Substance Abuse and Male Hypogonadism
Abstract
1. Introduction
2. Alcohol
2.1. Effects on Testosterone Production
2.2. Effects on Spermatogenesis
3. Cigarette Smoking
3.1. Effects on Testosterone Production
3.2. Effects on Spermatogenesis
4. Caffeine
4.1. Effects on Testosterone Production
4.2. Effects on Spermatogenesis
5. Cannabis
5.1. Effects on Testosterone Production
5.2. Effects on Spermatogenesis
6. Cocaine
6.1. Effects on Testosterone Production
6.2. Effects on Spermatogenesis
7. Amphetamine, Methamphetamine, and MDMA (Ecstasy)
7.1. Effects on Testosterone Production
7.2. Effects on Spermatogenesis
8. Opioids
8.1. Effects on Testosterone Production
8.2. Effects on Spermatogenesis
9. Anabolic-Androgenic Steroids
9.1. Effects on Testosterone Production
9.2. Effects on Spermatogenesis
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Travison, T.G.; Araujo, A.B.; O’Donnell, A.B.; Kupelian, V.; McKinlay, J.B. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 2007, 92, 196–202. [Google Scholar] [CrossRef]
- Andersson, A.M.; Jensen, T.K.; Juul, A.; Petersen, J.H.; Jørgensen, T.; Skakkebaek, N.E. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J. Clin. Endocrinol. Metab. 2007, 92, 4696–4705. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Araujo, A.B.; Hall, S.A.; McKinlay, J.B. Temporal trends in testosterone levels and treatment in older men. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Balercia, G.; Vicari, E.; Calogero, A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J. Androl. 2013, 15, 221–225. [Google Scholar] [CrossRef]
- Van Thiel, D.H.; Gavaler, J.S.; Cobb, C.F.; Santucci, L.; Graham, T.O. Ethanol, a Leydig cell toxin: Evidence obtained in vivo and in vitro. Pharmacol. Biochem. Behav. 1983, 18 (Suppl. 1), 317–323. [Google Scholar] [CrossRef]
- Muthusami, K.R.; Chinnaswamy, P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005, 84, 919–924. [Google Scholar] [CrossRef]
- Jana, K.; Jana, N.; De, D.K.; Guha, S.K. Ethanol induces mouse spermatogenic cell apoptosis in vivo through over-expression of Fas/Fas-L, p53, and caspase-3 along with cytochrome c translocation and glutathione depletion. Mol. Reprod. Dev. 2010, 77, 820–833. [Google Scholar] [CrossRef]
- Gordon, G.G.; Southren, A.L.; Vittek, J.; Lieber, C.S. The effect of alcohol ingestion on hepatic aromatase activity and plasma steroid hormones in the rat. Metabolism 1979, 28, 20–24. [Google Scholar] [CrossRef]
- Burra, P.; Franklyn, J.A.; Ramsden, D.B.; Elias, E.; Sheppard, M.C. Severity of alcoholic liver disease and markers of thyroid and steroid status. Postgrad. Med. J. 1992, 68, 804–810. [Google Scholar] [CrossRef][Green Version]
- Ida, Y.; Tsujimaru, S.; Nakamaura, K.; Shirao, I.; Mukasa, H.; Egami, H.; Nakazawa, Y. Effects of acute and repeated alcohol ingestion on hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal functioning in normal males. Drug Alcohol Depend. 1992, 31, 57–64. [Google Scholar] [CrossRef]
- Emanuele, M.A.; Emanuele, N.V. Alcohol’s effects on male reproduction. Alcohol Health Res. World 1998, 22, 195–201. [Google Scholar] [PubMed]
- Gianoulakis, C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur. J. Pharmacol. 1990, 180, 21–29. [Google Scholar] [CrossRef]
- Pajarinen, J.T.; Karhunen, P.J. Spermatogenic arrest and ‘Sertoli cell-only’ syndrome--common alcohol-induced disorders of the human testis. Int. J. Androl. 1994, 17, 292–299. [Google Scholar] [CrossRef]
- Pajarinen, J.; Karhunen, P.J.; Savolainen, V.; Lalu, K.; Penttilä, A.; Laippala, P. Moderate alcohol consumption and disorders of human spermatogenesis. Alcohol. Clin. Exp. Res. 1996, 20, 332–337. [Google Scholar] [CrossRef]
- Anderson, R.A., Jr.; Willis, B.R.; Oswald, C. Spontaneous recovery from ethanol-induced male infertility. Alcohol 1985, 2, 479–484. [Google Scholar] [CrossRef]
- Vicari, E.; Arancio, A.; Giuffrida, V.; D’Agata, R.; Calogero, A.E. A case of reversible azoospermia following withdrawal from alcohol consumption. J. Endocrinol. Investig. 2002, 25, 473–476. [Google Scholar] [CrossRef]
- Sermondade, N.; Elloumi, H.; Berthaut, I.; Mathieu, E.; Delarouzière, V.; Ravel, C.; Mandelbaum, J. Progressive alcohol-induced sperm alterations leading to spermatogenic arrest, which was reversed after alcohol withdrawal. Reprod. Biomed. Online 2010, 20, 324–327. [Google Scholar] [CrossRef]
- Guthauser, B.; Boitrelle, F.; Plat, A.; Thiercelin, N.; Vialard, F. Chronic excessive alcohol consumption and male fertility: A case report on reversible azoospermia and a literature review. Alcohol Alcohol. 2014, 49, 42–44. [Google Scholar] [CrossRef]
