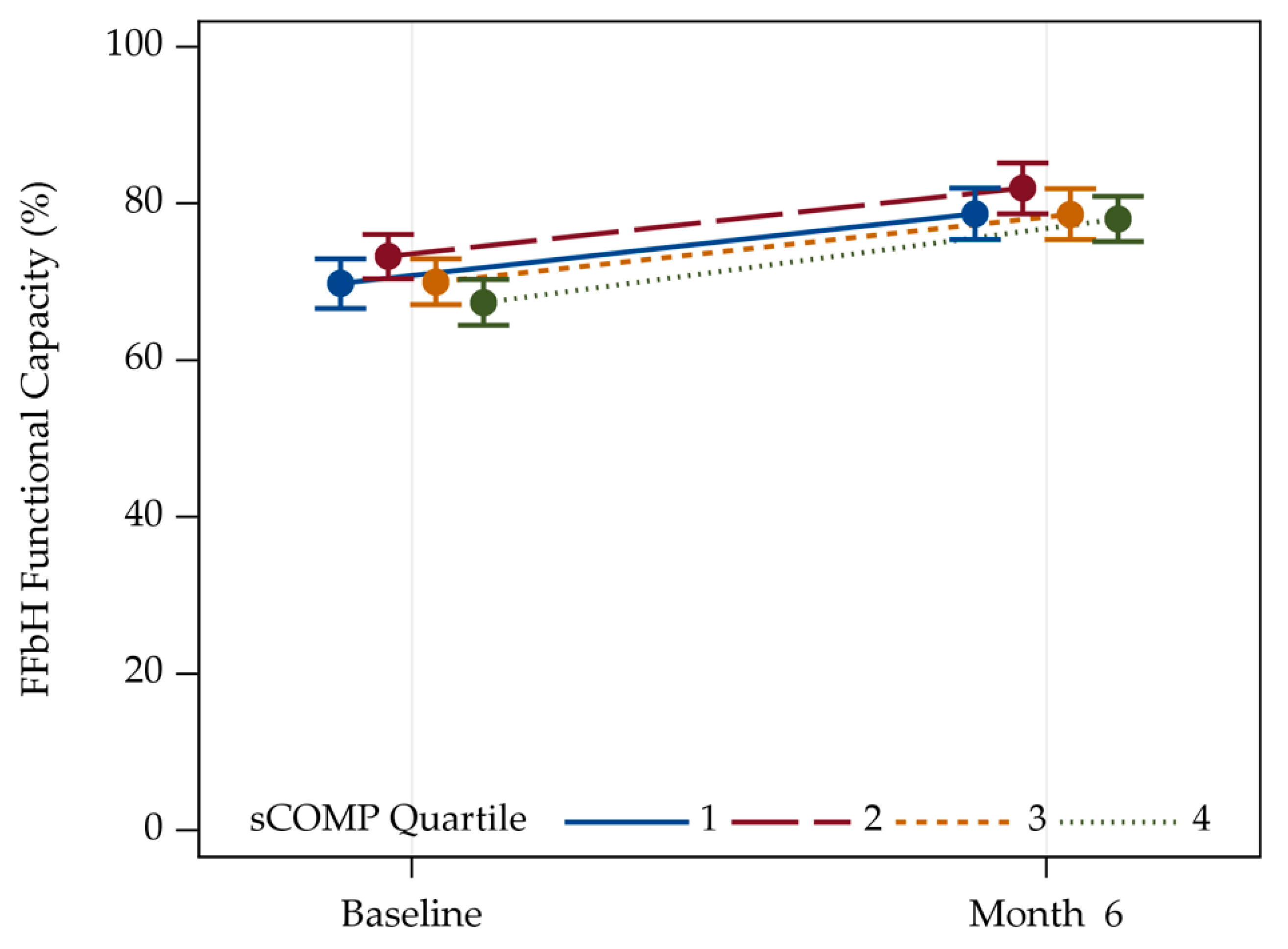
3.3. Correlation between sCOMP and FFbH Functionality for Patients with Hip and Knee OA
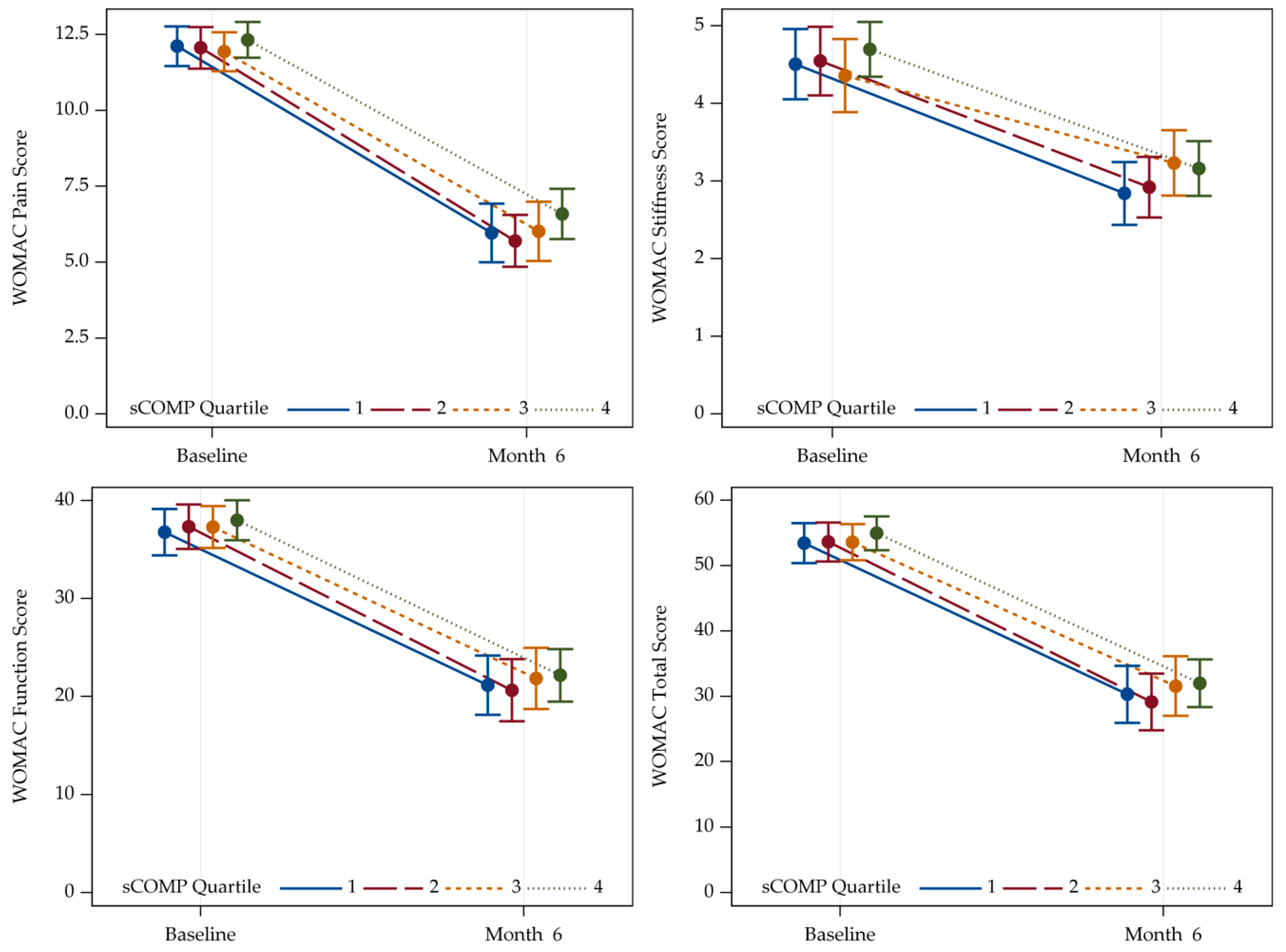
3.4. Association between sCOMP and WOMAC in Patients with Hip and Knee OA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| COMP Quarter | ||||||
|---|---|---|---|---|---|---|
| Parameter | Category/Statistic | Quarter 1 (N = 188) | Quarter 2 (N = 189) | Quarter 3 (N = 189) | Quarter 4 (N = 188) | Total (N = 754) |
| FFbH Functional | Baseline | 67.8 (15.0) | 67.1 (15.2) | 68.2 (15.7) | 66.2 (16.4) | 67.4 (15.5) |
| Capacity (%) | 6 months | 80.4 (14.1) | 82.5 (14.9) | 78.8 (15.7) | 79.1 (15.7) | 80.1 (15.2) |
| COMP Quarter | ||||||
|---|---|---|---|---|---|---|
| Parameter | Category/Statistic | Quarter 1 (N = 188) | Quarter 2 (N = 189) | Quarter 3 (N = 189) | Quarter 4 (N = 188) | Total (N = 754) |
| WOMAC Pain | Baseline | 12.0 (3.4) | 11.8 (3.3) | 11.4 (3.5) | 12.0 (3.5) | 11.8 (3.4) |
| 6 months | 4.7 (4.0) | 4.6 (3.7) | 5.0 (4.4) | 5.1 (4.2) | 4.8 (4.1) | |
| WOMAC Stiffness | Baseline | 4.6 (2.1) | 4.7 (2.0) | 4.5 (2.2) | 4.7 (2.0) | 4.6 (2.1) |
| 6 months | 2.4 (1.7) | 2.4 (1.8) | 2.9 (2.0) | 2.7 (1.8) | 2.6 (1.8) | |
| WOMAC Function | Baseline | 38.2 (11.0) | 39.7 (10.9) | 38.4 (11.9) | 38.3 (11.8) | 38.7 (11.2) |
| 6 months | 18.5 (12.5) | 16.9 (13.3) | 20.1 (14.0) | 19.0 (14.6) | 18.6 (13.6) | |
| WOMAC Total | Baseline | 54.8 (14.7) | 56.1 (14.4) | 54.3 (14.7) | 54.9 (15.3) | 55.0 (14.8) |
| 6 months | 25.9 (17.4) | 23.7 (17.9) | 28.5 (19.4) | 26.9 (19.4) | 26.2 (18.5) | |
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.F.; Lee, Y.Y.; Su, E.P.; McLawhorn, A.S. Quality-Adjusted Life Years After Hip and Knee Arthroplasty: Health-Related Quality of Life After 12,782 Joint Replacements. JBJS Open Access 2018, 3, e0007. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, H.; Kinghorn, P.; Oppong, R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Henderson, G.R.; Gaston, P.; MacDonald, D.; Howie, C.; Simpson, A.H. Comparative outcomes of total hip and knee arthroplasty: A prospective cohort study. Postgrad. Med. J. 2012, 88, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Dumenci, L.; Perera, R.A.; Keefe, F.J.; Ang, D.C.; Slover, J.; Jensen, M.P.; Riddle, D.L. Model-based pain and function outcome trajectory types for patients undergoing knee arthroplasty: A secondary analysis from a randomized clinical trial. Osteoarthr. Cartil. 2019, 27, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.E. Osteoarthritis biomarkers: Year in review. Osteoarthr. Cartil. 2018, 26, 312–318. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Thudium, C.S.; Mobasheri, A. Development and use of biochemical markers in osteoarthritis: current update. Curr. Opin. Rheumatol. 2018, 30, 121–128. [Google Scholar] [CrossRef]
