Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis and Assessment of the Risk of Bias
2.4. Sensitivity Analyses
2.5. Subgroup Analyses
2.6. Meta-Regression Analyses
3. Results
3.1. Study Characteristics
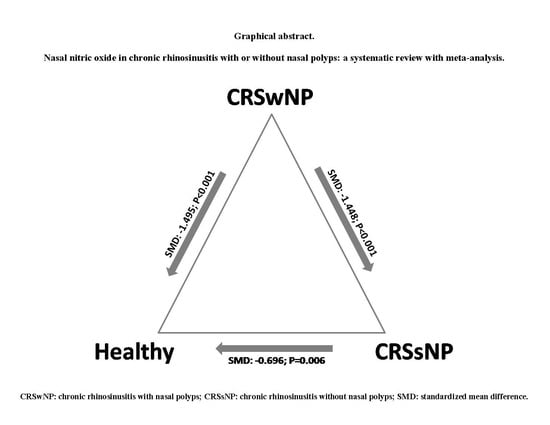
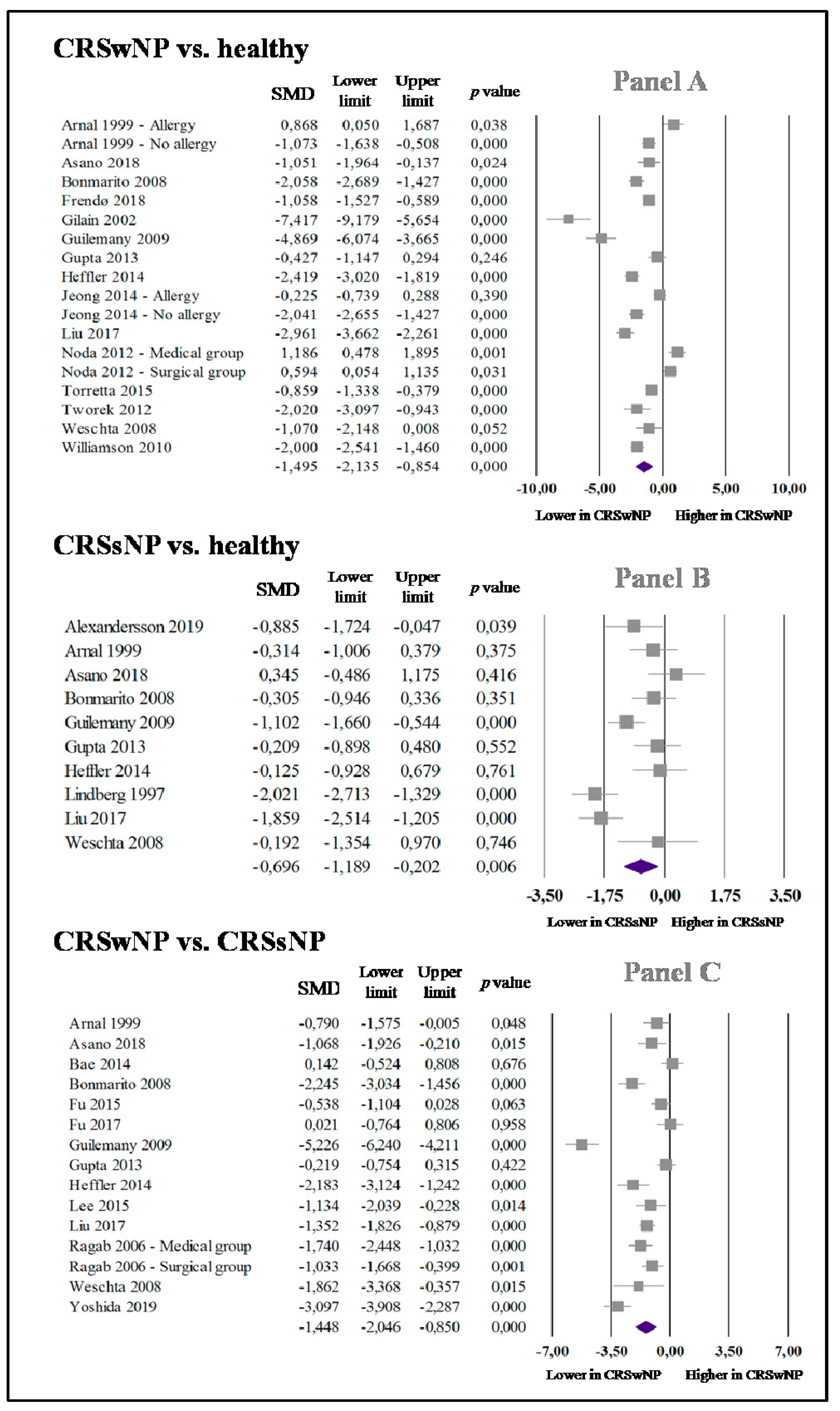
3.2. nNO in CRSwNP and Healthy Controls
3.3. nNO in CRSsNP and Healthy Subjects
3.4. nNO in CRSwNP and CRSsNP
3.5. Publication Bias
3.6. Sensitivity Analyses
3.7. Subgroup Analyses
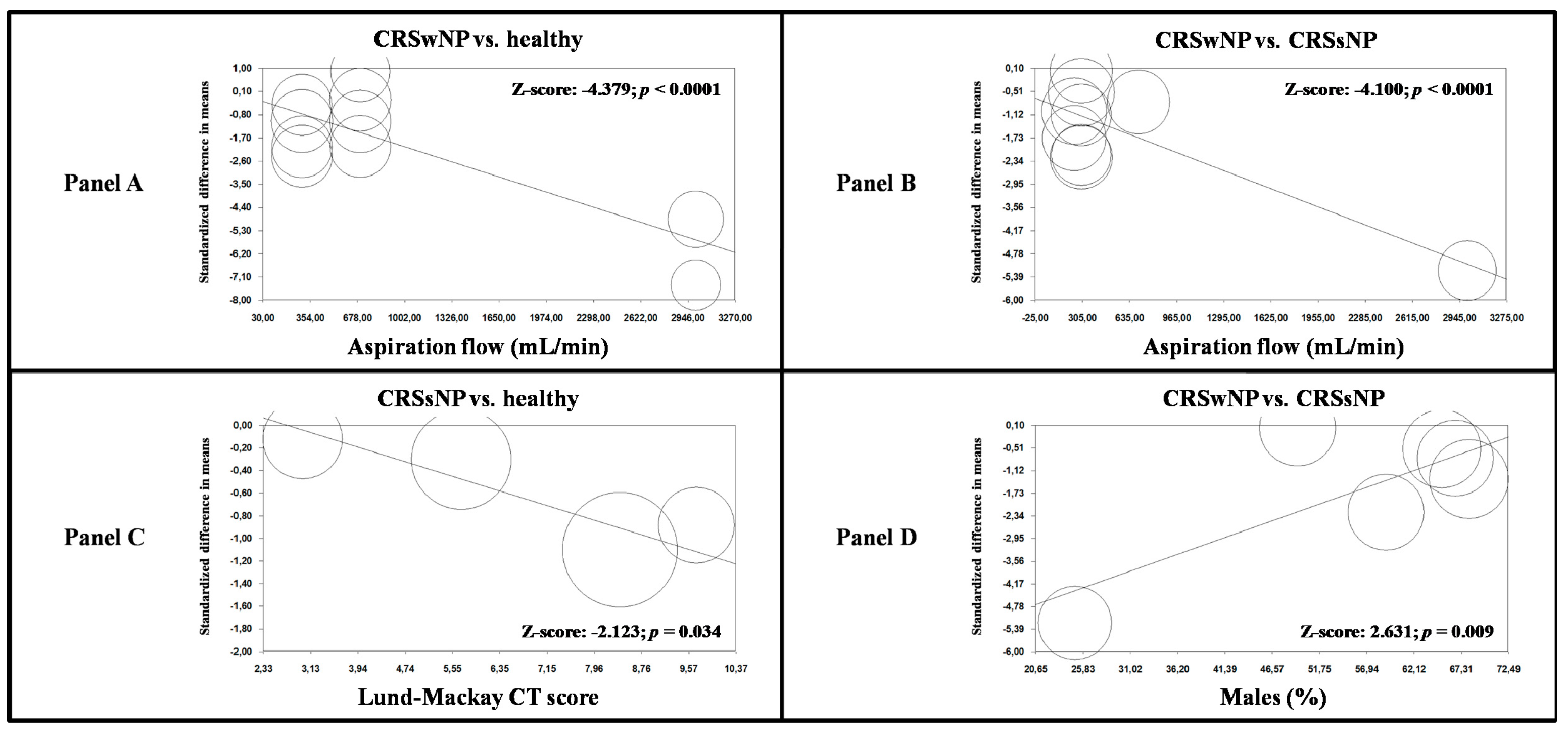
3.8. Meta-Regression Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, W.; Zhang, Y.; Zhang, L. Nasal nitric oxide is correlated with nasal patency and nasal symptoms. Allergy Asthma Immunol. Res. 2019, 11, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res. 2007, 56, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 1033–1041. [Google Scholar] [CrossRef]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Furukawa, K.; Harrison, D.G.; Saleh, D.; Shennib, H.; Chagnon, F.P.; Giaid, A. Expression of nitric oxide synthase in the human nasal mucosa. Am. J. Respir. Crit. Care Med. 1996, 153, 847–850. [Google Scholar] [CrossRef]
- Maniscalco, M.; Sofia, M.; Weitzberg, E.; De Laurentiis, G.; Stanziola, A.; Rossillo, V.; Lundberg, J.O. Humming-induced release of nasal nitric oxide for assessment of sinus obstruction in allergic rhinitis: Pilot study. Eur. J. Clin. Investig. 2004, 34, 555–560. [Google Scholar] [CrossRef]
- Palm, J.P.; Alving, K.; Lundberg, J.O. Characterization of airway nitric oxide in allergic rhinitis: The effect of intranasal administration of L-NAME. Allergy 2003, 58, 885–892. [Google Scholar] [CrossRef]
- Kharitonov, S.; Alving, K.; Barnes, P. Exhaled and nasal nitric oxide measurements: Recommendations. Eur. Respir. J. 1997, 10, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S. Recommendations for standardized procedures for online and offline measurementt of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children. Am. J. Respir. Crit. Care Med. 1999, 160, 2104–2117. [Google Scholar]
- Exhaled, N.O. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef]
- Maniscalco, M.; Bianco, A.; Mazzarella, G.; Motta, A. Recent advances on nitric oxide in the upper airways. Curr. Med. Chem. 2016, 23, 2736–2745. [Google Scholar] [CrossRef]
- Collins, S.A.; Gove, K.; Walker, W.; Lucas, J.S. Nasal nitric oxide screening for primary ciliary dyskinesia: Systematic review and meta-analysis. Eur. Respir. J. 2014, 44, 1589–1599. [Google Scholar] [CrossRef]
- Jeong, J.H.; Yoo, H.S.; Lee, S.H.; Kim, K.R.; Yoon, H.J.; Kim, S.H. Nasal and exhaled nitric oxide in chronic rhinosinusitis with polyps. Am. J. Rhinol. Allergy 2014, 28, e11–e16. [Google Scholar] [CrossRef]
