Nocturnal Hypoxemia Impacts Right Ventricle Diastolic Function in Obstructive Sleep Apnea: A Retrospective Observational Study
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Measurements
2.2.1. Night Cardiorespiratory Monitoring
- AHI: expressed as number of events/h, and OSA severity classified as mild (<15/h), moderate (15–29/h), or severe (≥30/h);
- Oxygen desaturation index (ODI), expressed as number of events/h;
- Mean nighttime oxyhemoglobin saturation (mean SpO2:), expressed as a percentage;
- Time elapsed with SpO2 < 90% (T90), expressed as a percentage of the total monitoring nocturnal time; two classes of patients were evaluated: non-desaturators (T90 < 30%) and desaturators (T90 ≥ 30%) [27];
- Mean desaturation peak values of nocturnal events (Mdes), expressed as a percentage, and relative subdivision into three severity groups: mild (≥89%), moderate (between 85% and 89%), or severe (≤85%) referred to, respectively, Mdes 89, Mdes 85–89, and Mdes 85;
- Apnea duration: mean duration time of sleep apnea/hypopneas, expressed in seconds.
2.2.2. Comprehensive Transthoracic Two-Dimensional and Doppler Echocardiography Examination
- EDT: deceleration time of the E-wave of the trans-tricuspid diastolic flow curve, acquired from the apical four-chamber view. This was the RVD function parameter (normal values for diastolic function <240 ms; PW Doppler modality);
- RV diameter: transverse diameter of the right ventricle, recorded in correspondence to the tract of inflow in the telediastolic phase, from apical four-chamber view (2D modality);
- RV wall thickness: parietal end-diastolic thickness of the RV, recorded from parasternal long axis view (M-Mode modality);
- RV-Sm: pulsed-wave TDI was recorded at the lateral tricuspid annulus from four-chamber view, in the ventricular ejection phase (TDI modality);
- Pulmonary arterial systolic pressure (PASP), calculated using the modified Bernoulli equation and conventional Doppler tricuspid regurgitation, adding the estimated right atrial pressure value, obtained from the vena cava collapsibility index (Doppler and M-Mode modality);