- Hosnijeh, F.S.; Siebuhr, A.S.; Uitterlinden, A.G.; Oei, E.H.G.; Hofman, A.; Karsdal, M.A.; Bierma-Zeinstra, S.M.; Bay-Jensen, A.C.; van Meurs, J.B.J. Association between biomarkers of tissue inflammation and progression of osteoarthritis: Evidence from the Rotterdam study cohort. Arthritis Res. Ther. 2016, 18, 81. [Google Scholar] [CrossRef]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five-year prospective cohort study. Arthritis Res. Ther. 2015, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.Q.; Zhang, J.F.; He, Q.Q.; Wang, Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2019, 27, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Yik, J.H.N.; Kishore, A.; Dinh, V.V.; Di Cesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: Interaction, regulation and role in chondrogenesis. Matrix Biol. 2014, 37, 102–111. [Google Scholar] [CrossRef]
- Posey, K.L.; Hecht, J.T. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets 2008, 9, 869–877. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013, 31, 999–1006. [Google Scholar] [CrossRef]
- Hoch, J.M.; Mattacola, C.G.; Bush, H.M.; Medina McKeon, J.M.; Hewett, T.E.; Lattermann, C. Longitudinal documentation of serum cartilage oligomeric matrix protein and patient-reported outcomes in collegiate soccer athletes over the course of an athletic season. Am. J. Sports Med. 2012, 40, 2583–2589. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Saxne, T.; Heinegard, D.K. Release of Cartilage Oligomeric Matrix Protein (Comp) into Joint Fluid after Knee Injury and in Osteoarthritis. Ann. Rheum. Dis. 1994, 53, 8–13. [Google Scholar] [CrossRef]
- Firner, S.; Zaucke, F.; Michael, J.; Dargel, J.; Schiwy-Bochat, K.H.; Heilig, J.; Rothschild, M.A.; Eysel, P.; Bruggemann, G.P.; Niehoff, A. Extracellular Distribution of Collagen II and Perifibrillar Adapter Proteins in Healthy and Osteoarthritic Human Knee Joint Cartilage. J. Histochem. Cytochem. 2017, 65, 593–606. [Google Scholar] [CrossRef]
- Koelling, S.; Clauditz, T.S.; Kaste, M.; Miosge, N. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, 56. [Google Scholar] [CrossRef]
