The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
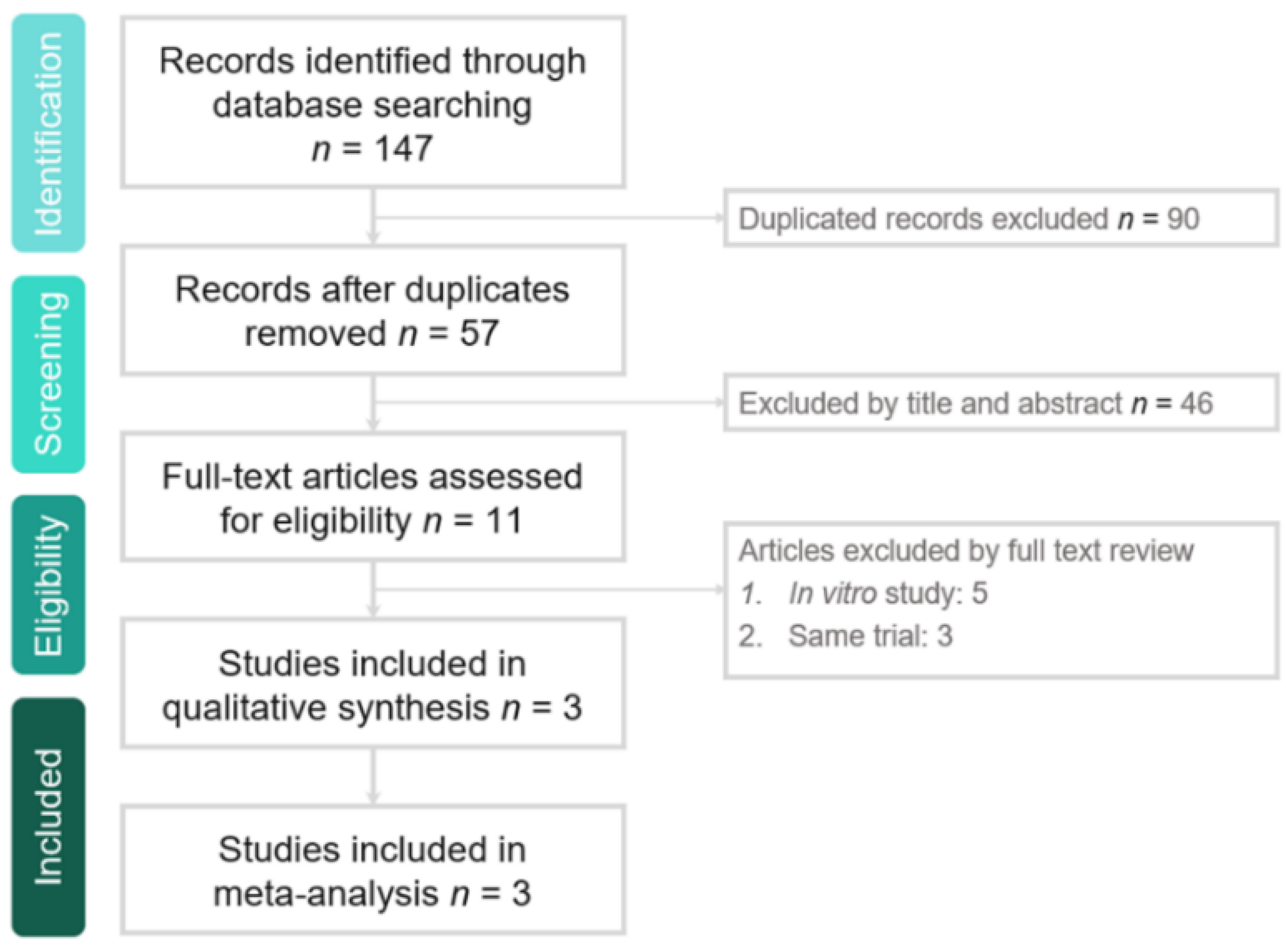
2.1. Study Search and Selection
2.2. Outcome Measurement
2.3. Data Analysis
3. Results
3.1. Study Selection and Characteristics
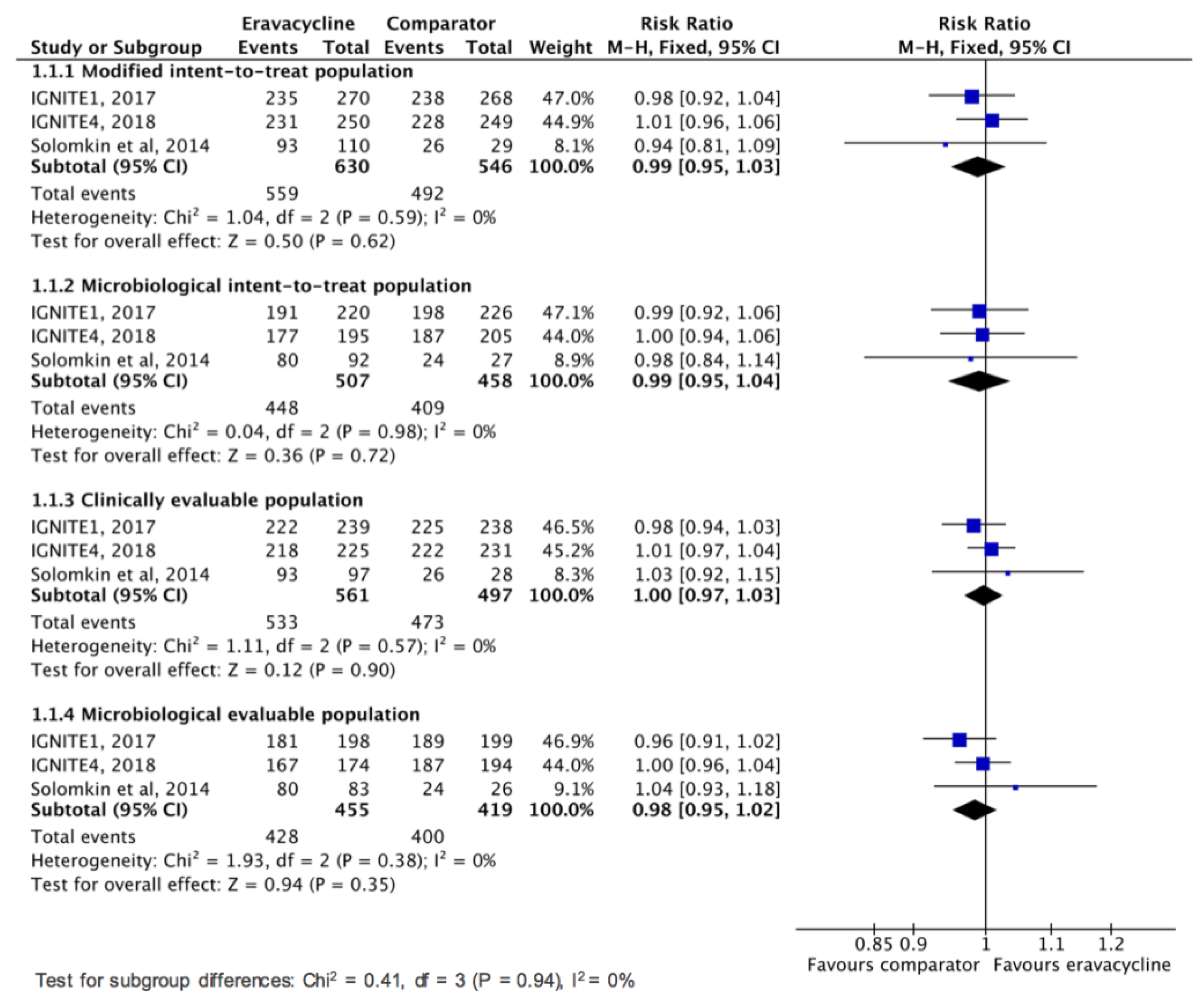
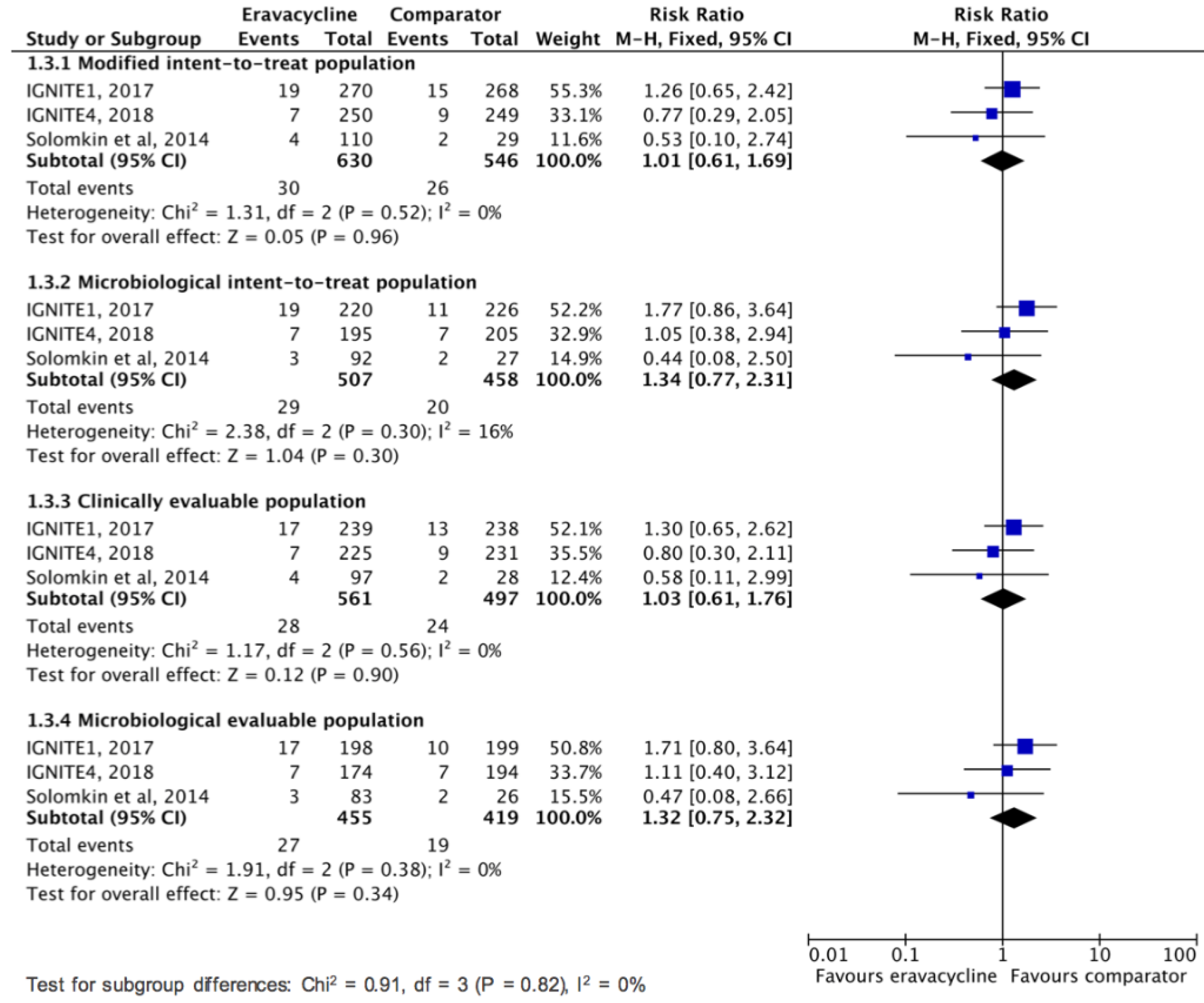
3.2. Clinical Efficacy and Microbiologic Response
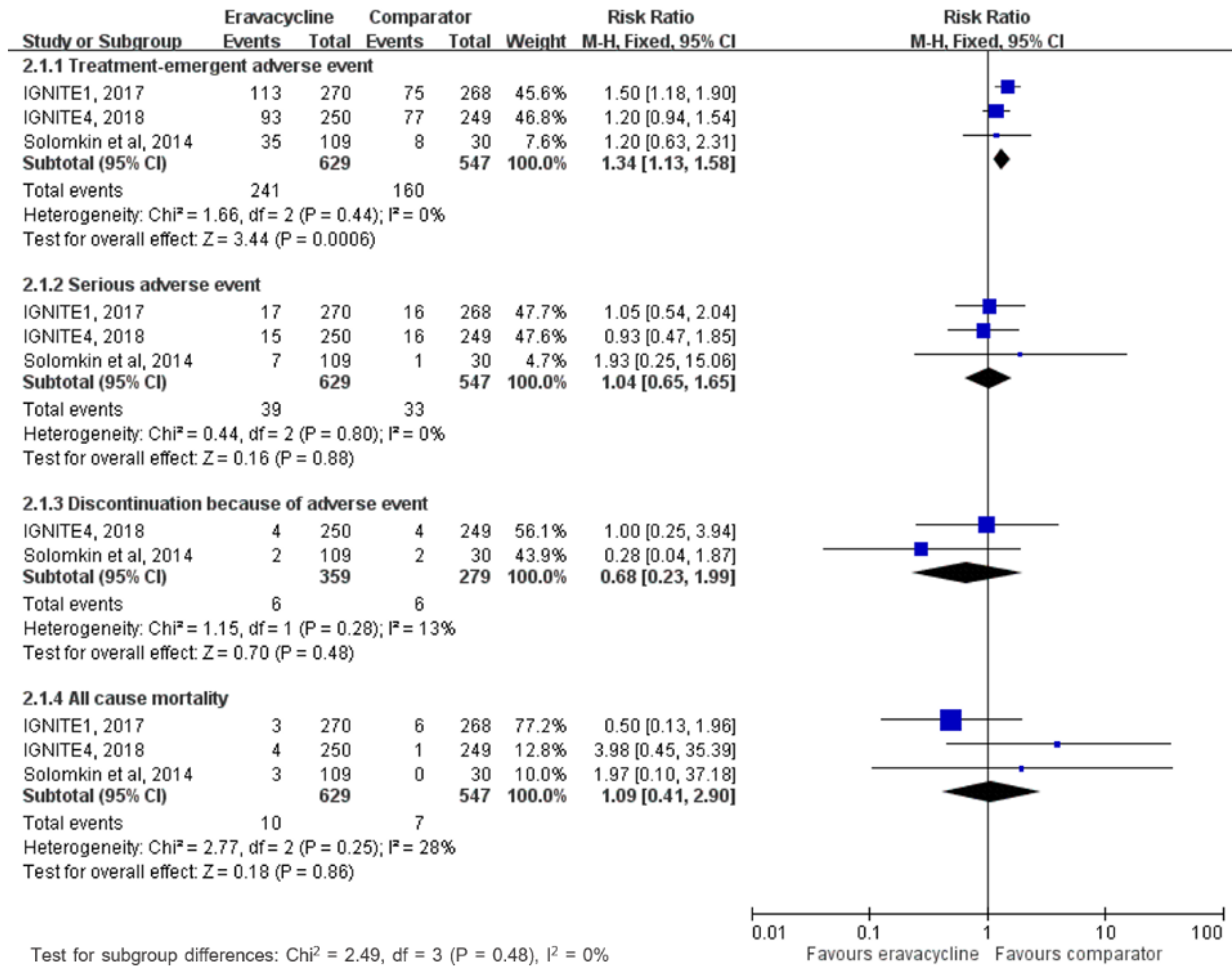
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A. Search Strategy
| PubMed Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | Eravacycline [Title/Abstract] OR TP-434 [Title/Abstract] OR Xerava [Title/Abstract] | 74 |
| 2 | abdom* [Title/Abstract] | 330,674 |
| 3 | 1 AND 2 | |
| 4 | Search (abdom* (Title/Abstract)) AND (((Eravacycline (Title/Abstract)) OR TP-434 (Title/Abstract)) OR Xerava (Title/Abstract)) | 22 |
| Web of Science Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | (Eravacycline) OR (Xerava) OR (TP-434) | 71 |
| 2 | (abdom*) | 269,250 |
| 3 | 1 AND 2 | |
| 4 | #1 AND #2 | 20 |
| EBSCO Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | AB Eravacycline OR AB Xerava OR AB TP-434 | 176 |
| 2 | AB abdom* | 495,125 |
| 3 | 1 AND 2 | |
| 4 | S1 AND S2 | 40 |