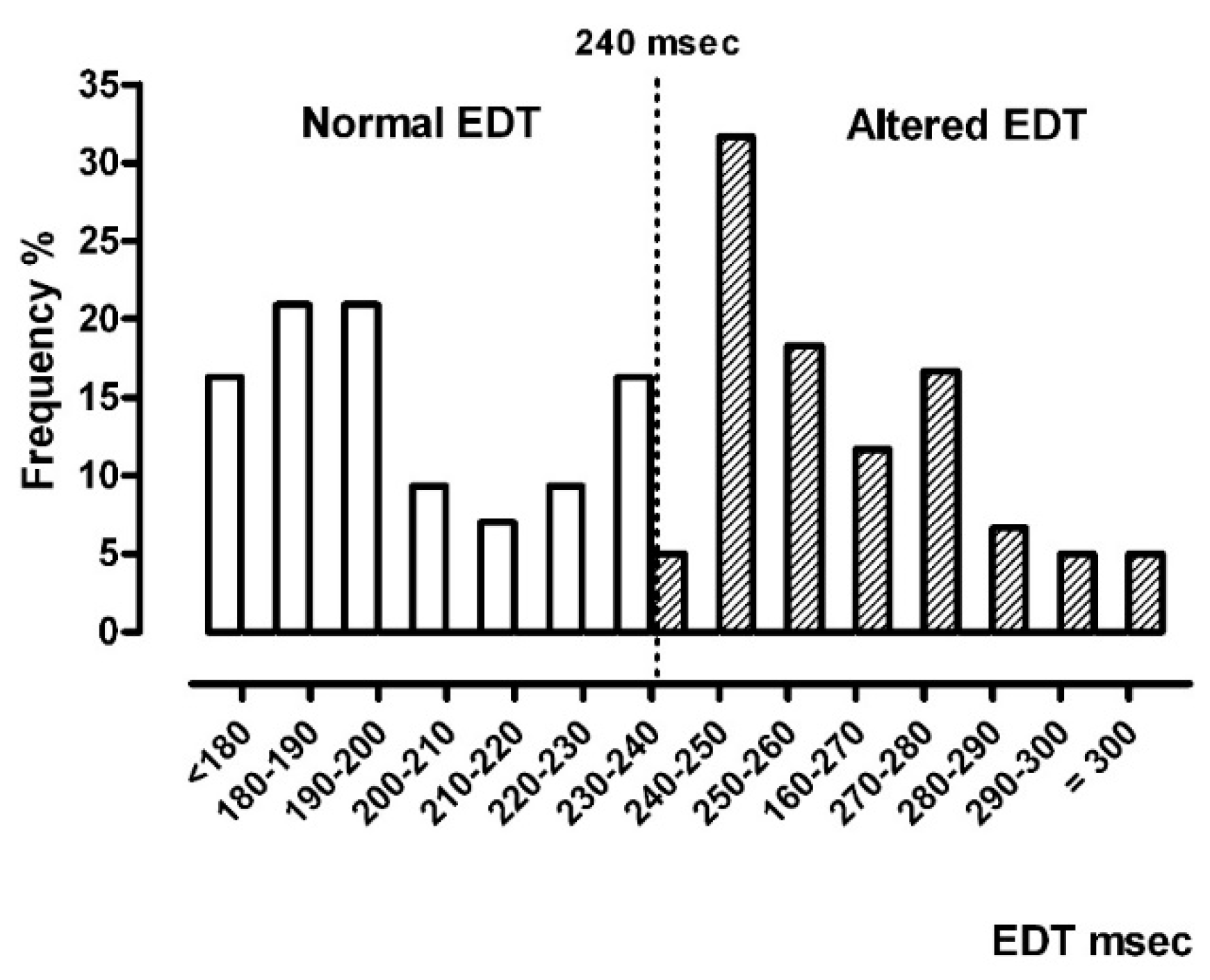
- For EDT, RV diameter, RV wall thickness, and PASP parameters, normal vs. altered classes were generated.
2.3. Statistical Analysis
3. Results
3.1. Primary Aim
- No association with sex (p = 0.847) or presence of systemic hypertension (p = 1.000);
- No significant difference in age: 61.9 years in normal EDT vs. 62.1 years in altered EDT (p = 0.948);
- A significant difference in body mass index (BMI): 30.9 in normal EDT vs. 34.6 Kg/m2 in altered EDT (p = 0.013; 95%CI −4.90:−0.70).
3.2. Secondary Aims
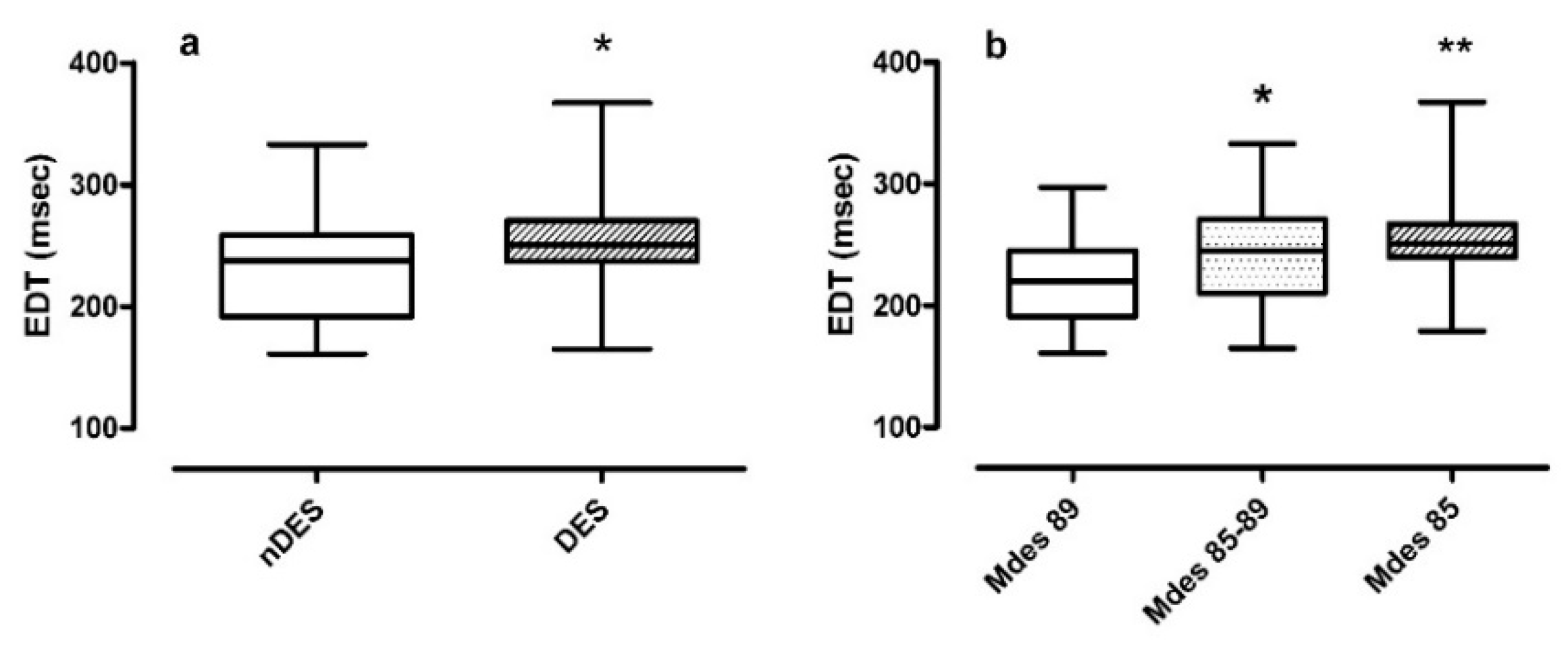
- Non-desaturators vs. desaturators in Figure 3 panel a. The median EDT in desaturators was 250.0 ms compared to the median EDT in non-desaturators 238.3 ms (p = 0.027);