- Jensen, T.K.; Swan, S.; Jørgensen, N.; Toppari, J.; Redmon, B.; Punab, M.; Drobnis, E.Z.; Haugen, T.B.; Zilaitiene, B.; Sparks, A.E.; et al. Alcohol and male reproductive health: A cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014, 29, 1801–1809. [Google Scholar] [CrossRef]
- Jensen, T.K.; Gottschau, M.; Madsen, J.O.; Andersson, A.M.; Lassen, T.H.; Skakkebæk, N.E.; Swan, S.H.; Priskorn, L.; Juul, A.; Jørgensen, N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open 2014, 4, e005462. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Dehò, F.; Montanari, E.; Montorsi, F.; Salonia, A. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef]
- Ricci, E.; Noli, S.; Ferrari, S.; La Vecchia, I.; Cipriani, S.; De Cosmi, V.; Somigliana, E.; Parazzini, F. Alcohol intake and semen variables: Cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology 2018, 6, 690–696. [Google Scholar] [CrossRef]
- Savolainen, V.T.; Pjarinen, J.; Perola, M.; Penttilä, A.; Karhunen, P.J. Glutathione-S-transferase GST M1 “null” genotype and the risk of alcoholic liver disease. Alcohol. Clin. Exp. Res. 1996, 20, 1340–1345. [Google Scholar] [CrossRef]
- Pajarinen, J.; Savolainen, V.; Perola, M.; Penttilä, A.; Karhunen, P.J. Glutathione S-transferase-M1 ‘null’ genotype and alcohol-induced disorders of human spermatogenesis. Int. J. Androl. 1996, 19, 155–163. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Our experience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Stabbert, R.; Dempsey, R.; Diekmann, J.; Euchenhofer, C.; Hagemeister, T.; Haussmann, H.J.; Knorr, A.; Mueller, B.P.; Pospisil, P.; Reininghaus, W.; et al. Studies on the contributions of smoke constituents, individually and in mixtures, in a range of in vitro bioactivity assays. Toxicol. In Vitro 2017, 42, 222–246. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Mongioì, L.M.; Li Volti, G.; Barbagallo, I.; Avola, R.; Calogero, A.E. Nicotine Effects and Receptor Expression on Human Spermatozoa: Possible Neuroendocrine Mechanism. Front. Physiol. 2017, 8, 177. [Google Scholar] [CrossRef]
- Pacifici, R.; Altieri, I.; Gandini, L.; Lenzi, A.; Pichini, S.; Rosa, M.; Zuccaro, P.; Dondero, F. Nicotine, cotinine, and trans-3-hydroxycotinine levels in seminal plasma of smokers: Effects on sperm parameters. Ther. Drug Monit. 1993, 15, 358–363. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Isoyama, E.; Sofikitis, N.; Miyagawa, I. Effects of smoking on testicular function and fertilizing potential in rats. Urol. Res. 1998, 26, 45–48. [Google Scholar] [CrossRef]
- Kavitharaj, N.K.; Vijayammal, P.L. Nicotine administration induced changes in the gonadal functions in male rats. Pharmacology 1999, 58, 2–7. [Google Scholar] [CrossRef]
- Oyeyipo, I.P.; Raji, Y.; Bolarinwa, A.F. Nicotine alters male reproductive hormones in male albino rats: The role of cessation. J. Hum. Reprod. Sci. 2013, 6, 40–44. [Google Scholar] [CrossRef]
- Yeh, J.; Barbieri, R.L.; Friedman, A.J. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. J. Steroid Biochem. 1989, 33, 627–630. [Google Scholar] [CrossRef]
- Kim, K.H.; Joo, K.J.; Park, H.J.; Kwon, C.H.; Jang, M.H.; Kim, C.J. Nicotine induces apoptosis in TM3 mouse Leydig cells. Fertil. Steril. 2005, 83 (Suppl. 1), 1093–1099. [Google Scholar] [CrossRef]
- Trummer, H.; Habermann, H.; Haas, J.; Pummer, K. The impact of cigarette smoking on human semen parameters and hormones. Hum. Reprod. 2002, 17, 1554–1559. [Google Scholar] [CrossRef]
- Svartberg, J.; Midtby, M.; Bønaa, K.H.; Sundsfjord, J.; Joakimsen, R.M.; Jorde, R. The associations of age, lifestyle factors and chronic disease with testosterone in men: The Tromsø Study. Eur. J. Endocrinol. 2003, 149, 145–152. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.H.; Thulstrup, A.M.; Aggerholm, A.S.; Jensen, M.S.; Toft, G.; Bonde, J.P. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum. Reprod. 2007, 22, 188–196. [Google Scholar] [CrossRef]
- Blanco-Muñoz, J.; Lacasaña, M.; Aguilar-Garduño, C. Effect of current tobacco consumption on the male reproductive hormone profile. Sci. Total Environ. 2012, 426, 100–105. [Google Scholar] [CrossRef]
- Lotti, F.; Corona, G.; Vitale, P.; Maseroli, E.; Rossi, M.; Fino, M.G.; Maggi, M. Current smoking is associated with lower seminal vesicles and ejaculate volume, despite higher testosterone levels, in male subjects of infertile couples. Hum. Reprod. 2015, 30, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Leung, J.Y.; Lin, S.L.; Schooling, C.M. Cigarette smoking and testosterone in men and women: A systematic review and meta-analysis of observational studies. Prev. Med. 2016, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vine, M.F.; Margolin, B.H.; Morrison, H.I.; Hulka, B.S. Cigarette smoking and sperm density: A meta-analysis. Fertil. Steril. 1994, 61, 35–43. [Google Scholar]