| Cochrane Library Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | (Eravacycline):ti,ab,kw OR (TP-434):ti,ab,kw OR (Xerava):ti,ab,kw | 12 |
| 2 | (abdom*):ti,ab,kw | 40,365 |
| 3 | 1 AND 2 | |
| 4 | #1 AND #2 | 5 |
| Ovid Medline Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | (Eravacycline or Xerava or TP-434).ab. | 82 |
| 2 | abdom*.ab | 373,974 |
| 3 | 1 AND 2 | |
| 4 | 1 and 2 | 24 |
| Embase Search Strategy—Last Searched on 26 May 2019 | Results | |
| 1 | eravacycline:ti,ab,kw OR xerava:ti,ab,kw OR ‘tp 434’:ti,ab,kw | 87 |
| 2 | abdom*:ti,ab,kw | 508,670 |
| 3 | 1 AND 2 | |
| 4 | #1 AND #2 | 27 |
| ClinicalTrials.gov Search Strategy—Last Searched on May 26, 2019 | Results | |
| 1 | Eravacycline (completed studies) | 9 |
References
- Solomkin, J.S.; Mazuski, J.E.; Baron, E.J.; Sawyer, R.G.; Nathens, A.B.; DiPiro, J.T.; Buchman, T.; Dellinger, E.P.; Jernigan, J.; Gorbach, S.; et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin. Infect. Dis. 2003, 37, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.J.; Botha, R.F.; Poswa, X.; Senekal, M.; Badal, R.E.; Grolman, D.C.; Richards, G.A.; Feldman, C.; Boffard, K.D.; Veller, M.; et al. Antimicrobial susceptibility of Gram-negative pathogens isolated from patients with complicated