- Mdes severity classes in Figure 3 panel b. The median EDT value increased as Mdes decreased, from 219.5 ms in mild to 245.0 ms in moderate and reaching 251.0 ms in severe Mdes (p = 0.005). Post-hoc analysis confirmed a significance between moderate and mild Mdes (p = 0.032) and severe and mild Mdes (p = 0.001).
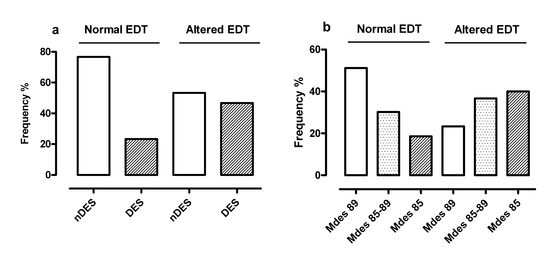
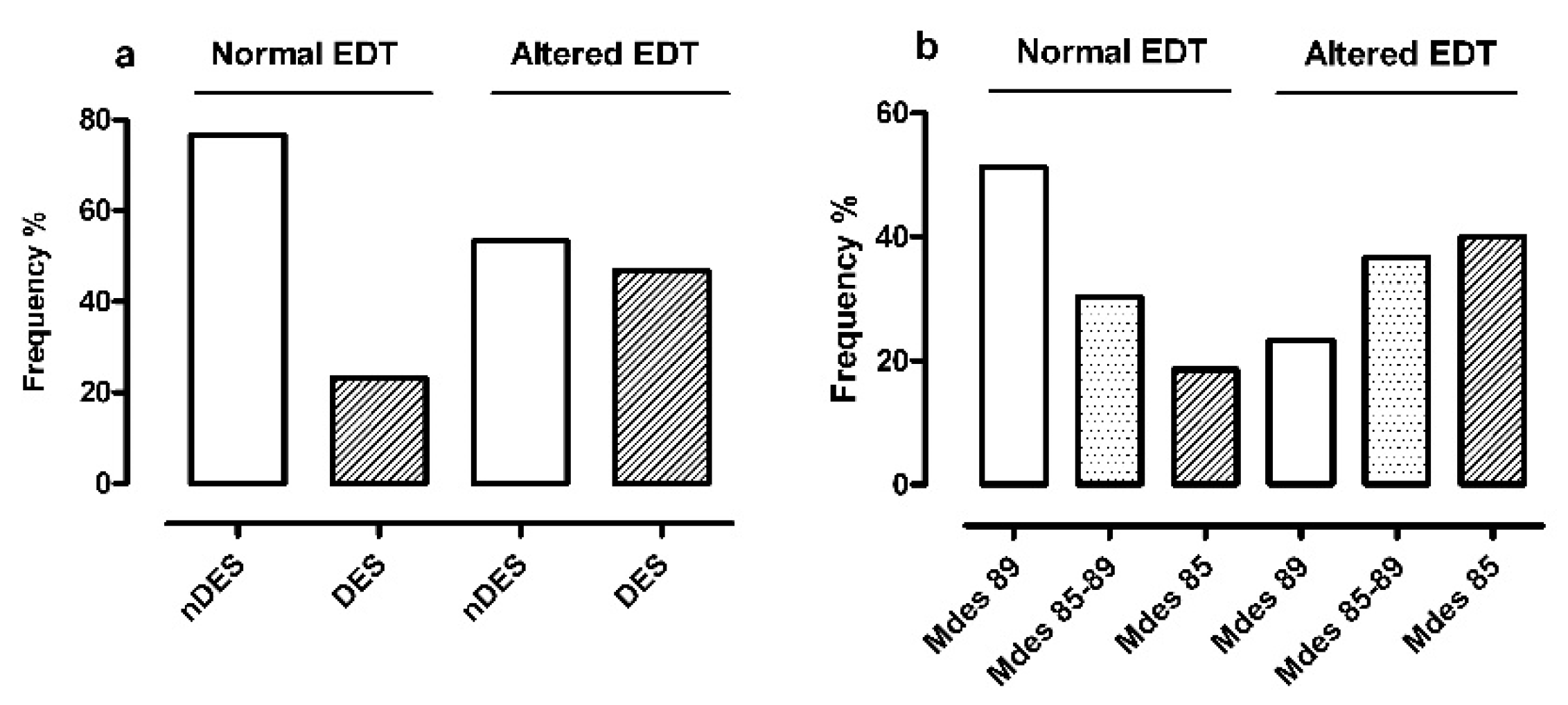
- Non-desaturators and desaturators (Figure 4 panel a) resulted differently distributed in the two EDT classes (p = 0.026): there was a lower frequency of desaturators in normal EDT (10/43 cases, 23%) than altered EDT (28/60 cases, 47%). Of note, the OR for altered EDT in desaturators with respect to non-desaturators was 2.86 (95%CI 1.12:7.72);
- The severity classes of Mdes (Figure 4 panel b) were characterized by an exactly opposite distribution in the two EDT classes (p = 0.008): severe group (Mdes 85) was the most represented in altered EDT (24/60 cases, 40%) and the least represented in normal EDT (8/43 cases, 19%). In addition, the OR for altered EDT in medium/severe Mdes with respect to mild Mdes was 3.40 (p = 0.007; 95%CI 1.36:8.77).