- Sturmer, T.; Sun, Y.; Sauerland, S.; Zeissig, I.; Gunther, K.P.; Puhl, W.; Brenner, H. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J. Rheumatol. 1998, 25, 1827–1832. [Google Scholar] [PubMed]
- Buchele, G.; Gunther, K.P.; Brenner, H.; Puhl, W.; Sturmer, T.; Rothenbacher, D.; Brenner, R.E. Osteoarthritis-patterns, cardio-metabolic risk factors and risk of all-cause mortality: 20 years follow-up in patients after hip or knee replacement. Sci. Rep. 2018, 8, 5253. [Google Scholar] [CrossRef] [PubMed]
- Gunther, K.P.; Sturmer, T.; Sauerland, S.; Zeissig, I.; Sun, Y.; Kessler, S.; Scharf, H.P.; Brenner, H.; Puhl, W. Prevalence of generalised osteoarthritis in patients with advanced hip and knee osteoarthritis: The Ulm Osteoarthritis Study. Ann. Rheum. Dis. 1998, 57, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sturmer, T.; Brenner, H.; Koenig, W.; Gunther, K.P. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann. Rheum. Dis. 2004, 63, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X.; Becker, D.M.; Bouchard, C.; Carleton, R.A.; Colditz, G.A.; Dietz, W.H.; Foreyt, J.P.; Garrison, R.J.; Grundy, S.M.; Hansen, B.C.; et al. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation-Study of Womac—A Health-Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug-Therapy in Patients with Osteo-Arthritis of the Hip or Knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Inker, L.A.; Levey, A.S. Pro: Estimating GFR using the chronic kidney disease epidemiology collaboration (CKD-EPI) 2009 creatinine equation: The time for change is now. Nephrol. Dial. Transpl. 2013, 28, 1390–1394. [Google Scholar] [CrossRef][Green Version]
- Fernandes, F.A.; Pucinelli, M.L.; da Silva, N.P.; Feldman, D. Serum cartilage oligomeric matrix protein (COMP) levels in knee osteoarthritis in a Brazilian population: Clinical and radiological correlation. Scand. J. Rheumatol. 2007, 36, 211–215. [Google Scholar] [CrossRef]
- Kluzek, S.; Bay-Jensen, A.C.; Judge, A.; Karsdal, M.A.; Shorthose, M.; Spector, T.; Hart, D.; Newton, J.L.; Arden, N.K. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers 2015, 20, 557–564. [Google Scholar] [CrossRef]
- Sharif, M.; Granell, R.; Johansen, J.; Clarke, S.; Elson, C.; Kirwan, J.R. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology (Oxford) 2006, 45, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Misumi, K.; Tagami, M.; Kamimura, T.; Miyakoshi, D.; Helal, I.E.; Arai, K.; Fujiki, M. Urine cartilage oligomeric matrix protein (COMP) measurement is useful in discriminating the osteoarthritic Thoroughbreds. Osteoarthr. Cartil. 2006, 14, 1174–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleck, C.; Janz, A.; Schweitzer, F.; Karge, E.; Schwertfeger, M.; Stein, G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in renal failure patients. Kidney Int. Suppl. 2001, 78, S14–S18. [Google Scholar] [CrossRef] [PubMed]