- Künzle, R.; Mueller, M.D.; Hänggi, W.; Birkhäuser, M.H.; Drescher, H.; Bersinger, N.A. Semen quality of male smokers and nonsmokers in infertile couples. Fertil. Steril. 2003, 79, 287–291. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Sobreiro, B.P.; Hallak, J.; Pasqualotto, E.B.; Lucon, A.M. Cigarette smoking is related to a decrease in semen volume in a population of fertile men. BJU Int. 2006, 97, 324–326. [Google Scholar] [CrossRef]
- De Jong, A.M.; Menkveld, R.; Lens, J.W.; Nienhuis, S.E.; Rhemrev, J.P. Effect of alcohol intake and cigarette smoking on sperm parameters and pregnancy. Andrologia 2014, 46, 112–117. [Google Scholar] [CrossRef]
- Gaur, D.S.; Talekar, M.S.; Pathak, V.P. Alcohol intake and cigarette smoking: Impact of two major lifestyle factors on male fertility. Indian J. Pathol. Microbiol. 2010, 53, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, H.; Li, Y.; Cao, J. Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil. Steril. 2011, 95, 116–123. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Janoo, G.; Bhurtu, A.; Teeluck, A.R.; Soogund, M.Z.S.; Pursun, M.; Huang, F. Tobacco smoking and semen quality in infertile males: A systematic review and meta-analysis. BMC Public Health 2019, 19, 36. [Google Scholar] [CrossRef]
- Calogero, A.; Polosa, R.; Perdichizzi, A.; Guarino, F.; La Vignera, S.; Scarfia, A.; Fratantonio, E.; Condorelli, R.; Bonanno, O.; Barone, N.; et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod. Biomed. Online 2009, 19, 564–571. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Vicari, E.; Mongioì, L.; Calogero, A.E. In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. Int. J. Immunopathol. Pharmacol. 2013, 26, 739–746. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Duca, Y.; Zanghi, G.N.; Calogero, A.E. Nicotine Receptors as a Possible Marker for Smoking-related Sperm Damage. Protein Pept. Lett. 2018, 25, 451–454. [Google Scholar] [CrossRef]
- Aydos, K.; Güven, M.C.; Can, B.; Ergün, A. Nicotine toxicity to the ultrastructure of the testis in rats. BJU Int. 2001, 88, 622–626. [Google Scholar] [CrossRef]
- Dai, J.B.; Wang, Z.X.; Qiao, Z.D. The hazardous effects of tobacco smoking on male fertility. Asian J. Androl. 2015, 17, 954–960. [Google Scholar] [CrossRef]
- Kiziler, A.R.; Aydemir, B.; Onaran, I.; Alici, B.; Ozkara, H.; Gulyasar, T.; Akyolcu, M.C. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol. Trace Elem. Res. 2007, 120, 82–91. [Google Scholar] [CrossRef]
- Yu, B.; Chen, J.; Liu, D.; Zhou, H.; Xiao, W.; Xia, X.; Huang, Z. Cigarette smoking is associated with human semen quality in synergy with functional NRF2 polymorphisms. Biol. Reprod. 2013, 89, 5. [Google Scholar] [CrossRef]
- Karmon, A.E.; Toth, T.L.; Chiu, Y.H.; Gaskins, A.J.; Tanrikut, C.; Wright, D.L.; Hauser, R.; Chavarro, J.E.; Earth Study Team. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology 2017, 5, 354–361. [Google Scholar] [CrossRef]
- Beach, C.A.; Bianchine, J.R.; Gerber, N. The excretion of caffeine in the semen of men: Pharmacokinetics and comparison of the concentrations in blood and semen. J. Clin. Pharmacol. 1984, 24, 120–126. [Google Scholar] [CrossRef]
- Jensen, T.K.; Swan, S.H.; Skakkebaek, N.E.; Rasmussen, S.; Jørgensen, N. Caffeine intake and semen quality in a population of 2554 young Danish men. Am. J. Epidemiol. 2010, 171, 883–891. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.H.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J.; Bech, B.H. Semen quality according to prenatal coffee and present caffeine exposure: Two decades of follow-up of a pregnancy cohort. Hum. Reprod. 2008, 23, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, R.L.; Barrett-Connor, E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am. J. Epidemiol. 1998, 147, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Pollard, I. Increases in plasma concentrations of steroids in the rat after the administration of caffeine: Comparison with plasma disposition of caffeine. J. Endocrinol. 1988, 119, 275–280. [Google Scholar] [CrossRef]
- Oluwole, O.F.; Salami, S.A.; Ogunwole, E.; Raji, Y. Implication of caffeine consumption and recovery on the reproductive functions of adult male Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 483–491. [Google Scholar] [CrossRef]