- Lindberg, S.; Cervin, A.; Runer, T. Nitric Oxide (NO) production in the upper airways is decreased in chronic sinusitis. Acta Oto-Laryngol. 1997, 117, 113–117. [Google Scholar] [CrossRef]
- Lee, J.M.; McKnight, C.L.; Aves, T.; Yip, J.; Grewal, A.S.; Gupta, S. Nasal nitric oxide as a marker of sinus mucosal health in patients with nasal polyposis. Int. Forum Allergy Rhinol. 2015, 5, 894–899. [Google Scholar] [CrossRef]
- Yoshida, K.; Takabayashi, T.; Imoto, Y.; Sakashita, M.; Narita, N.; Fujieda, S. Reduced nasal nitric oxide levels in patients with eosinophilic chronic rhinosinusitis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2019, 68, 225–232. [Google Scholar] [CrossRef]
- Fu, C.H.; Tseng, H.J.; Huang, C.C.; Chang, P.H.; Chen, Y.W.; Lee, T.J. Nasal nitric oxide in unilateral sinus disease. PLoS ONE 2017, 12, e0171965. [Google Scholar] [CrossRef]
- Gupta, N.; Drusch, J.; Landis, B.N.; Hummel, T. Nasal nitric oxide levels do not allow for discrimination between olfactory loss due to various etiologies. Laryngoscope 2013, 123, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells GA, S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 1 December 2019).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Alexandersson, C.; Tuomi, L.; Olin, A.C. Measurement of nasal nitric oxide in chronic rhinosinusitis and its relationship to patient-reported outcome: A longitudinal pilot study. Ear Nose Throat J. 2019, 145561319880624. [Google Scholar] [CrossRef]
- Arnal, J.F.; Flores, P.; Rami, J.; Murris-Espin, M.; Bremont, F.; Aguilla, I.P.; Serrano, E.; Didier, A. Nasal nitric oxide concentration in paranasal sinus inflammatory diseases. Eur. Respir. J. 1999, 13, 307–312. [Google Scholar] [CrossRef]
- Asano, T.; Takemura, M.; Kanemitsu, Y.; Yokota, M.; Fukumitsu, K.; Takeda, N.; Ichikawa, H.; Hijikata, H.; Uemura, T.; Takakuwa, O.; et al. Combined measurements of fractional exhaled nitric oxide and nasal nitric oxide levels for assessing upper airway diseases in asthmatic patients. J. Asthma 2018, 55, 300–309. [Google Scholar] [CrossRef]
- Bommarito, L.; Guida, G.; Heffler, E.; Badiu, I.; Nebiolo, F.; Usai, A.; De Stefani, A.; Rolla, G. Nasal nitric oxide concentration in suspected chronic rhinosinusitis. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2008, 101, 358–362. [Google Scholar] [CrossRef]
- Frendo, M.; Hakansson, K.; Schwer, S.; Ravn, A.T.; Meteran, H.; Porsbjerg, C.; Backer, V.; von Buchwald, C. Exhaled and nasal nitric oxide in chronic rhinosinusitis patients with nasal polyps in primary care. Rhinology 2018, 56, 59–64. [Google Scholar] [CrossRef]