- Bjurman, C.; Petzold, M.; Venge, P.; Farbemo, J.; Fu, M.L.; Hammarsten, O. High-sensitive cardiac troponin, NT-proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin. Biochem. 2015, 48, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Brenner, H.; Sauerland, S.; Gunther, K.P.; Puhl, W.; Sturmer, T. Serum uric acid and patterns of radiographic osteoarthritis--the Ulm Osteoarthritis Study. Scand. J. Rheumatol. 2000, 29, 380–386. [Google Scholar]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar]
- Veronese, N.; Cereda, E.; Maggi, S.; Luchini, C.; Solmi, M.; Smith, T.; Denkinger, M.; Hurley, M.; Thompson, T.; Manzato, E.; et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheu. 2016, 46, 160–167. [Google Scholar] [CrossRef]
- Fu, Y.; Kong, W. Cartilage Oligomeric Matrix Protein: Matricellular and Matricrine Signaling in Cardiovascular Homeostasis and Disease. Curr. Vasc. Pharmacol. 2017, 15, 186–196. [Google Scholar] [CrossRef]
- Wang, F.F.; Ha, L.; Yu, H.Y.; Mi, L.; Han, J.L.; Gao, W. Altered serum level of cartilage oligomeric matrix protein and its association with coronary calcification in patients with coronary heart disease. J. Geriatr. Cardiol. 2017, 14, 87–92. [Google Scholar]
- Du, Y.; Gao, C.; Liu, Z.; Wang, L.; Liu, B.; He, F.; Zhang, T.; Wang, Y.; Wang, X.; Xu, M.; et al. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2580–2588. [Google Scholar] [CrossRef]
- Ueland, T.; Laugsand, L.E.; Vatten, L.J.; Janszky, I.; Platou, C.; Michelsen, A.E.; Damas, J.K.; Aukrust, P.; Asvold, B.O. Extracellular matrix markers and risk of myocardial infarction: The HUNT Study in Norway. Eur. J. Prev. Cardiol. 2017, 24, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fu, Y.; Qi, R.; Wang, M.; Yang, N.; He, L.; Yu, F.; Zhang, J.; Yun, C.H.; Wang, X.; et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood 2015, 126, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Morrow, D.A.; Braunwald, E.; Davies, R.Y.; Goodrich, E.L.; Murphy, S.A.; Cannon, C.P.; O’Donoghue, M.L. Cystatin C for Risk Stratification in Patients After an Acute Coronary Syndrome. J. Am. Heart Assoc. 2018, 7, e009077. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, E.; Cheng, X.W.; Urakawa, H.; Arai, E.; Yamada, Y.; Kitamura, S.; Sato, K.; Kuzuya, M.; Ishiguro, N.; Nishida, Y. Increased expression and activation of cathepsin K in human osteoarthritic cartilage and synovial tissues. J. Orthop. Res. 2016, 34, 127–134. [Google Scholar] [CrossRef]