- Oldereid, N.B.; Rui, H.; Purvis, K. Life styles of men in barren couples and their relationship to sperm quality. Int. J. Fertil. 1992, 37, 343–349. [Google Scholar] [PubMed]
- Jensen, T.K.; Henriksen, T.B.; Hjollund, N.H.; Scheike, T.; Kolstad, H.; Giwercman, A.; Ernst, E.; Bonde, J.P.; Skakkebaek, N.E.; Olsen, J. Caffeine intake and fecundability: A follow-up study among 430 Danish couples planning their first pregnancy. Reprod. Toxicol. 1998, 12, 289–295. [Google Scholar] [CrossRef]
- Sobreiro, B.P.; Lucon, A.M.; Pasqualotto, F.F.; Hallak, J.; Athayde, K.S.; Arap, S. Semen analysis in fertile patients undergoing vasectomy: Reference values and variations according to age, length of sexual abstinence, seasonality, smoking habits and caffeine intake. Sao Paulo Med. J. 2005, 123, 161–166. [Google Scholar] [CrossRef]
- Dias, T.R.; Alves, M.G.; Bernardino, R.L.; Martins, A.D.; Moreira, A.C.; Silva, J.; Barros, A.; Sousa, M.; Silva, B.M.; Oliveira, P.F. Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: Relevance for male fertility. Toxicology 2015, 328, 12–20. [Google Scholar] [CrossRef]
- Park, B.; McPartland, J.M.; Glass, M. Cannabis, cannabinoids and reproduction. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 189–197. [Google Scholar] [CrossRef]
- Battista, N.; Pasquariello, N.; Di Tommaso, M.; Maccarrone, M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J. Neuroendocrinol. 2008, 20 (Suppl. 1), 82–89. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, R.C.; Masters, W.H.; Kolodner, R.M.; Toro, G. Depression of plasma testosterone levels after chronic intensive marihuana use. N. Engl. J. Med. 1974, 290, 872–874. [Google Scholar] [CrossRef]
- Cone, E.J.; Johnson, R.E.; Moore, J.D.; Roache, J.D. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol. Biochem. Behav. 1986, 24, 1749–1754. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Kuehnle, J.; Ellingboe, J.; Babor, T.F. Plasma testosterone levels before, during and after chronic marihuana smoking. N. Engl. J. Med. 1974, 291, 1051–1055. [Google Scholar] [CrossRef]
- Cushman, P., Jr. Plasma testosterone levels in healthy male marijuana smokers. Am. J. Drug Alcohol Abus. 1975, 2, 269–275. [Google Scholar] [CrossRef]
- Friedrich, G.; Nepita, W.; André, T. Serum testosterone concentrations in cannabis and opiate users. Beitr. Gerichtl. Med. 1990, 48, 57–66. [Google Scholar] [PubMed]
- Thistle, J.E.; Graubard, B.I.; Braunlin, M.; Vesper, H.; Trabert, B.; Cook, M.B.; McGlynn, K.A. Marijuana use and serum testosterone concentrations among U.S. males. Andrology 2017, 5, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, T.D.; Jørgensen, N.; Andersson, A.M.; Bang, A.K.; Nordkap, L.; Skakkebæk, N.E.; Priskorn, L.; Juul, A.; Jensen, T.K. Association Between Use of Marijuana and Male Reproductive Hormones and Semen Quality: A Study Among 1215 Healthy Young Men. Am. J. Epidemiol. 2015, 182, 473–481. [Google Scholar] [CrossRef]
- Rajanahally, S.; Raheem, O.; Rogers, M.; Brisbane, W.; Ostrowski, K.; Lendvay, T.; Walsh, T. The relationship between cannabis and male infertility, sexual health, and neoplasm: A systematic review. Andrology 2019, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fasano, S.; Meccariello, R.; Cobellis, G.; Chianese, R.; Cacciola, G.; Chioccarelli, T.; Pierantoni, R. The endocannabinoid system: An ancient signaling involved in the control of male fertility. Ann. N. Y. Acad. Sci. 2009, 1163, 112–124. [Google Scholar] [CrossRef]
- Wenger, T.; Ledent, C.; Csernus, V.; Gerendai, I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem. Biophys. Res. Commun. 2001, 284, 363–368. [Google Scholar] [CrossRef]
- Farkas, I.; Kalló, I.; Deli, L.; Vida, B.; Hrabovszky, E.; Fekete, C.; Moenter, S.M.; Watanabe, M.; Liposits, Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 5818–5829. [Google Scholar] [CrossRef] [PubMed]
- List, A.; Nazar, B.; Nyquist, S.; Harclerode, J. The effects of delta9-tetrahydrocannabinol and cannabidiol on the metabolism of gonadal steroids in the rat. Drug Metab. Dispos. 1977, 5, 268–272. [Google Scholar]
- Jakubovic, A.; McGeer, E.G.; McGeer, P.L. Effects of cannabinoids on testosterone and protein synthesis in rat testis Leydig cells in vitro. Mol. Cell. Endocrinol. 1979, 15, 41–50. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, A.; Srivastava, P.; Turner, H.; Krishna, A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 195–205. [Google Scholar] [CrossRef]
- Close, C.E.; Roberts, P.L.; Berger, R.E. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J. Urol. 1990, 144, 900–903. [Google Scholar] [CrossRef]