- Gilain, L.; Bedu, M.; Jouaville, L.; Guichard, C.; Advenier, D.; Mom, T.; Laurent, S.; Caillaud, D. Analysis of nasal and exhaled nitric oxide concentration in nasal polyposis. Ann. Otolaryngol. Chir. Cervicofac. 2002, 119, 234–242. [Google Scholar] [PubMed]
- Guilemany, J.M.; Angrill, J.; Alobid, I.; Centellas, S.; Pujols, L.; Bartra, J.; Bernal-Sprekelsen, M.; Valero, A.; Picado, C.; Mullol, J. United airways again: High prevalence of rhinosinusitis and nasal polyps in bronchiectasis. Allergy 2009, 64, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Pizzimenti, S.; Badiu, I.; Guida, G.; Ricciardolo, F.L.; Bucca, C.; Rolla, G. Nasal nitric oxide is a marker of poor asthma control. J. Breath Res. 2013, 7, 026009. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, M.; He, F.; Wang, X.; Zhang, L. Role of exhaled nasal nitric oxide in distinguishing between chronic rhinosinusitis with and without nasal polyps. Am. J. Rhinol. Allergy 2017, 31, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Takeno, S.; Fukuiri, T.; Hirakawa, K. Monitoring of oral and nasal exhaled nitric oxide in eosinophilic chronic rhinosinusitis: A prospective study. Am. J. Rhinol. Allergy 2012, 26, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Torretta, S.; Cappadona, M.; Carioli, D.; Pignataro, L. Airborne nitric oxide and nasal cytology in patients with chronic rhinosinusitis and nasal polyps. J. Biol. Regul. Homeost. Agents 2015, 29, 969–976. [Google Scholar] [PubMed]
- Tworek, D.; Kuna, P. Nasal nitric oxide measurements in the assessment of nasal allergen challenge. J. Investig. Allergol. Clin. Immunol. 2012, 22, 102–108. [Google Scholar]
- Weschta, M.; Deutschle, T.; Riechelmann, H. Nasal fractional exhaled nitric oxide analysis with a novel hand-held device. Rhinology 2008, 46, 23–27. [Google Scholar]
- Williamson, P.A.; Vaidyanathan, S.; Clearie, K.; Stewart, M.; Lipworth, B.J. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2010, 105, 162–167. [Google Scholar] [CrossRef]
- Bae, W.Y. Measurement of nasal and exhaled nitric oxide in chronic rhinosinusitis and its comparison according to the presence of nasal polyps. J. Allergy Clin. Immunol. 2014, 133, 129. [Google Scholar] [CrossRef]