intra-abdominal infections in South African hospitals (SMART Study 2004–2009): Impact of the new carbapenem breakpoints. Surg. Infect. (Larchmt) 2012, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Chen, Y.S.; Toh, H.S.; Huang, C.C.; Liu, Y.M.; Ho, C.M.; Lu, P.L.; Ko, W.C.; Chen, Y.H.; Wang, J.H.; et al. Antimicrobial susceptibility of pathogens isolated from patients with complicated intra-abdominal infections at five medical centers in Taiwan that continuously participated in the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2006 to 2010. Int. J. Antimicrob. Agents 2012, 40, S29–S36. [Google Scholar] [PubMed]
- Sheng, W.H.; Badal, R.E.; Hsueh, P.R. Distribution of extended-spectrum beta-lactamases, AmpC beta-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: Results of the study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob. Agents Chemother. 2013, 57, 2981–2988. [Google Scholar] [PubMed]
- Zhanel, G.G.; Cheung, D.; Adam, H.; Zelenitsky, S.; Golden, A.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Walkty, A.; Gin, A.S.; et al. Review of Eravacycline, a Novel Fluorocycline Antibacterial Agent. Drugs 2016, 76, 567–588. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Landman, D.; Quale, J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob. Agents Chemother. 2015, 59, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In vitro activity of eravacycline against Carbapenem-Resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3840–3844. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Baxter, M.R.; Adam, H.J.; Sutcliffe, J.; Karlowsky, J.A. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014–2015. Diagn. Microbiol. Infect. Dis. 2018, 91, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P.A. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE 1) trial: A randomized clinical trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Montravers, P.; Sway, A.; Evans, D.; Tsai, L. IGNITE4: Results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs. meropenem in the treatment of complicated intra-abdominal infections. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Ramesh, M.K.; Cesnauskas, G.; Novikovs, N.; Stefanova, P.; Sutcliffe, J.A.; Walpole, S.M.; Horn, P.T. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob. Agents Chemother. 2014, 58, 1847–1854. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Citron, D.M.; Tyrrell, K.L. In vitro activity of eravacycline and comparator antimicrobials against 143 recent strains of Bacteroides and Parabacteroides species. Anaerobe 2018, 52, 122–124. [Google Scholar] [CrossRef]