3.3. Influence of Minor Comorbidities
4. Discussion
4.1. Limitations
4.2. Practical Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strohl, K.P.; Redline, S. Recognition of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1996, 154, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Commissione Paritetica Associazione Italiana Medicina del Sonno (AIMS) e Associazione Italiana Pneumologi Ospedalieri (AIPO). Linee Guida di Procedura Diagnostica nella Sindrome delle Apnee Ostruttive nel Sonno dell’Adulto. Rassegna di Patologia dell’Apparato Respiratorio 2001, 16, 104–114. [Google Scholar]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Hudgel, D.; Sateia, M.; Schwab, R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S. Medicine ftAAoS. In The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Ryan, C.M.; Bradley, T.D. Pathogenesis of obstructive sleep apnea. J. Appl. Physiol. 2005, 99, 2440–2450. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar]
- Peker, Y.; Hedner, J.; Norum, J.; Kraiczi, H.; Carlson, J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: A 7-year follow-up. Am. J. Respir. Crit. Care Med. 2002, 166, 159–165. [Google Scholar] [CrossRef]
- Hopps, E.; Caimi, G. Obstructive Sleep Apnea Syndrome: Links Betwen Pathophysiology and Cardiovascular Complications. Clin. Investig. Med. 2015, 38, E362–E370. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Maripov, A.; Mamazhakypov, A.; Sartmyrzaeva, M.; Akunov, A.; Muratali Uulu, K.; Duishobaev, M.; Cholponbaeva, M.; Sydykov, A.; Sarybaev, A. Right Ventricular Remodeling and Dysfunction in Obstructive Sleep Apnea: A Systematic Review of the Literature and Meta-Analysis. Can Respir. J. 2017, 2017, 1587865. [Google Scholar] [CrossRef] [PubMed]
- Grote, L.; Hedner, J.; Peter, J.H. Mean blood pressure, pulse pressure and grade of hypertension in untreated hypertensive patients with sleep-related breathing disorder. J. Hypertens. 2001, 19, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P.; Herer, P.; Hoffstein, V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 2000, 320, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.E.; Andrus, B.W. Obesity and pulmonary hypertension: A review of pathophysiologic mechanisms. J. Obes. 2012, 2012, 505274. [Google Scholar] [CrossRef] [PubMed]
- Sascau, R.; Zota, I.M.; Statescu, C.; Boisteanu, D.; Roca, M.; Mastaleru, A.; Leon Constantin, M.M.; Vasilcu, T.F.; Gavril, R.S.; Mitu, F. Review of Echocardiographic Findings in Patients with Obstructive Sleep Apnea. Can. Respir. J. 2018, 2018, 1206217. [Google Scholar] [CrossRef]
- Nahmias, J.; Lao, R.; Karetzky, M. Right ventricular dysfunction in obstructive sleep apnoea: Reversal with nasal continuous positive airway pressure. Eur. Respir. J. 1996, 9, 945–951. [Google Scholar] [CrossRef]
- Malone, S.; Liu, P.P.; Holloway, R.; Rutherford, R.; Xie, A.; Bradley, T.D. Obstructive sleep apnoea in patients with dilated cardiomyopathy: Effects of continuous positive airway pressure. Lancet 1991, 338, 1480–1484. [Google Scholar]
- Shim, C.Y.; Kim, D.; Park, S.; Lee, C.J.; Cho, H.J.; Ha, J.W.; Cho, Y.J.; Hong, G.R. Effects of continuous positive airway pressure therapy on left ventricular diastolic function: A randomised, sham-controlled clinical trial. Eur. Respir. J. 2018, 51, 1701774. [Google Scholar] [CrossRef]
- Bayram, N.A.; Ciftci, B.; Bayram, H.; Keles, T.; Durmaz, T.; Akcay, M.; Yeter, E.; Bozkurt, E. Effects of continuous positive airway pressure therapy on right ventricular function assessment by tissue Doppler imaging in patients with obstructive sleep apnea syndrome. Echocardiography 2008, 25, 1071–1078. [Google Scholar] [CrossRef]
- Massironi, L.; Pes, R.; Sciomer, S. Capitolo 4: Ecocardiogramma normale: Funzione sisto-diastolica ventricolare destra. Linee Guida SIEC 2011. 2011, pp. 1–21. Available online: https://www.siec.it/ (accessed on 5 January 2020).