- Dixit, V.P.; Gupta, C.L.; Agrawal, M. Testicular degeneration and necrosis induced by chronic administration of cannabis extract in dogs. Endokrinologie 1977, 69, 299–305. [Google Scholar]
- Lewis, S.E.; Paro, R.; Borriello, L.; Simon, L.; Robinson, L.; Dincer, Z.; Riedel, G.; Battista, N.; Maccarrone, M. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. Int. J. Androl. 2012, 35, 731–740. [Google Scholar] [CrossRef]
- López-Cardona, A.P.; Ibarra-Lecue, I.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Urigüen, L.; Agirregoitia, N.; Gutiérrez-Adán, A.; Agirregoitia, E. Effect of chronic THC administration in the reproductive organs of male mice, spermatozoa and in vitro fertilization. Biochem. Pharmacol. 2018, 157, 294–303. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Sanchez-Yague, J.; Paniagua, R. Effects of cocaine on testicular structure in the rat. Reprod. Toxicol. 1992, 6, 51–55. [Google Scholar] [CrossRef]
- Samplaski, M.K.; Bachir, B.G.; Lo, K.C.; Grober, E.D.; Lau, S.; Jarvi, K.A. Cocaine Use in the Infertile Male Population: A Marker for Conditions Resulting in Subfertility. Curr. Urol. 2015, 8, 38–42. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Sholar, M.B.; Mutschler, N.H.; Jaszyna-Gasior, M.; Goletiani, N.V.; Siegel, A.J.; Mello, N.K. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone, and prolactin in men. J. Pharmacol. Exp. Ther. 2003, 307, 339–348. [Google Scholar] [CrossRef]
- Goletiani, N.V.; Mendelson, J.H.; Sholar, M.B.; Siegel, A.J.; Mello, N.K. Opioid and cocaine combined effect on cocaine-induced changes in HPA and HPG axes hormones in men. Pharmacol. Biochem. Behav. 2009, 91, 526–536. [Google Scholar] [CrossRef]
- Wisniewski, A.B.; Brown, T.T.; John, M.; Frankowicz, J.K.; Cofranceso, J., Jr.; Golub, E.T.; Ricketts, E.P.; Dobs, A.S. Hypothalamic-pituitary-gonadal function in men and women using heroin and cocaine, stratified by HIV status. Gend. Med. 2007, 4, 35–44. [Google Scholar] [CrossRef]
- Insel, J.R.; Dhanjal, N. Pituitary infarction resulting from intranasal cocaine abuse. Endocr. Pract. 2004, 10, 478–482. [Google Scholar] [CrossRef]
- De Lange, T.E.; Simsek, S.; Kramer, M.H.; Nanayakkara, P.W. A case of cocaine-induced panhypopituitarism with human neutrophil elastase-specific anti-neutrophil cytoplasmic antibodies. Eur. J. Endocrinol. 2009, 160, 499–502. [Google Scholar] [CrossRef]
- Gordon, L.A.; Mostofsky, D.I.; Gordon, G.G. Changes in testosterone levels in the rat following intraperitoneal cocaine HCl. Int. J. Neurosci. 1980, 11, 139–141. [Google Scholar] [CrossRef]
- George, V.K.; Li, H.; Teloken, C.; Grignon, D.J.; Lawrence, W.D.; Dhabuwala, C.B. Effects of long-term cocaine exposure on spermatogenesis and fertility in peripubertal male rats. J. Urol. 1996, 155, 327–331. [Google Scholar] [CrossRef]
- Mello, N.K.; Mendelson, J.H.; Negus, S.S.; Kelly, M.; Knudson, I.; Roth, M.E. The effects of cocaine on gonadal steroid hormones and LH in male and female rhesus monkeys. Neuropsychopharmacology 2004, 29, 2024–2034. [Google Scholar] [CrossRef]
- Bracken, M.B.; Eskenazi, B.; Sachse, K.; McSharry, J.E.; Hellenbrand, K.; Leo-Summers, L. Association of cocaine use with sperm concentration, motility, and morphology. Fertil. Steril. 1990, 53, 315–322. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Rajpurkar, A.; Dunbar, J.C.; Dhabuwala, C.B. Cocaine induced apoptosis in rat testes. J. Urol. 1999, 162, 213–216. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Rajpurkar, A.; Tefilli, M.V.; Dunbar, J.C.; Dhabuwala, C.B. Lipid peroxidation and antioxidant activities in rat testis after chronic cocaine administration. Urology 1999, 54, 925–928. [Google Scholar] [CrossRef]
- Liechti, M. Novel psychoactive substances (designer drugs): Overview and pharmacology of modulators of monoamine signaling. Swiss Med. Wkly. 2015, 145, w14043. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Mechan, A.O.; Elliott, J.M.; O’Shea, E.; Colado, M.I. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol. Rev. 2003, 55, 463–508. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Chiao, Y.C.; Lu, C.C.; Doong, M.L.; Chen, Y.H.; Shih, H.C.; Liaw, C.; Wang, S.W.; Wang, P.S. Inhibition by amphetamine of testosterone secretion through a mechanism involving an increase of cyclic AMP production in rat testes. Br. J. Pharmacol. 1996, 118, 984–988. [Google Scholar] [CrossRef]
- Tsai, S.C.; Chen, J.J.; Chiao, Y.C.; Lu, C.C.; Lin, H.; Yeh, J.Y.; Lo, M.J.; Kau, M.M.; Wang, S.W.; Wang, P.S. The role of cyclic AMP production, calcium channel activation and enzyme activities in the inhibition of testosterone secretion by amphetamine. Br. J. Pharmacol. 1997, 122, 949–955. [Google Scholar] [CrossRef]