- Wislowska, M.; Jablonska, B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin. Rheumatol. 2005, 24, 278–284. [Google Scholar] [CrossRef]
- Das Gupta, E.; Ng, W.R.; Wong, S.F.; Bhurhanudeen, A.K.; Yeap, S.S. Correlation of serum cartilage oligomeric matrix protein (COMP) and interleukin-16 (IL-16) levels with disease severity in primary knee osteoarthritis: A pilot study in a Malaysian population. PLoS ONE 2017, 12, e0184802. [Google Scholar]
- Garnero, P.; Piperno, M.; Gineyts, E.; Christgau, S.; Delmas, P.D.; Vignon, E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: Relations with disease activity and joint damage. Ann. Rheum. Dis. 2001, 60, 619–626. [Google Scholar] [CrossRef]
- Sowers, M.F.; Karvonen-Gutierrez, C.A.; Yosef, M.; Jannausch, M.; Jiang, Y.; Garnero, P.; Jacobson, J. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthr. Cartil. 2009, 17, 1609–1614. [Google Scholar] [CrossRef]
| Association to COMP [1] | Association to COMP [2] | ||||
|---|---|---|---|---|---|
| Parameter | Total (N = 754) Median (Q1, Q3) | Rho | p-Value | Rho | p-Value |
| COMP (ng/mL) | 799.6 (600.4, 1068.3) | ||||
| hsCRP (mg/L) | 2.5 (1.3, 5.0) | −0.065 | 0.074 | −0.078 | 0.036 |
| Uric Acid (µmol/L) | 315.4 (267.8, 378.0) | 0.044 | 0.264 | 0.023 | 0.559 |
| AST (IU/L) | 10.0 (8.0, 12.0) | 0.128 | <0.001 | 0.139 | <0.001 |
| ALT (IU/L) | 11.0 (9.0, 16.0) | 0.010 | 0.788 | 0.027 | 0.466 |
| Alkaline Phosphatase (IU/L) | 103.0 (85.0, 125.0) | 0.076 | 0.055 | 0.071 | 0.076 |
| Calcium (mmol/L) | 2.4 (2.3, 2.4) | 0.021 | 0.589 | 0.043 | 0.274 |
| Phosphate (mmol/L) | 1.0 (0.9, 1.2) | 0.031 | 0.464 | 0.035 | 0.414 |
| GDF-15 (ng/L) | 1007.5 (780.9, 1280.5) | 0.066 | 0.096 | 0.039 | 0.339 |
| hs-cTnT (ng/L) | 3.2 (1.5, 6.2) | 0.020 | 0.603 | 0.017 | 0.663 |
| hs-cTnI (ng/L) | 3.9 (2.9, 5.7) | 0.045 | 0.222 | 0.042 | 0.263 |
| NT-proBNP (ng/L) | 96.9 (51.9, 180.2) | 0.102 | 0.006 | 0.095 | 0.011 |
| Cystatin C (mg/L) | 0.9 (0.8, 1.0) | 0.171 | <0.001 | 0.136 | <0.001 |
| Creatinine (µmol/L) | 77.0 (67.0, 88.4) | 0.095 | 0.010 | ||
| eGFR (mL/min/1.73 m²) | 78.6 (64.9, 92.0) | −0.097 | 0.009 | ||
