Effects of Dapagliflozin on Volume Status When Added to Renin–Angiotensin System Inhibitors
Abstract
1. Introduction
2. Experimental Section
2.1. Design and Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Changes in HbA1c, Renal Function, and Markers of Volume Status
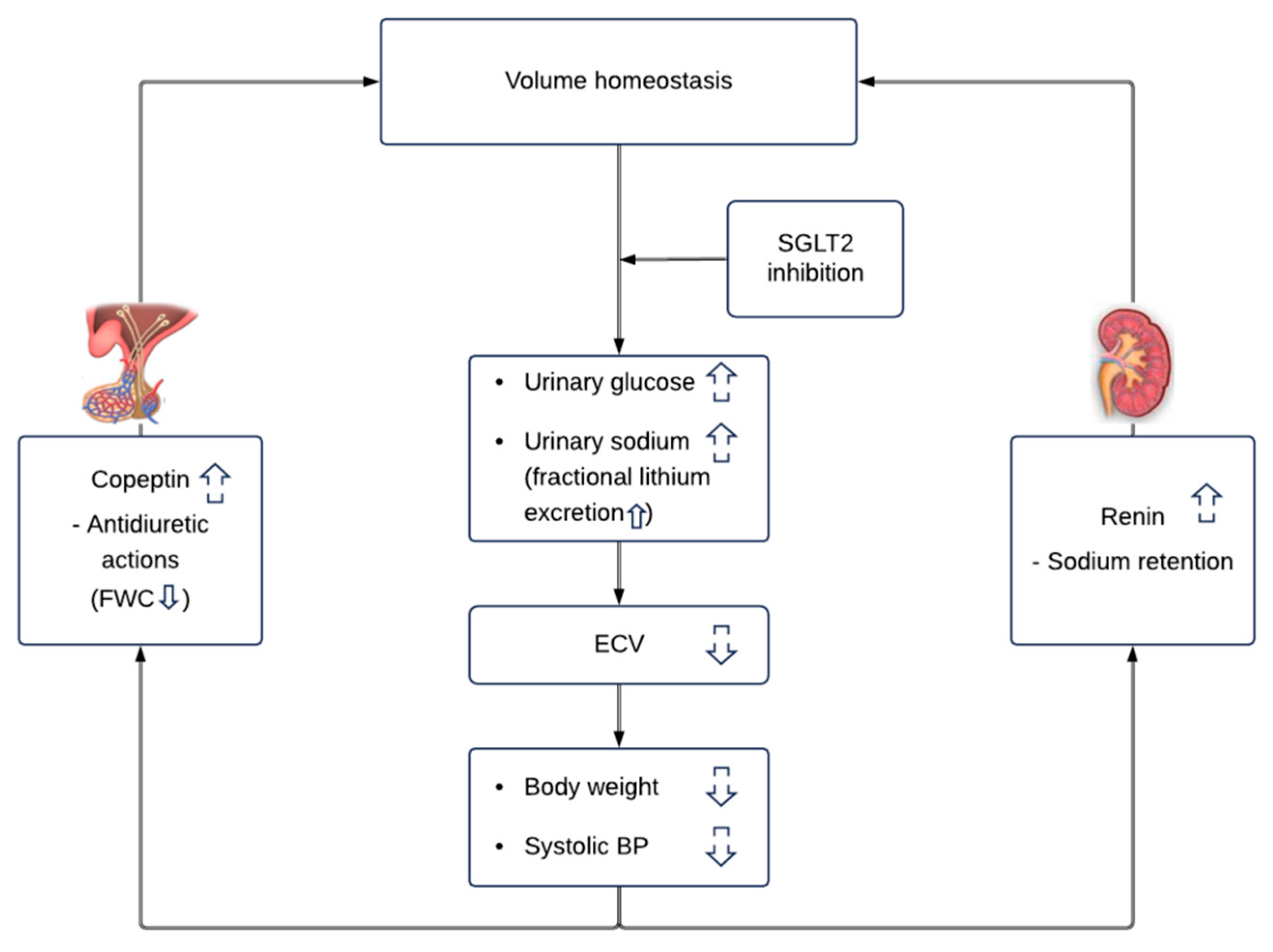
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Bonaca, O.; Kato, E.T.; Cahn, A.; Silverman, M.S.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Jordan, J.; Wanner, C.; Heer, C.; Macha, S.; Biomath, M.M.D.; Lund, M.M.; Woerle, H.J.; Broedl, U.C. Pharmacodynamic Effects of Single and Multiple Doses of Empagliflozin in Patients with Type 2 Diabetes. Clin. Ther. 2016, 38, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Shen, W.; Boulton, D.W.; Leslie, B.L.; Griffen, S.C. Interaction Between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Giannini, L.; Seghieri, M.; Seghieri, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Petrykiv, S.I.; Laverman, G.D.; de Zeeuw, D.; Heerspink, H.J.L. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes. Metab. 2017, 19, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, M.K.; Olsen, F.J.; Frimodt-Møller, M.; Diaz, L.J.; Faber, J.; Jensen, M.T.; Rossing, P.; Persson, F. Effect of Dapagliflozin on Cardiac Function in Patients with Type 2 Diabetes and Albuminuria. Presented at EASD, Berlin, Germany, 1–5 October 2018. [Google Scholar]
- Heida, J.E.; Boesten, L.S.M.; Ettema, E.M.; Kobold, A.C.M.; Franssen, C.F.M.; Gansevoort, R.T.; Zittema, D. Comparison of ex vivo stability of copeptin and vasopressin. Clin. Chem. Lab. Med. 2017, 55, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Koomans, H.A.; Boer, W.H.; Dorhout Mees, E.J. Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int. 1989, 36, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.; Jan Danser, A.H.; Nussberger, J.; Dole, W.P.; Hollenberg, N.K. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 2008, 117, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.G.; Danser, A.H.J. Juxtaglomerular Cell Phenotypic Plasticity. High Blood Press. Cardiovasc. Prev. 2017, 24, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J.; Zou, A.P. Influence of the renal medullary circulation on the control of sodium excretion. Am. J. Physiol. 1993, 265, R963–R973. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Polidori, D.; Heise, T.; Natarajan, J.; Farrell, K.; Wang, S.S.; Sica, D.; Rothenberg, P.; Plum-Mörschel, L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2014, 16, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Masuda, T.; Murakami, T.; Imai, T.; Yoshizawa, H.; Nakagawa, S.; Okada, M.; Miki, A.; Myoga, A.; Sugase, T.; et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: A comparison study with furosemide and tolvaptan. Nephrology 2018. [Google Scholar] [CrossRef] [PubMed]

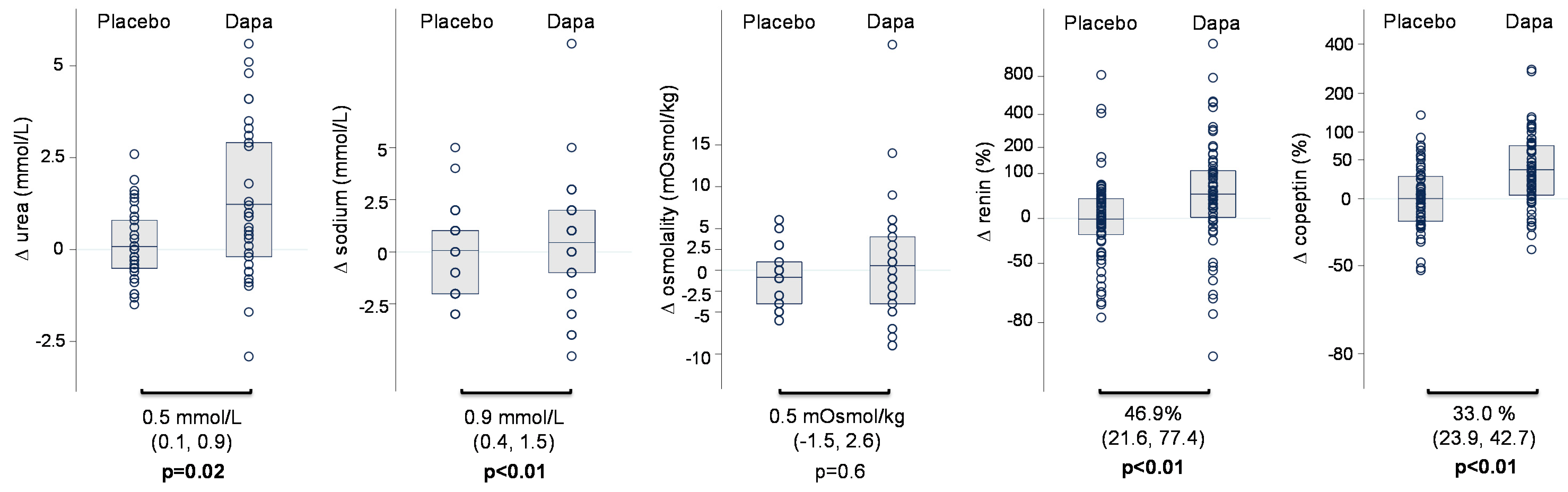
| Characteristics | At Baseline (n = 69) | End of Placebo Treatment | End of Dapagliflozin Treatment | Change during Dapagliflozin vs. Placebo (95% CI; p-Value) |
|---|---|---|---|---|
| Weight (kg) | 99.2 (21.5) | 98.9 (21.2) | 97.9 (21.2) | −1.3 (−1.8, 0.9; p < 0.01) |
| Body mass index (kg/m2) | 31.9 (5.7) | 31.8 (5.7) | 31.5 (5.8) | −0.39 (−0.6, −0.2; p < 0.01) |
| Systolic blood pressure (mmHg) | 141.2 (15.2) | 140.4 (14.5) | 134.7 (15.9) | −5.7 (−9.1, −2.3; p < 0.01) |
| Diastolic blood pressure (mmHg) | 79.8 (8.6) | 78.1 (9.4) | 76.8 (8.3) | −1.2 (−2.9, 0.5; p = 0.2) |
| Fasting plasma glucose (mmoL/L) | 9.8 (3.6) | 10.0 (3.4) | 8.2 (2.8) | −1.8 (−2.6, −0.9; p < 0.01) |
| HbA1c (mmoL/moL) | 65.4 (15.0) | 66.6 | 61.3 | −5.2 (−7.2, −3.2; p < 0.01) |