- Chen, L.Y.; Huang, Y.L.; Liu, M.Y.; Leu, S.F.; Huang, B.M. Effects of amphetamine on steroidogenesis in MA-10 mouse Leydig tumor cells. Life Sci. 2003, 72, 1983–1995. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, K.; Hayase, T. Effect of methamphetamine on male mice fertility. J. Obstet. Gynaecol. Res. 1999, 25, 353–358. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, Y.H.; Liao, P.C.; Lin, Y.C.; Tsai, T.F.; Chou, K.Y.; Chen, H.E.; Tsai, S.C.; Hwang, T.I. Induction of testicular damage by daily methamphetamine administration in rats. Chin. J. Physiol. 2014, 57, 19–30. [Google Scholar] [CrossRef]
- Kaewman, P.; Nudmamud-Thanoi, S.; Thanoi, S. GABAergic Alterations in the Rat Testis after Methamphetamine Exposure. Int. J. Med. Sci. 2018, 15, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.M.; Walker, D.M.; Reveron, M.E.; Duvauchelle, C.L.; Gore, A.C. The recreational drug ecstasy disrupts the hypothalamic-pituitary-gonadal reproductive axis in adult male rats. Neuroendocrinology 2008, 88, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Barenys, M.; Macia, N.; Camps, L.; de Lapuente, J.; Gomez-Catalan, J.; Gonzalez-Linares, J.; Borras, M.; Rodamilans, M.; Llobet, J.M. Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats. Toxicol. Lett. 2009, 191, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamamoto, K.; Hayase, T.; Abiru, H.; Shiota, K.; Mori, C. Methamphetamine induces apoptosis in seminiferous tubules in male mice testis. Toxicol. Appl. Pharmacol. 2002, 178, 155–160. [Google Scholar] [CrossRef]
- Alavi, S.H.; Taghavi, M.M.; Moallem, S.A. Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. Syst. Biol. Reprod. Med. 2008, 54, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nudmamud-Thanoi, S.; Thanoi, S. Methamphetamine induces abnormal sperm morphology, low sperm concentration and apoptosis in the testis of male rats. Andrologia 2011, 43, 278–282. [Google Scholar] [CrossRef]
- Saberi, A.; Sepehri, G.; Safi, Z.; Razavi, B.; Jahandari, F.; Divsalar, K.; Salarkia, E. Effects of Methamphetamine on Testes Histopathology and Spermatogenesis Indices of Adult Male Rats. Addict. Health 2017, 9, 199–205. [Google Scholar]
- Nudmamud-Thanoi, S.; Sueudom, W.; Tangsrisakda, N.; Thanoi, S. Changes of sperm quality and hormone receptors in the rat testis after exposure to methamphetamine. Drug Chem. Toxicol. 2016, 39, 432–438. [Google Scholar] [CrossRef]
- Mobaraki, F.; Seghatoleslam, M.; Fazel, A.; Ebrahimzadeh-Bideskan, A. Effects of MDMA (ecstasy) on apoptosis and heat shock protein (HSP70) expression in adult rat testis. Toxicol. Mech. Methods 2018, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Van Uum, S.H.; O’Dell, L.E.; Lutfy, K.; Friedman, T.C. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr. Rev. 2010, 31, 98–132. [Google Scholar] [CrossRef]
- Hsieh, A.; DiGiorgio, L.; Fakunle, M.; Sadeghi-Nejad, H. Management Strategies in Opioid Abuse and Sexual Dysfunction: A Review of Opioid-Induced Androgen Deficiency. Sex. Med. Rev. 2018, 6, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Zhu, V.; Vorsanger, G.; Eichenbaum, G. Effect of Opioids on Testosterone Levels: Cross-Sectional Study using NHANES. Pain Med. 2015, 16, 2235–2242. [Google Scholar] [CrossRef][Green Version]
- Fronczak, C.M.; Kim, E.D.; Barqawi, A.B. The insults of illicit drug use on male fertility. J. Androl. 2012, 33, 515–528. [Google Scholar] [CrossRef]
- Abs, R.; Verhelst, J.; Maeyaert, J.; Van Buyten, J.P.; Opsomer, F.; Adriaensen, H.; Verlooy, J.; Van Havenbergh, T.; Smet, M.; Van Acker, K. Endocrine consequences of long-term intrathecal administration of opioids. J. Clin. Endocrinol. Metab. 2000, 85, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Lafisca, S.; Bolelli, G.; Franceschetti, F.; Danieli, A.; Tagliaro, F.; Marigo, M.; Flamigni, C. Free and bound testosterone in male heroin addicts. In Receptors and Other Targets for Toxic Substances; Springer: Berlin/Heidelberg, Germany, 1985; pp. 394–397. [Google Scholar]
- Rasheed, A.; Tareen, I.A. Effects of heroin on thyroid function, cortisol and testosterone level in addicts. Pol. J. Pharmacol. 1995, 47, 441–444. [Google Scholar] [PubMed]
- Daniell, H.W. Hypogonadism in men consuming sustained-action oral opioids. J. Pain 2002, 3, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Asgari, S.A.; Farshi, A.; Ghaedi, G.; Kolahi, A.A.; Iravani, S.; Khoshdel, A.R. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod. Toxicol. 2013, 36, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bliesener, N.; Albrecht, S.; Schwager, A.; Weckbecker, K.; Lichtermann, D.; Klingmüller, D. Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J. Clin. Endocrinol. Metab. 2005, 90, 203–206. [Google Scholar] [CrossRef]