| sCOMP Quartile | sCOMP, ng/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Category/Statistic | Quartile 1 (N = 188) | Quartile 2 (N = 189) | Quartile 3 (N = 189) | Quartile 4 (N = 188) | Total (N = 754) | Median | p-Value | p-Value |
| Age (years) | Median (Q1, Q3) | 61.5 (55.0, 68.0) | 65.0 (57.0, 69.0) | 66.0 (59.0, 71.0) | 67.0 (62.0, 71.0) | 65.0 (58.0, 70.0) | |||
| ≥25 to <60 | 80 (42.6%) | 61 (32.3%) | 48 (25.4%) | 32 (17.0%) | 221 (29.3%) | 697.95 | |||
| ≥60 to <70 | 69 (36.7%) | 81 (42.9%) | 83 (43.9%) | 88 (46.8%) | 321 (42.6%) | 821.71 | |||
| ≥70 to <80 | 39 (20.7%) | 47 (24.9%) | 58 (30.7%) | 68 (36.2%) | 212 (28.1%) | 885.53 | <0.0001 a | <0.001 c | |
| Sex | Male | 60 (31.9%) | 72 (38.1%) | 72 (38.1%) | 81 (43.1%) | 285 (37.8%) | 815.13 | ||
| Female | 128 (68.1%) | 117 (61.9%) | 117 (61.9%) | 107 (56.9%) | 469 (62.2%) | 779.53 | 0.047 a | <0.001 c | |
| BMI (kg/m²) | Median (Q1, Q3) | 27.1 (25.0, 29.5) | 28.0 (25.7, 30.8) | 28.1 (25.6, 31.1) | 28.3 (25.7, 32.0) | 27.8 (25.4, 30.8) | |||
| ≥0 to <25 | 48 (25.5%) | 40 (21.2%) | 43 (22.8%) | 39 (20.7%) | 170 (22.5%) | 787.03 | |||
| ≥25 to <30 | 99 (52.7%) | 93 (49.2%) | 84 (44.4%) | 78 (41.5%) | 354 (46.9%) | 759.79 | |||
| ≥30 to <35 | 33 (17.6%) | 47 (24.9%) | 45 (23.8%) | 54 (28.7%) | 179 (23.7%) | 830.58 | |||
| ≥35 | 8 (4.3%) | 9 (4.8%) | 17 (9.0%) | 17 (9.0%) | 51 (6.8%) | 905.45 | 0.002 a | 0.005 c | |
| Localization of OA | Hip | 109 (58.0%) | 104 (55.0%) | 101 (53.4%) | 80 (42.6%) | 394 (52.3%) | 777.75 | ||
| Knee | 79 (42.0%) | 85 (45.0%) | 88 (46.6%) | 108 (57.4%) | 360 (47.7%) | 839.53 | 0.338 b | 0.487 d | |
| Laterality of OA | Unilateral OA | 30 (16.0%) | 32 (16.9%) | 21 (11.1%) | 22 (11.7%) | 105 (13.9%) | 718.09 | ||
| Bilateral OA | 143 (76.1%) | 141 (74.6%) | 154 (81.5%) | 144 (76.6%) | 582 (77.2%) | 806.22 | 0.443 b | 0.426 d | |
| Unknown | 15 (8.0%) | 16 (8.5%) | 14 (7.4%) | 22 (11.7%) | 67 (8.9%) | ||||
| Generalization of OA | Not-generalized OA | 118 (62.8%) | 116 (61.4%) | 106 (56.1%) | 101 (53.7%) | 441 (58.5%) | 781.15 | ||
| Generalized OA | 28 (14.9%) | 39 (20.6%) | 44 (23.3%) | 49 (26.1%) | 160 (21.2%) | 875.00 | 0.110 b | 0.063 d | |
| Unknown | 42 (22.3%) | 34 (18.0%) | 39 (20.6%) | 38 (20.2%) | 153 (20.3%) | ||||
| Cause of OA | Primary OA | 108 (57.4%) | 124 (65.6%) | 117 (61.9%) | 108 (57.4%) | 457 (60.6%) | 792.86 | ||
| Secondary OA | 75 (39.9%) | 59 (31.2%) | 68 (36.0%) | 72 (38.3%) | 274 (36.3%) | 815.03 | 0.353 b | 0.417 d | |
| Unknown | 5 (2.7%) | 6 (3.2%) | 4 (2.1%) | 8 (4.3%) | 23 (3.1%) | ||||
| Smoking Status | Never | 115 (61.2%) | 110 (58.2%) | 103 (54.5%) | 109 (58.0%) | 437 (58.0%) | 784.81 | ||