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713, quiz 786–788. [Google Scholar]
- Venkatachalam, S.; Wu, G.; Ahmad, M. Echocardiographic assessment of the right ventricle in the current era: Application in clinical practice. Echocardiography 2017, 34, 1930–1947. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Levi-Valensi, P.; Weitzenblum, E.; Rida, Z.; Aubry, P.; Braghiroli, A.; Donner, C.; Aprill, M.; Zielinski, J.; Wurtemberger, G. Sleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patients. Eur. Respir. J. 1992, 5, 301–307. [Google Scholar]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- R-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Wachter, R.; Luthje, L.; Klemmstein, D.; Luers, C.; Stahrenberg, R.; Edelmann, F.; Holzendorf, V.; Hasenfuss, G.; Andreas, S.; Pieske, B. Impact of obstructive sleep apnoea on diastolic function. Eur. Respir. J. 2013, 41, 376–383. [Google Scholar] [CrossRef]
- Arias, M.A.; Garcia-Rio, F.; Alonso-Fernandez, A.; Mediano, O.; Martinez, I.; Villamor, J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: Effects of nasal continuous positive airway pressure in men. Circulation 2005, 112, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Sanner, B.M.; Konermann, M.; Sturm, A.; Muller, H.J.; Zidek, W. Right ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur. Respir. J. 1997, 10, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Pellikka, P.A.; Olson, E.J.; Bailey, K.R.; Korinek, J.; Orban, M.; Sierra-Johnson, J.; Kato, M.; Amin, R.S.; et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest 2007, 132, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Bodez, D.; Lang, S.; Meuleman, C.; Boyer-Chatenet, L.; Nguyen, X.L.; Soulat-Dufour, L.; Boccara, F.; Fleury, B.; Cohen, A. Left ventricular diastolic dysfunction in obstructive sleep apnoea syndrome by an echocardiographic standardized approach: An observational study. Arch. Cardiovasc. Dis. 2015, 108, 480–490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haruki, N.; Takeuchi, M.; Nakai, H.; Kanazawa, Y.; Tsubota, N.; Shintome, R.; Lang, R.M.; Otsuji, Y. Overnight sleeping induced daily repetitive left ventricular systolic and diastolic dysfunction in obstructive sleep apnoea: Quantitative assessment using tissue Doppler imaging. Eur. J. Echocardiogr. 2009, 10, 769–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonauro, A.; Galderisi, M.; Santoro, C.; Canora, A.; Bocchino, M.L.; Lo Iudice, F.; Lembo, M.; Esposito, R.; Castaldo, S.; Trimarco, B.; et al. Obstructive sleep apnoea and right ventricular function: A combined assessment by speckle tracking and three-dimensional echocardiography. Int. J. Cardiol. 2017, 243, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, A.; Guzel, D.; Yildirimturk, O.; Aytekin, S. Evaluation of right ventricular systolic and diastolic function in patients with newly diagnosed obstructive sleep apnea syndrome without hypertension. Cardiology 2009, 113, 184–192. [Google Scholar] [CrossRef]