| Sodium (mmoL/L) | 139.2 (2.7) | 139.6 (2.8) | 140.5 (2.8) | 0.9 (0.4, 1.5; p < 0.01) |
| Potassium (mmoL/L) | 4.3 (0.5) | 4.3 (0.4) | 4.2 (0.4) | −0.02 (−0.1, 0.1; p = 0.61) |
| Urea (mmoL/L) | 6.4 (2.2) | 6.6 (2.4) | 7.1 (2.6) | 0.5 (0.1, 0.9; p = 0.02) |
| Osmolality (mOsmoL/kg) | 294.8 (14.4) | 291.1 (8.6) | 291.6 (7.3) | 0.5 (−1.5, 2.6; p =0.61) |
| Copeptin (pmoL/L) ‡ | 8.3 (5.7, 11.2) | 8.3 (5.4, 12.6) | 11.6 (6.8, 16.6) | 33.0% (23.9, 42.7; p < 0.01) |
| Renin (ng/L) ‡ | 37.1 (17.1, 85.0) | 33.6 (16.0, 70.1) | 59.3 (21.1, 101.0) | 46.9% (21.6, 77.4; p < 0.01) |
| NT-proBNP (ng/L) ‡ | 103.0 (35.0, 205.5) | 107.5 (43.8, 227.0) | 105.0 (48.0, 185) | −5.2% (−19.6, 8.1; p = 0.4) |
| Estimated GFR (mL/min/1.73 m2) | 79.4 (19.3) | 80.1 (18.8) | 76.1 (20.8) | −4.1 (−5.9, −2.4; p < 0.01) |
| UACR (mg/g) ‡ | 199.7 (102.3, 405.3) | 202.3 (106.3, 480.0) | 133.7 (75.3, 282.3) | −52.0% (−72.3, −34.0; p < 0.01) |
| Urinary volume (mL/24 h) | 2057 (762) | 2120 (741) | 2394 (804) | 266.3 (100.6, 432.0; p < 0.01) |
| Urine glucose excretion (mmoL/24 h) ‡ | 21.5 (2.0, 130.2) | 23.0 (2.0, 154.0) | 211.3 (121.1, 512.5) | 217.2 (155.7, 278.7; p < 0.01) |
| Urinary osmolality (mOsmoL/kg) | 560.7 (177.3) | 553.4 (175.6) | 614.2 (131.7) | 60.4 (30.0, 90.9; p < 0.01) |
| Urinary sodium excretion (mmoL/24 h) | 205.2 (110.6) | 200.5 (84.5) | 195.9 (98.3) | −4.5 (−27.5, 18.5; p = 0.70) |
| Fractional sodium excretion (%) | 937.8 (321.2) | 898.9 (335.1) | 1006.3 (384.8) | 104.2% (19.0, 189.4; p = 0.02) |
| Fractional lithium excretion (%)‡# | 11,318.7 (8984.9, 17,344.4) | 10,484.6 (8648.9, 13,734.9) | 12,437.4 (10,461.9, 16,275.4) | 19.6% (6.7, 34.2; p < 0.01) |
| Free water clearance (FWC) (mL/24 h) | −1727.1 (−1335.4) | −1724.3 (−1230.6) | −2606.1 (−1390.7) | −885.3 (−1156.2, −614.3; p < 0.01) |