| Former | 48 (25.5%) | 62 (32.8%) | 57 (30.2%) | 54 (28.7%) | 221 (29.3%) | 800.03 | |||
| Current | 25 (13.3%) | 17 (9.0%) | 29 (15.3%) | 25 (13.3%) | 96 (12.7%) | 822.34 | 0.192 b | 0.210 d | |
| Comorbidities | |||||||||
| Diabetes | No | 178 (94.7%) | 176 (93.1%) | 166 (87.8%) | 169 (89.9%) | 689 (91.4%) | 787.22 | ||
| Yes | 10 (5.3%) | 13 (6.9%) | 22 (11.6%) | 19 (10.1%) | 64 (8.5%) | 857.81 | 0.446 b | 0.741 d | |
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.1%) | ||||
| Hypertension | No | 93 (49.5%) | 105 (55.6%) | 95 (50.3%) | 78 (41.5%) | 371 (49.2%) | 772.54 | ||
| Yes | 95 (50.5%) | 84 (44.4%) | 94 (49.7%) | 110 (58.5%) | 383 (50.8%) | 818.21 | 0.918 b | 0.852 d | |
| Cardiac | No | 181 (96.3%) | 180 (95.2%) | 181 (95.8%) | 180 (95.7%) | 722 (95.8%) | 799.56 | ||
| Infarction | Yes | 7 (3.7%) | 9 (4.8%) | 7 (3.7%) | 8 (4.3%) | 31 (4.1%) | 776.07 | 0.668 b | 0.538 d |
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.1%) | ||||
| Cardiac | No | 161 (85.6%) | 159 (84.1%) | 152 (80.4%) | 138 (73.4%) | 610 (80.9%) | 785.20 | ||
| Insufficiency | Yes | 27 (14.4%) | 30 (15.9%) | 36 (19.0%) | 50 (26.6%) | 143 (19.0%) | 877.47 | 0.382 b | 0.525 d |
| Unknown | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.1%) | ||||
| Baseline | Follow-Up 6 Months | |||||
|---|---|---|---|---|---|---|
| Predictors | β-Coefficients | CI | p-Value | β-Coefficients | CI | p-Value |
| ln (COMP) | −3.87 | −7.60–−0.15 | 0.042 | −1.46 | −5.34–2.43 | 0.463 |
| Age | −0.35 | −0.64–−0.07 | 0.016 | −0.27 | −0.59–0.05 | 0.098 |
| Sex: Female | −7.07 | −11.07–−3.07 | 0.001 | −5.63 | −9.79–−1.47 | 0.009 |
| BMI | −0.30 | −0.73–0.12 | 0.164 | −0.46 | −0.91–−0.01 | 0.048 |
| eGFR | −0.07 | −0.17–0.04 | 0.199 | −0.08 | −0.19–0.03 | 0.162 |
| Observations | 297 | 236 | ||||
| R2/adjusted R2 | 0.096/0.080 | 0.067/0.046 | ||||
| (A) WOMAC Scores—Baseline; Knee OA Patients. | ||||||||||||
| Pain Score | Stiffness Score | Function Score | Total Score | |||||||||
| Predictors | β-coefficients | CI | p | β-coefficients | CI | p | β-coefficients | CI | p | β-coefficients | CI | p |
| ln (COMP) | 0.72 | −0.06–1.50 | 0.071 | 0.40 | −0.15–0.94 | 0.154 | 3.56 | 0.83–6.29 | 0.011 | 4.42 | 0.87–7.97 | 0.015 |
| Age | −0.07 | −0.13–−0.01 | 0.031 | −0.02 | −0.06–0.02 | 0.311 | −0.12 | −0.32–0.09 | 0.270 | −0.18 | −0.45–0.09 | 0.184 |
| Sex: Female | 2.25 | 1.38–3.11 | <0.001 | 0.82 | 0.22–1.43 | 0.008 | 7.02 | 3.97–10.06 | <0.001 | 9.12 | 5.17–13.07 | <0.001 |