- Fu, C.H.; Huang, C.C.; Chen, Y.W.; Chang, P.H.; Lee, T.J. Nasal nitric oxide in relation to quality-of-life improvements after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2015, 29, e187–e191. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.M.; Lund, V.J.; Saleh, H.A.; Scadding, G. Nasal nitric oxide in objective evaluation of chronic rhinosinusitis therapy. Allergy 2006, 61, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lou, H.; Wang, Y.; He, F.; Cao, F.; Wang, C.; Zhang, L. Nasal ventilation is an important factor in evaluating the diagnostic value of nasal nitric oxide in allergic rhinitis. Int. Forum Allergy Rhinol. 2018, 8, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Vlad, D.; Albu, S. Arginase isoform expression in chronic rhinosinusitis. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Chen, S.S.; Jou, L.S.; Weng, P.K.; Wang, H.W. Immunolocalization of inducible nitric oxide synthase and 3-nitrotyrosine in the nasal mucosa of patients with rhinitis. Eur. Arch. Otorhinolaryngol. 2000, 257, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Takeno, S.; Yajin, K. Increased expression of inducible nitric oxide synthase in nasal epithelial cells in patients with allergic rhinitis. Laryngoscope 1999, 109, 2015–2020. [Google Scholar] [CrossRef]
- Kawamoto, H.; Takumida, M.; Takeno, S.; Watanabe, H.; Fukushima, N.; Yajin, K. Localization of nitric oxide synthase in human nasal mucosa with nasal allergy. Acta Oto-Laryngol. 1998, 539, 65–70. [Google Scholar] [CrossRef]
- Mahr, T.A.; Malka, J.; Spahn, J.D. Inflammometry in pediatric asthma: A review of fractional exhaled nitric oxide in clinical practice. Allergy Asthma Proc. 2013, 34, 210–219. [Google Scholar] [CrossRef]
- Baraldi, E.; Azzolin, N.M.; Carra, S.; Dario, C.; Marchesini, L.; Zacchello, F. Effect of topical steroids on nasal nitric oxide production in children with perennial allergic rhinitis: A pilot study. Respir. Med. 1998, 92, 558–561. [Google Scholar] [CrossRef]
- Takeno, S.; Noda, N.; Hirakawa, K. Measurements of nasal fractional exhaled nitric oxide with a hand-held device in patients with allergic rhinitis: Relation to cedar pollen dispersion and laser surgery. Allergol. Int. 2012, 61, 93–100. [Google Scholar] [CrossRef]
- Colantonio, D.; Brouillette, L.; Parikh, A.; Scadding, G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Struben, V.M.; Wieringa, M.H.; Mantingh, C.J.; de Jongste, J.C.; Feenstra, L. Nasal NO measurement by direct sampling from the nose during breathhold: Aspiration flow, nasal resistance and reproducibility. Eur. Arch. Otorhinolaryngol. Head Neck 2006, 263, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Chatkin, J.M.; Qian, W.; Cole, P.; Zamel, N.; McClean, P.; Furlott, H.; Haight, J.S. Aerodynamic influences on nasal nitric oxide output measurements. Acta Oto-Laryngol. 1999, 119, 479–485. [Google Scholar] [CrossRef]
- Maniscalco, M.; Pelaia, G.; Sofia, M. Exhaled nasal nitric oxide during humming: Potential clinical tool in sinonasal disease? Biomark. Med. 2013, 7, 261–266. [Google Scholar] [CrossRef]