| Baseline Subgroups | Mean Baseline Urinary Osmolality (mOsmoL/kg) (SD) | Change in Urinary Osmolality (mOsmoL/kg) (95% CI; p-Value) | Median Baseline NT-proBNP (ng/L) (IQR) | Change in NT-proBNP (%) (95% CI; p-Value) | Median Baseline Copeptin (pmoL/L) (IQR) | Change in Copeptin (%) (95% CI; p-Value) | Median Baseline Renin (ng/L) (IQR) | Change in Renin (%) (95% CI; p-Value) |
|---|---|---|---|---|---|---|---|---|
| Diuretics use Yes (N = 44) No (N = 25) | 554 (168) 573 (196) | 63.1 (24.7, 101.5; p < 0.01) 59.1 (8.2, 110.0; p = 0.02) | 75 (35, 201) 113 (38, 206) | −3.8% (−22.2, 13.5; p = 0.65) 7.1% (−33.6, 16.4; p = 0.54) | 7.3 (5.4, 11.2) 9.0 (5.9, 10.9) | 29.6% (18.7, 41.6; p < 0.01) 39.1% (23.9, 56.1; p < 0.01) | 38 (19, 85) 29 (17, 72) | 52.6% (20.4, 93.4; p < 0.01) 38.7% (1.6, 89.3; p = 0.04) |
| p for interaction | p = 0.19 | p = 0.39 | p = 0.41 | p = 0.08 | ||||
| HbA1c (mmoL/moL) <63 ≥63 | 532 (175) 589 (177) | 71.7 (27.7, 115.8; p < 0.01) 51.4 (9.0, 93.8; p = 0.02) | 110 (35, 209) 100 (35, 201) | 4.1% (-16.2, 25.8; p=0.68) −13.0% (−35.0, 5.8; p = 0.18) | 7.5 (5.6, 11.2) 9.0 (5.8, 14.0) | 32.0% (19.0, 46.5; p < 0.01) 35.3% (19.0, 53.7; p < 0.01) | 29 (19, 87) 40 (15, 85) | 32.2% (1.07, 73.0; p = 0.04) 62.7% (25.3, 111.2; p < 0.01) |
| p for interaction | p = 0.51 | p = 0.76 | p = 0.49 | p = 0.56 | ||||
| Estimated GFR (mL/min/1.73 m2) <82 ≥82 | 509 (152) 611 (188) | 65.4 (21.2, 109.5; p < 0.01) 57.1 (14.6, 99.6; p < 0.01) | 118 (53, 263) 66 (35, 150) | −4.8% (−26.1, 14.7; p = 0.61) −4.6% (−26.2, 15.3; p = 0.63) | 9.2 (6.3, 16.4) 7.1 (4.5, 10.0) | 36.7% (23.6, 51.2; p < 0.01) 29.5% (17.4, 42.8; p < 0.01) | 29 (20, 113) 41 (11, 70) | 48.5% (13.3, 94.7; p < 0.01) 46.0% (12.2, 90.0; p = 0.01) |
| p for interaction | p = 0.68 | p = 0.85 | p = b0.46 | p = 0.66 | ||||