| BMI | 0.02 | −0.07–0.11 | 0.700 | 0.00 | −0.06–0.07 | 0.911 | 0.05 | −0.26–0.36 | 0.747 | 0.02 | −0.38–0.42 | 0.931 |
| eGFR | 0.01 | −0.01–0.03 | 0.418 | 0.01 | −0.01–0.02 | 0.495 | 0.04 | −0.04–0.11 | 0.338 | 0.04 | −0.06–0.14 | 0.478 |
| Observations | 278 | 278 | 262 | 278 | ||||||||
| R2/adjusted R2 | 0.097/0.080 | 0.031/0.013 | 0.088/0.070 | 0.082/0.065 | ||||||||
| (B) WOMAC Scores—Follow-up Six Months; Knee OA Patients. | ||||||||||||
| Pain Score | Stiffness Score | Function Score | Total Score | |||||||||
| Predictors | β-coefficients | CI | p | β-coefficients | CI | p | β-coefficients | CI | p | β-coefficients | CI | p |
| ln (COMP) | 0.93 | −0.20–2.06 | 0.109 | 0.53 | 0.03–1.02 | 0.037 | 1.98 | −1.74–5.70 | 0.297 | 3.10 | −2.09–8.30 | 0.243 |
| Age | −0.04 | −0.13–0.05 | 0.379 | −0.04 | −0.09–−0.00 | 0.029 | 0.10 | −0.20–0.39 | 0.523 | 0.03 | −0.38–0.45 | 0.879 |
| Sex: Female | 0.75 | −0.45–1.95 | 0.222 | 0.11 | −0.41–0.64 | 0.673 | -0.06 | −4.07–3.96 | 0.977 | 0.42 | −5.17–6.01 | 0.884 |
| BMI | 0.07 | −0.06–0.19 | 0.283 | 0.02 | −0.04–0.07 | 0.507 | 0.48 | 0.07–0.89 | 0.021 | 0.67 | 0.10–1.23 | 0.021 |
| eGFR | 0.03 | −0.00–0.06 | 0.091 | 0.01 | −0.01–0.02 | 0.233 | 0.12 | 0.00–0.23 | 0.042 | 0.14 | −0.01–0.30 | 0.072 |
| Observations | 242 | 253 | 214 | 208 | ||||||||
| R2/adjusted R2 | 0.034/0.014 | 0.045/0.026 | 0.052/0.029 | 0.050/0.027 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riegger, J.; Rehm, M.; Büchele, G.; Brenner, H.; Günther, K.-P.; Rothenbacher, D.; Brenner, R.E. Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers. J. Clin. Med. 2020, 9, 268. https://doi.org/10.3390/jcm9010268
Riegger J, Rehm M, Büchele G, Brenner H, Günther K-P, Rothenbacher D, Brenner RE. Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers. Journal of Clinical Medicine. 2020; 9(1):268. https://doi.org/10.3390/jcm9010268
Chicago/Turabian StyleRiegger, Jana, Martin Rehm, Gisela Büchele, Hermann Brenner, Klaus-Peter Günther, Dietrich Rothenbacher, and Rolf E. Brenner. 2020. "Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers" Journal of Clinical Medicine 9, no. 1: 268. https://doi.org/10.3390/jcm9010268
APA StyleRiegger, J., Rehm, M., Büchele, G., Brenner, H., Günther, K.-P., Rothenbacher, D., & Brenner, R. E. (2020). Serum Cartilage Oligomeric Matrix Protein in Late-Stage Osteoarthritis: Association with Clinical Features, Renal Function, and Cardiovascular Biomarkers. Journal of Clinical Medicine, 9(1), 268. https://doi.org/10.3390/jcm9010268