| Study | Pop (n) | Males (%) | Age (Years) | Smoking (%) | Atopy (%) | Asthma (%) | Intranasal CCS (%) | Lund–Mackay CT Score | SNOT-22 Score | FEV1 (% Predicted) | FEV1/FVC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRSwNP vs. HEALTHY | ||||||||||||
| Arnal 1999—No allergy [28] | Pts | 20 | 65.0 | 48.0 | 15.0 | 0 | 10.0 | 55.0 | - | - | - | - |
| Controls | 42 | 52.4 | 42.0 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Arnal 1999—Allergy [28] | Pts | 7 | 71.4 | 42.0 | 15.0 | 100 | 71.4 | 14.3 | - | - | - | - |
| Controls | 42 | 52.4 | 42.0 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Asano 2018 [29] | Pts | 11 | - | - | 0 | 100 | 100 | 0 | - | - | - | - |
| Controls | 10 | - | - | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Bommarito 2008 [30] | Pts | 30 | 63.3 | 52.0 | 0 | 57.0 | - | 0 | 13.5 | - | - | - |
| Controls | 29 | 31.0 | 39.0 | 0 | 55.0 | N/A | N/A | N/A | N/A | - | - | |
| Frendø 2018 [31] | Pts | 57 | 70.2 | 49.3 | - | 32.0 | 44.0 | 0 | - | - | - | - |
| Controls | 30 | 36.7 | 34.5 | - | 0 | N/A | N/A | N/A | N/A | - | - | |
| Gilian 2002 * [32] | Pts | 18 | 77.8 | - | - | - | 0 | - | - | - | - | - |
| Controls | 21 | 33.3 | - | - | - | N/A | N/A | N/A | N/A | - | - | |
| Guilemany 2009 [33] | Pts | 22 | 50.0 | 49.0 | 4.5 | - | - | - | 10.8 | - | 80.0 | 92.0 |
| Controls | 20 | 50.0 | 59.0 | 0 | - | N/A | N/A | N/A | N/A | 90.0 | 96.0 | |
| Gupta 2013 [21] | Pts | 24 | - | 54.5 | - | - | - | - | - | 28.2 | - | - |
| Controls | 11 | - | 45.7 | - | - | N/A | N/A | N/A | N/A | - | - | |
| Heffler 2014 [34] | Pts | 34 | - | - | 0 | - | 100 | 0 | 13.0 | - | - | - |
| Controls | 40 | 25.0 | 48.7 | 0 | 20.0 | N/A | N/A | N/A | N/A | 97.2 | 82.3 | |
| Jeong 2014—No allergy [16] | Pts | 30 | 53.3 | 33.4 | - | 0 | 0 | 0 | 14.6 | - | - | - |
| Controls | 30 | 50.0 | 27.3 | - | 0 | N/A | N/A | N/A | N/A | - | - | |
| Jeong 2014—Allergy [16] | Pts | 27 | 77.8 | 33.4 | - | 100 | 0 | 0 | 11.6 | - | - | - |
| Controls | 30 | 50.0 | 27.3 | - | 0 | N/A | N/A | N/A | N/A | - | - | |
| Liu 2017 [35] | Pts | 54 | 61.1 | - | 0 | 27.7 | 0 | 0 | - | - | - | - |
| Controls | 20 | 85.0 | 35.5 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Noda 2012—Medical group [36] | Pts | 12 | - | 57.9 | - | - | 75.0 | 0 | 14.2 | - | - | - |
| Controls | 32 | - | 50.0 | - | - | N/A | N/A | N/A | N/A | - | - | |
| Noda 2012—Surgical group [36] | Pts | 24 | 56.2 | - | - | 58.3 | 0 | 16.9 | - | - | - | |
| Controls | 32 | - | 50.0 | - | - | N/A | N/A | N/A | N/A | - | - | |