- Hallinan, R.; Byrne, A.; Agho, K.; McMahon, C.G.; Tynan, P.; Attia, J. Hypogonadism in men receiving methadone and buprenorphine maintenance treatment. Int. J. Androl. 2009, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.; Loh, H.S.; Danaee, M.; Riahi, S.; Ng, C.G.; Sulaiman, A.H. Plasma Testosterone and Sexual Function in Southeast Asian Men Receiving Methadone and Buprenorphine Maintenance Treatment. J. Sex. Med. 2018, 15, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bawor, M.; Bami, H.; Dennis, B.B.; Plater, C.; Worster, A.; Varenbut, M.; Daiter, J.; Marsh, D.C.; Steiner, M.; Anglin, R.; et al. Testosterone suppression in opioid users: A systematic review and meta-analysis. Drug Alcohol Depend. 2015, 149, 1–9. [Google Scholar] [CrossRef]
- Rubinstein, A.L.; Carpenter, D.M.; Minkoff, J.R. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin. J. Pain 2013, 29, 840–845. [Google Scholar] [CrossRef]
- Li, S.; Pelletier, G. Opioid regulation of gonadotropin-releasing hormone gene expression in the male rat brain as studied by in situ hybridization. Neuroreport 1993, 4, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.H.; Purohit, V.; Ahluwalia, B.S. Methadone blocks dopamine-mediated release of gonadotropins in rat hypothalamus. Neuroendocrinology 1982, 34, 347–352. [Google Scholar] [CrossRef]
- Adams, M.L.; Sewing, B.; Forman, J.B.; Meyer, E.R.; Cicero, T.J. Opioid-induced suppression of rat testicular function. J. Pharmacol. Exp. Ther. 1993, 266, 323–328. [Google Scholar] [PubMed]
- Abdellatief, R.B.; Elgamal, D.A.; Mohamed, E.E. Effects of chronic tramadol administration on testicular tissue in rats: An experimental study. Andrologia 2015, 47, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnia, H.; Akhavan Rezayat, A.; Hoseyni, M.; Sharifi, N.; Khajedalooee, M.; Akhavan Rezayat, A. Short-Period Influence of Chronic Morphine Exposure on Serum Levels of Sexual Hormones and Spermatogenesis in Rats. Nephro-Urol. Mon. 2016, 8, e38052. [Google Scholar] [CrossRef]
- Agirregoitia, E.; Valdivia, A.; Carracedo, A.; Casis, L.; Gil, J.; Subiran, N.; Ochoa, C.; Irazusta, J. Expression and localization of delta-, kappa-, and mu-opioid receptors in human spermatozoa and implications for sperm motility. J. Clin. Endocrinol. Metab. 2006, 91, 4969–4975. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Salah-Eldin, A.E. Chronic Addiction to Tramadol and Withdrawal Effect on the Spermatogenesis and Testicular Tissues in Adult Male Albino Rats. Pharmacology 2019, 103, 202–211. [Google Scholar] [CrossRef]
- Cicero, T.J.; Davis, L.A.; LaRegina, M.C.; Meyer, E.R.; Schlegel, M.S. Chronic opiate exposure in the male rat adversely affects fertility. Pharmacol. Biochem. Behav. 2002, 72, 157–163. [Google Scholar] [CrossRef]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Cannarella, R.; Duca, Y.; Calogero, A.E. Sport, doping and female fertility. Reprod. Biol. Endocrinol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Coward, R.M.; Rajanahally, S.; Kovac, J.R.; Smith, R.P.; Pastuszak, A.W.; Lipshultz, L.I. Anabolic steroid induced hypogonadism in young men. J. Urol. 2013, 190, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Christou, M.A.; Christou, P.A.; Markozannes, G.; Tsatsoulis, A.; Mastorakos, G.; Tigas, S. Effects of Anabolic Androgenic Steroids on the Reproductive System of Athletes and Recreational Users: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Hudson, J.I.; Pope, H.G., Jr. Illicit anabolic-androgenic steroid use. Horm. Behav. 2010, 58, 111–121. [Google Scholar] [CrossRef]
- Gårevik, N.; Strahm, E.; Garle, M.; Lundmark, J.; Ståhle, L.; Ekström, L.; Rane, A. Long term perturbation of endocrine parameters and cholesterol metabolism after discontinued abuse of anabolic androgenic steroids. J. Steroid Biochem. Mol. Biol. 2011, 127, 295–300. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Selmer, C.; Østergren, P.B.; Pedersen, K.B.; Schou, M.; Gustafsson, F.; Faber, J.; Juul, A.; Kistorp, C. Former Abusers of Anabolic Androgenic Steroids Exhibit Decreased Testosterone Levels and Hypogonadal Symptoms Years after Cessation: A Case-Control Study. PLoS ONE 2016, 11, e0161208. [Google Scholar] [CrossRef]
- Feinberg, M.J.; Lumia, A.R.; McGinnis, M.Y. The effect of anabolic-androgenic steroids on sexual behavior and reproductive tissues in male rats. Physiol. Behav. 1997, 62, 23–30. [Google Scholar] [CrossRef]