- Snydman, D.R.; McDermott, L.A.; Jacobus, N.V.; Kerstein, K.; Grossman, T.H.; Sutcliffe, J.A. Evaluation of the in vitro activity of eravacycline against a broad spectrum of recent clinical anaerobic isolates. Antimicrob. Agents Chemother. 2018, 62, e02206-17. [Google Scholar] [CrossRef]
- Tetraphase Pharmaceuticals Inc. Xerava (Eravacycline): US prescribing information. 2018. Available online: http://www.fda.gov (accessed on 23 May 2019).
| Study, Published Year | Study Design | Study Site | Study Period | No. of Patients (ITT population) | Dose Regimen | ||
|---|---|---|---|---|---|---|---|
| Eravacycline | Comparator | Eravacycline | Comparator | ||||
| Solomkin et al, 2014 | Randomized, double-blind trial | 19 sites in 6 countries | 2011–2012 | 56 (1.5 mg/kg), 57 (1.0 mg/kg) | 30 | 1.5 mg/kg or 1.0 mg/kg q24 h | Ertapenem 1 g q24 h |
| IGNITE1, 2017 | Randomized, double-blind trial | 66 sites in 11 countries | 2013–2014 | 270 | 271 | 1.0 mg/kg q12 h | Ertapenem 1 g q24 h |
| IGNITE4, 2018 | Randomized, double-blind trial | 65 sites in 11 countries | 2016–2017 | 250 | 250 | 1.0 mg/kg q12 h | Meropenem 1 g q8 h |
| Risk of Bias | Study | ||
|---|---|---|---|
| IGNITE1, 2017 | IGNITE4, 2018 | Solomkin et al, 2014 | |
| Random sequence generation | low | low | low |
| Allocation concealment | low | low | low |
| Blinding of participants and personnel | low | low | low |
| Blinding of outcome assessment | low | low | low |
| Incomplete outcome data | low | low | low |
| Selective reporting | low | low | low |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.-H.; Chang, S.-P.; Lai, C.-C.; Lu, L.-C.; Chao, C.-M. The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2019, 8, 866. https://doi.org/10.3390/jcm8060866
Lan S-H, Chang S-P, Lai C-C, Lu L-C, Chao C-M. The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2019; 8(6):866. https://doi.org/10.3390/jcm8060866
Chicago/Turabian StyleLan, Shao-Huan, Shen-Peng Chang, Chih-Cheng Lai, Li-Chin Lu, and Chien-Ming Chao. 2019. "The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 8, no. 6: 866. https://doi.org/10.3390/jcm8060866
APA StyleLan, S.-H., Chang, S.-P., Lai, C.-C., Lu, L.-C., & Chao, C.-M. (2019). The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 8(6), 866. https://doi.org/10.3390/jcm8060866