| Torretta 2015 * [37] | Pts | 37 | - | - | - | - | - | - | - | - | - | - |
| Controls | 36 | - | - | - | - | N/A | N/A | N/A | N/A | - | - | |
| Tworek 2012 [38] | Pts | 10 | 50.0 | 38.9 | 0 | 70.0 | 0 | 0 | - | - | 89.7 | - |
| Controls | 10 | 60.0 | 41.0 | 0 | 60.0 | N/A | N/A | N/A | N/A | 92.2 | - | |
| Weschta 2008 [39] | Pts | 6 | - | - | - | - | - | 0 | - | - | - | - |
| Controls | 10 | 30.0 | 38.0 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Williamson 2010 [40] | Pts | 38 | 55.3 | 52.0 | - | 57.9 | 0 | 0 | - | - | 101.1 | - |
| Controls | 41 | 46.3 | 27.0 | - | 0 | N/A | N/A | N/A | N/A | 98.4 | - | |
| CRSsNP vs. HEALTHY | ||||||||||||
| Alexandersson 2019 [27] | Pts | 12 | 33.0 | 46.0 | 0 | 33.0 | 17.0 | 67.0 | 9.7 | 50.7 | - | - |
| Controls | 12 | 42.0 | 35.0 | 0 | 50.0 | N/A | N/A | N/A | N/A | |||
| Arnal 1999 [28] | Pts | 10 | 70.0 | 47.0 | 20.0 | 0 | 10.0 | 70.0 | - | - | - | - |
| Controls | 42 | 52.4 | 42.0 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Asano 2008 [29] | Pts | 13 | - | - | 0 | 100 | 100 | 0 | - | - | - | - |
| Controls | 10 | - | - | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Bommarito 2008 [30] | Pts | 14 | 50.0 | 42.0 | 0 | 50.0 | - | 0 | 5.7 | - | - | - |
| Controls | 29 | 31.0 | 39.0 | 0 | 55.0 | N/A | N/A | N/A | N/A | - | - | |
| Guilemany 2009 [33] | Pts | 46 | 13.0 | 56.0 | 9.0 | - | - | - | 8.4 | - | 81.0 | 92.0 |
| Controls | 20 | 50.0 | 59.0 | 0 | - | N/A | N/A | N/A | N/A | 90.0 | 96.0 | |
| Gupta 2013 [21] | Pts | 31 | - | 52.3 | - | - | - | - | - | 25.2 | - | - |
| Controls | 11 | - | 45.7 | - | - | N/A | N/A | N/A | N/A | - | - | |
| Heffler 2013 [34] | Pts | 7 | - | - | 0 | - | 100 | 0 | 3.0 | - | - | - |
| Controls | 40 | 25.0 | 48.7 | 0 | 20.0 | N/A | N/A | N/A | N/A | 97.2 | 82.3 | |
| Lindenberg 1997 [17] | Pts | 12 | 50.0 | 46.8 | 25.0 | 22.7 | - | 0 | - | - | - | - |
| Controls | 66 | 50.0 | 34.6 | 9.1 | 16.7 | N/A | N/A | N/A | N/A | - | - | |
| Liu 2017 [35] | Pts | 34 | 79.4 | - | 0 | 38.2 | 0 | 0 | - | - | - | - |
| Controls | 20 | 85.0 | 35.5 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| Weschta 2008 [39] | Pts | 4 | - | - | - | - | - | 0 | - | - | ||
| Controls | 10 | 30.0 | 38.0 | 0 | 0 | N/A | N/A | N/A | N/A | - | - | |
| CRSwNP vs. CRSsNP | ||||||||||||
| Arnal 1999 [28] | Pts | 20 | 65.0 | 48.0 | 15.0 | 0 | 10.0 | 55.0 | - | - | - | - |
| Controls | 10 | 70.0 | 47.0 | 20.0 | 0 | 10.0 | 70.0 | - | - | - | - | |
| Asano 2008 [29] | Pts | 11 | - | - | 0 | 100 | 100 | 0 | - | - | - | - |
| Controls | 13 | - | - | 0 | 100 | 100 | 0 | - | - | - | - | |
| Bae 2014 * [41] | Pts | 16 | - | - | - | - | - | - | - | - | - | - |