- De Souza, G.L.; Hallak, J. Anabolic steroids and male infertility: A comprehensive review. BJU Int. 2011, 108, 1860–1865. [Google Scholar] [CrossRef]
- Shokri, S.; Aitken, R.J.; Abdolvahhabi, M.; Abolhasani, F.; Ghasemi, F.M.; Kashani, I.; Ejtemaeimehr, S.; Ahmadian, S.; Minaei, B.; Naraghi, M.A.; et al. Exercise and supraphysiological dose of nandrolone decanoate increase apoptosis in spermatogenic cells. Basic Clin. Pharmacol. Toxicol. 2010, 106, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Knuth, U.A.; Maniera, H.; Nieschlag, E. Anabolic steroids and semen parameters in bodybuilders. Fertil. Steril. 1989, 52, 1041–1047. [Google Scholar] [CrossRef]
- Torres-Calleja, J.; González-Unzaga, M.; DeCelis-Carrillo, R.; Calzada-Sánchez, L.; Pedrón, N. Effect of androgenic anabolic steroids on sperm quality and serum hormone levels in adult male bodybuilders. Life Sci. 2001, 68, 1769–1774. [Google Scholar] [CrossRef]
- Liu, P.Y.; Swerdloff, R.S.; Christenson, P.D.; Handelsman, D.J.; Wang, C.; Hormonal Male Contraception Summit Group. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: An integrated analysis. Lancet 2006, 367, 1412–1420. [Google Scholar] [CrossRef]
- Nieschlag, E.; Vorona, E. MECHANISMS IN ENDOCRINOLOGY: Medical consequences of doping with anabolic androgenic steroids: Effects on reproductive functions. Eur. J. Endocrinol. 2015, 173, R47–R58. [Google Scholar] [CrossRef] [PubMed]
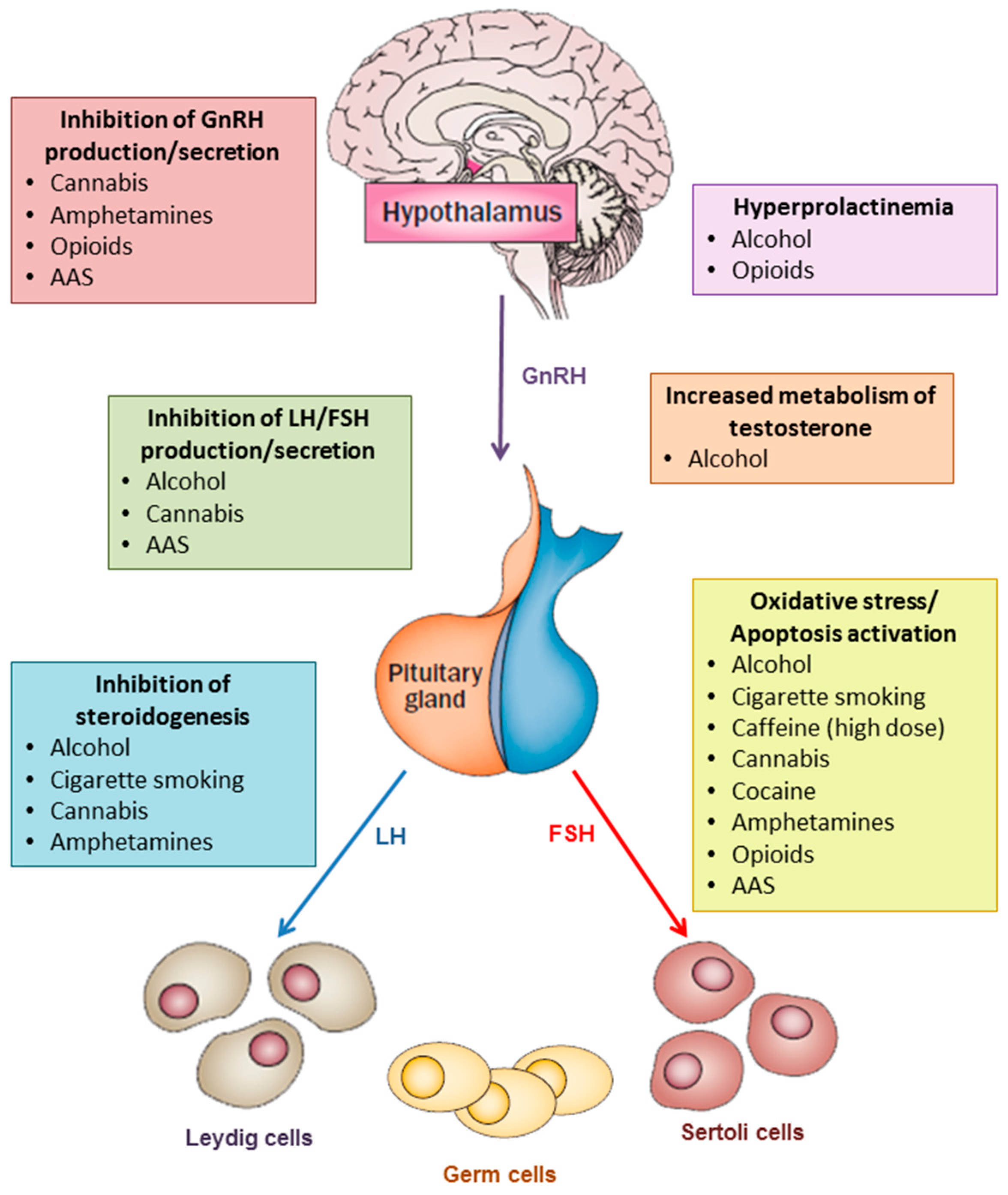
| Substance | Effect on Testosterone | Hypothesized Mechanisms | Effect on Sperm Concentration | Hypothesized Mechanisms |
|---|---|---|---|---|
| Alcohol | ↓ | Suppression of β-LH gene expression [14] Prolactin increase after acute ingestion [9] Inhibition of 3β-hydroxysteroid dehydrogenase and 17-ketosteroid reductase [9] Suppressed expression of StAR via ROS [10] Induction of the enzyme aromatase [11] | ↓ | Induction of apoptosis [10] Pro-oxidant effect [29] |
| Cigarette smoking | ↑ | Enhanced GnRH or LH release [38,41] Inhibition of prolactin release [38] Competitive inhibition of testosterone glucuronidation [43] | ↓ | Induction of apoptosis [52,53] Pro-oxidant effect [56,57,58] |
| Caffeine | ↑ | Induction of a stress-like hormonal pattern [64] | ↔ | Pro-oxidant effect at very high doses [69] |
| Cannabis | ↔ | Inhibition of GnRH and LH in animal models [81,83] Reduced expression of LH receptor on testis in animal models [86] Reduced activity of testicular 3β-hydroxysteroid dehydrogenase in animal models [86] | ↓ | Induction of apoptosis [71] |
| Cocaine | ↔ | Panhypopituitarism for pituitary infarction or inflammation (case reports) [96,97] | ↓ | Testicular vasoconstriction and ischemia [99] Induction of apoptosis [102] Pro-oxidant effect (reperfusion injury) [103] |
| Amphetamines | ↓ | Decreased expression of GnRH mRNA [112] Activation of adenylate cyclase [106] Inhibition of 3b-hydroxysteroid dehydrogenase, P450c17, and 17-ketosteroid reductase [107] Reduced Ca2+ influx [107] Increased testicular GABA concentration [111] | ↓ | Induction of apoptosis [114,115,116] Pro-oxidant effect [110] Testicular thermic damage [119] Increased testicular serotonin concentration [115] Increased testicular GABA concentration [111] Reduced testicular expression of progesterone and estrogen receptors [116] |
| Opioids | ↓ | Inhibition of GnRH secretion [120,134] Hyperprolactinemia [120] | ↓ | Induction of apoptosis [128,140] Pro-oxidant effect [128,129,130,131,132,133,134,135,136,137,138,139,140] |
| Anabolic-androgenic steroids (AAS) | ↓ | Inhibition of GnRH secretion [143] Inhibition of LH and FSH secretion [145] Depletion of Leydig cells [149] | ↓ | Reduction of intra-testicular testosterone levels [150,151] Induction of apoptosis [151] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duca, Y.; Aversa, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Substance Abuse and Male Hypogonadism. J. Clin. Med. 2019, 8, 732. https://doi.org/10.3390/jcm8050732
Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. Substance Abuse and Male Hypogonadism. Journal of Clinical Medicine. 2019; 8(5):732. https://doi.org/10.3390/jcm8050732
Chicago/Turabian StyleDuca, Ylenia, Antonio Aversa, Rosita Angela Condorelli, Aldo Eugenio Calogero, and Sandro La Vignera. 2019. "Substance Abuse and Male Hypogonadism" Journal of Clinical Medicine 8, no. 5: 732. https://doi.org/10.3390/jcm8050732
APA StyleDuca, Y., Aversa, A., Condorelli, R. A., Calogero, A. E., & La Vignera, S. (2019). Substance Abuse and Male Hypogonadism. Journal of Clinical Medicine, 8(5), 732. https://doi.org/10.3390/jcm8050732