| UACR (mg/g) <199.7 ≥199.7 | 591 (199) 532 (151) | 76.8 (33.6, 120.0; p < 0.01) 46.6 (3.7, 89.5; p = 0.03) | 75 (35, 201) 112 (53, 263) | −10.3% −33.3, 9.5; p = 0.30) −0.1% (−19.8, 20.0; p = 0.99) | 7.7 (5.8, 10.9) 8.9 (5.7, 11.2) | 32.5% (19.9, 46.4; p < 0.01) 33.5% (20.8, 47.5; p < 0.01) | 38 (17, 67) 37 (19, 85) | 45.4% (11.3, 89.8; p < 0.01) 49.0% (14.1, 94.7, p < 0.01) |
| p for interaction | p = 0.92 | p = 0.71 | p = 0.29 | p = 0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eickhoff, M.K.; Dekkers, C.C.J.; Kramers, B.J.; Laverman, G.D.; Frimodt-Møller, M.; Jørgensen, N.R.; Faber, J.; Danser, A.H.J.; Gansevoort, R.T.; Rossing, P.; et al. Effects of Dapagliflozin on Volume Status When Added to Renin–Angiotensin System Inhibitors. J. Clin. Med. 2019, 8, 779. https://doi.org/10.3390/jcm8060779
Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Møller M, Jørgensen NR, Faber J, Danser AHJ, Gansevoort RT, Rossing P, et al. Effects of Dapagliflozin on Volume Status When Added to Renin–Angiotensin System Inhibitors. Journal of Clinical Medicine. 2019; 8(6):779. https://doi.org/10.3390/jcm8060779
Chicago/Turabian StyleEickhoff, Mie K., Claire C. J. Dekkers, Bart J. Kramers, Gozewijn Dirk Laverman, Marie Frimodt-Møller, Niklas Rye Jørgensen, Jens Faber, A. H. Jan Danser, Ron T. Gansevoort, Peter Rossing, and et al. 2019. "Effects of Dapagliflozin on Volume Status When Added to Renin–Angiotensin System Inhibitors" Journal of Clinical Medicine 8, no. 6: 779. https://doi.org/10.3390/jcm8060779
APA StyleEickhoff, M. K., Dekkers, C. C. J., Kramers, B. J., Laverman, G. D., Frimodt-Møller, M., Jørgensen, N. R., Faber, J., Danser, A. H. J., Gansevoort, R. T., Rossing, P., Persson, F., & Heerspink, H. J. L. (2019). Effects of Dapagliflozin on Volume Status When Added to Renin–Angiotensin System Inhibitors. Journal of Clinical Medicine, 8(6), 779. https://doi.org/10.3390/jcm8060779