| Controls | 19 | - | - | - | - | - | - | - | - | - | - | |
| Bommarito 2008 [30] | Pts | 30 | 63.3 | 52.0 | 0 | 57.0 | - | 0 | 13.5 | - | - | - |
| Controls | 14 | 50.0 | 42.0 | 0 | 50.0 | 0 | 0 | 5,7 | - | - | - | |
| Fu 2015 [42] | Pts | 53 | 67.9 | 44.5 | 17.0 | 18.9 | 20.8 | - | 16.4 | 42.7 | - | - |
| Controls | 16 | 56.2 | 45.6 | 18.0 | 25.0 | 12.5 | - | 11.8 | 47.7 | - | - | |
| Fu 2017 [20] | Pts | 12 | 45.9 | 50.0 | 33.3 | 41.7 | 0 | - | 9.8 | 39.3 | - | - |
| Controls | 13 | 52.6 | 53.8 | 30.7 | 30.7 | 0 | - | 6.5 | 37.6 | - | - | |
| Guilemany 2009 [33] | Pts | 22 | 50.0 | 49.0 | 4.5 | - | - | - | 10.8 | - | 80.0 | 92.0 |
| Controls | 46 | 13.0 | 56.0 | 9.0 | - | - | - | 8.4 | - | 81.0 | 92.0 | |
| Gupta 2013 [21] | Pts | 24 | - | 54.5 | - | - | - | - | - | 28.2 | - | - |
| Controls | 31 | - | 52.3 | - | - | - | - | - | 25.2 | - | - | |
| Heffler 2013 [34] | Pts | 34 | - | - | 0 | - | 100 | 0 | 13.0 | - | - | - |
| Controls | 7 | - | - | 0 | - | 100 | 0 | 3.0 | - | - | - | |
| Lee 2015 [18] | Pts | 33 | - | - | - | - | - | 100 | - | - | - | - |
| Controls | 6 | - | - | - | - | 0 | 0 | - | - | - | - | |
| Liu 2017 [35] | Pts | 54 | 61.1 | - | 0 | 27.7 | 0 | 0 | - | - | - | - |
| Controls | 34 | 79.4 | - | 0 | 38.2 | 0 | 0 | - | - | - | - | |
| Ragab 2006—Medical group [43] | Pts | 16 | - | - | - | - | - | - | - | - | - | - |
| Controls | 29 | - | - | - | - | 0 | - | - | - | - | - | |
| Ragab 2006—Surgical group [43] | Pts | 19 | - | - | - | - | - | - | - | - | - | - |
| Controls | 25 | - | - | - | - | 0 | - | - | - | - | - | |
| Weschta 2008 [39] | Pts | 6 | - | - | - | - | - | 0 | - | - | - | - |
| Controls | 4 | - | - | - | - | - | 0 | - | - | - | - | |
| Yoshida 2019 [19] | Pts | 22 | - | - | - | - | - | 0 | - | - | - | - |
| Controls | 30 | - | - | - | - | - | 0 | - | - | - | - | |
| N of Studies | N of Patients | Effect Size SMD (95% CI) in nNO | |
|---|---|---|---|
| “High Quality” Studies | Panel A | ||
| CRSwNP vs. healthy | 12 | 370 pts | SMD: −1.567 (−2.155, −0.978); p < 0.0001 |
| (14 data-sets) | 295 controls | I2 = 90.5%; p <0.0001 | |
| CRSsNP vs. healthy | 9 | 171 pts | SMD: −0.550 (−1.002, −0.097); p = 0.017 |
| (9 data-sets) | 194 controls | I2 = 70.6%; p < 0.0001 | |
| CRSwNP vs. CRSsNP | 13 | 356 pts | SMD: −1.565 (−2.174, −0.957); p < 0.0001 |
| (14 data-sets) | 278 controls | I2 = 89.5%; p < 0.0001 | |
| Exclusion of duplicate populations | Panel B | ||
| CRSwNP vs. healthy | 15 | 461 pts | SMD: −1.495 (−2.135, −0.854); p < 0.0001 |
| (18 data-sets) | 384 controls | I2 = 93.9%; p < 0.0001 | |
| CRSsNP vs. healthy | 10 | 183 pts | SMD: −0.696 (−1.189, −0.202); p = 0.006 |