- Zhou, N.W.; Pan, C.Z.; Kong, D.H.; Li, Z.; Li, W.J.; Gong, X.; Chen, H.Y.; Zhao, W.P.; Wang, X.L.; Li, S.Q.; et al. A novel method for sensitive determination of subclinical right ventricular systolic dysfunction in patients with obstructive sleep apnea. Clin. Respir. J. 2017, 11, 951–959. [Google Scholar] [CrossRef]
- Chung, S.; Yoon, I.Y.; Lee, C.H.; Kim, J.W. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration 2010, 79, 363–369. [Google Scholar] [CrossRef]
- Zakhama, L.; Herbegue, B.; Abouda, M.; Antit, S.; Slama, I.; Boussabah, E.; Thameur, M.; Masmoudi, M.; Abdelaali, N.; Charfi, M.R.; et al. Impact of obstructive sleep apnea on the right ventricle. Tunis. Med. 2016, 94, 612–615. [Google Scholar]
| Mean (SD) | |
|---|---|
| Age, years | 62.04 (10.99) |
| BMI, Kg/m2 | 33.86 (6.08) |
| EDT, ms | 237.03 (39.01) |
| RV diameter, mm | 30.53 (4.25) |
| RV wall tickness, mm | 4.46 (10.6) |
| RV-Sm, m/s | 0.15 (0.03) |
| PASP, mmHg | 28.65 (6.54) |
| AHI, events/h | 37.51 (26.77) |
| mean SpO2, % | 90.34 (5.10) |
| T90 (TimeSpO2), % | 29.10 (30.65) |
| Mdes, % | 85.77 (6.24) |
| Apnea duration, s | 20.42 (5.07) |
| Frequency (n.) | |
|---|---|
| Sex, M/F | 72/31 |
| Hypertension, no/yes | 23/80 |
| EDT, normal (<240 ms)/altered | 43/60 |
| RV diameter, normal (<35 mm)/altered | 91/12 |
| RV wall tickness, normal (<5 mm)/altered | 76/27 |
| PASP, normal (<35 mmHg)/altered | 85/18 |
| OSA severity mild/moderate/severe | 29/21/53 |
| Respiratory insufficiency, nDES (T90 < 30%)/DES | 65/38 |
| Mdes severity, mild/moderate/severe | 36/35/32 |
| Normal EDT (n = 43) | Altered EDT (n = 60) | p (95%CI) | |
|---|---|---|---|
| AHI, events/h | 26.80 | 35.00 | 0.062 (−18.70:0.40) |
| ODI, events/h | 28.90 | 37.80 | 0.068 (−19.70:0.40) |
| mean SpO2, % | 92.50 | 91.25 | 0.002 (0.70:3.20) |
| T90 (TimeSpO2), % | 9.10 | 28.45 | 0.003 (−24.80:−2.90) |
| Mdes, % | 89.10 | 87.05 | 0.002 (0.90:3.90) |
| Apnea duration, s | 19.30 | 19.85 | 0.383 (−2.50:1.00) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotti, C.; Porta, R.; Olivares, A.; Comini, L.; Cinelli, A.; Scalvini, S.; Vitacca, M. Nocturnal Hypoxemia Impacts Right Ventricle Diastolic Function in Obstructive Sleep Apnea: A Retrospective Observational Study. J. Clin. Med. 2020, 9, 162. https://doi.org/10.3390/jcm9010162
Scotti C, Porta R, Olivares A, Comini L, Cinelli A, Scalvini S, Vitacca M. Nocturnal Hypoxemia Impacts Right Ventricle Diastolic Function in Obstructive Sleep Apnea: A Retrospective Observational Study. Journal of Clinical Medicine. 2020; 9(1):162. https://doi.org/10.3390/jcm9010162
Chicago/Turabian StyleScotti, Carla, Roberto Porta, Adriana Olivares, Laura Comini, Angelo Cinelli, Simonetta Scalvini, and Michele Vitacca. 2020. "Nocturnal Hypoxemia Impacts Right Ventricle Diastolic Function in Obstructive Sleep Apnea: A Retrospective Observational Study" Journal of Clinical Medicine 9, no. 1: 162. https://doi.org/10.3390/jcm9010162
APA StyleScotti, C., Porta, R., Olivares, A., Comini, L., Cinelli, A., Scalvini, S., & Vitacca, M. (2020). Nocturnal Hypoxemia Impacts Right Ventricle Diastolic Function in Obstructive Sleep Apnea: A Retrospective Observational Study. Journal of Clinical Medicine, 9(1), 162. https://doi.org/10.3390/jcm9010162