| (10 data-sets) | 260 controls | I2 = 78.2%; p < 0.0001 | |
| CRSwNP vs. CRSsNP | 13 | 360 pts | SMD: −1.554 (−2.171, −0.936); p < 0.0001 |
| (14 data-sets) | 284 controls | I2 = 90.0%; p < 0.0001 | |
| Exclusion of patients receiving intranasal CCS | Panel C | ||
| CRSwNP vs. healthy | 10 | 333 pts | SMD: −1.254 (−1.974, −0.534); p = 0.001 |
| (12 data-sets) | 318 controls | I2 = 93.3%; p < 0.0001 | |
| CRSsNP vs. healthy | 5 | 72 pts | SMD: −0.456 (−1.278, 0.367); p =0.277 |
| (5 data-sets) | 109 controls | I2 = 81.7%; p < 0.0001 | |
| CRSwNP vs. CRSsNP | 6 | 157 pts | SMD: −1.953 (−2.588, −1.318); p < 0.0001 |
| (6 data-sets) | 102 controls | I2 = 90.0%; p < 0.0001 | |
| N of Studies | N of Patients | Effect Size SMD (95% CI) in nNO | |
|---|---|---|---|
| Nasal Aspiration | Panel A | ||
| CRSwNP vs. healthy | 12 | 408 pts | SMD: −1.885 (−2.547, −1.223); p < 0.0001 |
| (14 data-sets) | 332 controls | I2 = 93.0%; p < 0.0001 | |
| CRSsNP vs. healthy | 8 | 166 pts | SMD: −0.862 (−1.384, −0.340); p = 0.001 |
| (8 data-sets) | 240 controls | I2 = 78.4%; p < 0.0001 | |
| CRSwNP vs. CRSsNP | 10 | 320 pts | SMD: −1.451 (−2.124, −0.779); p < 0.0001 |
| (11 data-sets) | 231 controls | I2 = 90.3%; p < 0.0001 | |
| Nasal exhalation | Panel B | ||
| CRSwNP vs. healthy | 3 | 53 pts | SMD: −0.022 (−1.067, 1.022); p = 0.966 |
| (4 data-sets) | 52 controls | I2 = 86.2%; p < 0.0001 | |
| CRSsNP vs. healthy | 2 | 17 pts | SMD: 0.163 (−0.512, 0.839); p = 0.636 |
| (2 data-sets) | 20 controls | I2 = 0%; p = 0.461 | |
| CRSwNP vs. CRSsNP | 4 | 52 pts | SMD: −1.448 (−3.018, 0.121); p = 0.071 |
| (4 data-sets) | 66 controls | I2 = 92.0%; p < 0.0001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosino, P.; Molino, A.; Spedicato, G.A.; Parrella, P.; Formisano, R.; Motta, A.; Di Minno, M.N.D.; Maniscalco, M. Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 200. https://doi.org/10.3390/jcm9010200
Ambrosino P, Molino A, Spedicato GA, Parrella P, Formisano R, Motta A, Di Minno MND, Maniscalco M. Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2020; 9(1):200. https://doi.org/10.3390/jcm9010200
Chicago/Turabian StyleAmbrosino, Pasquale, Antonio Molino, Giorgio Alfredo Spedicato, Paolo Parrella, Roberto Formisano, Andrea Motta, Matteo Nicola Dario Di Minno, and Mauro Maniscalco. 2020. "Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 9, no. 1: 200. https://doi.org/10.3390/jcm9010200
APA StyleAmbrosino, P., Molino, A., Spedicato, G. A., Parrella, P., Formisano, R., Motta, A., Di Minno, M. N. D., & Maniscalco, M. (2020). Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 9(1), 200. https://doi.org/10.3390/jcm9010200