Pretransplant Cardiac Evaluation Using Novel Technology
Abstract
1. Introduction
2. Current State of Cardiovascular Screening for Cardiac Structure and Function Prior to HSCT
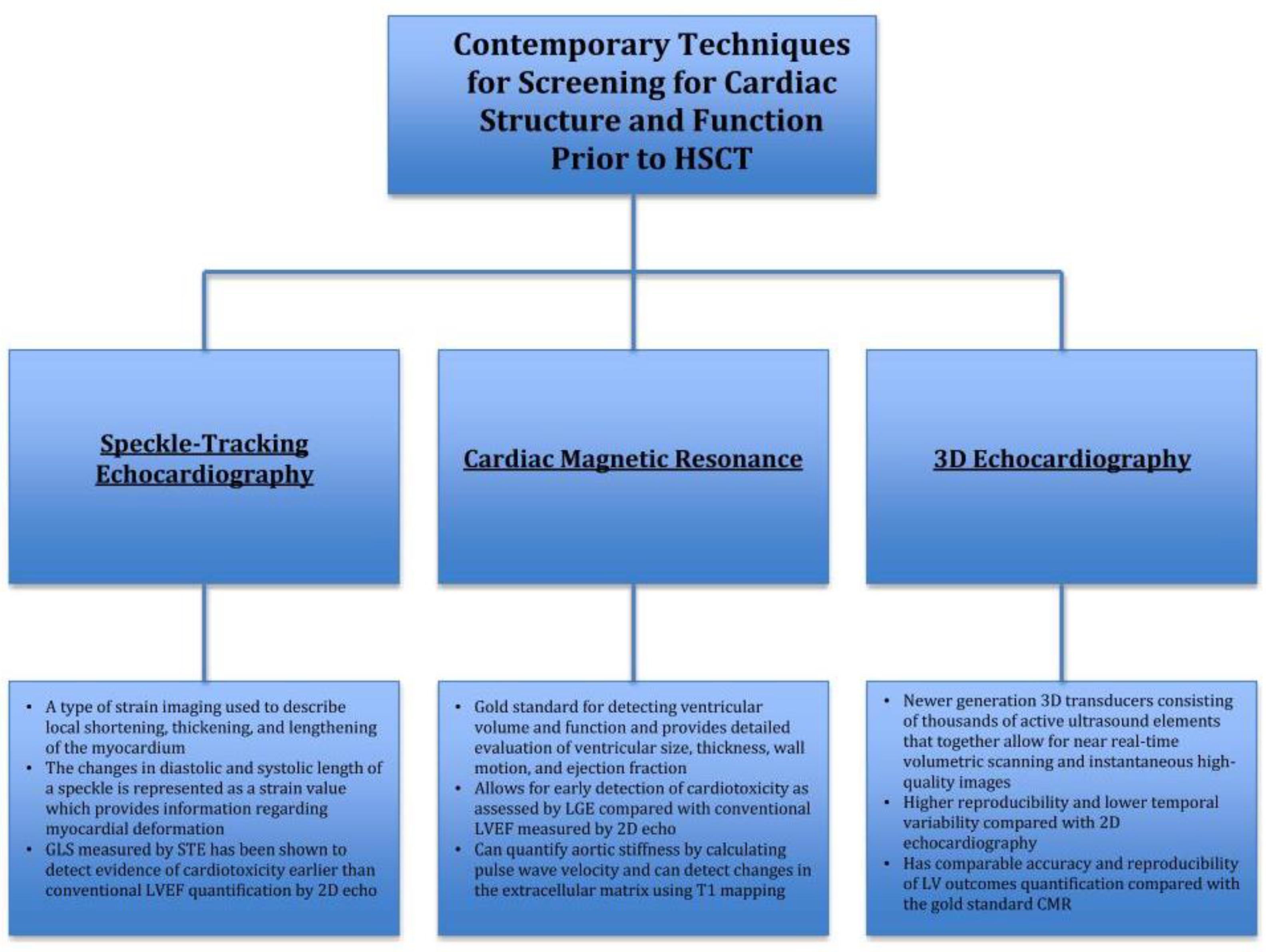
3. Contemporary Techniques for Screening for Cardiac Structure and Function Prior to HSCT
3.1. Speckle-Tracking Echocardiography
3.2. Cardiac Magnetic Resonance (CMR)
3.3. D Echocardiography (3D Echo)
4. Current State of Screening for Cardiac Ischemia Prior to HSCT
4.1. Stress Echocardiography
4.2. Single-Photon Emission Computed Tomography (SPECT)
5. Contemporary Techniques for Screening for Cardiac Ischemia Prior to HSCT using Cardiac Computer Tomography (CCT)
6. Coronary Calcium Scoring (CCS)
Coronary Computed Tomography Angiogram (CCTA)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blaes, A.; Konety, S.; Hurley, P. Cardiovascular complications of hematopoietic stem cell transplantation. Curr. Treat. Opt. Cardiovasc. Med. 2016, 18, 25. [Google Scholar] [CrossRef]
- Tonorezos, E.J.; Stillwell, E.E.; Calloway, J.J.; Glew, T.; Wessler, J.D.; Rebolledo, B.J.; Pham, A.; Steingart, R.M.; Lazarus, H.; Gale, R.P.; et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transpl. 2015, 50, 1212–1216. [Google Scholar] [CrossRef]
- Sureddi, R.K.; Amani, F.; Hebbar, P.; Williams, D.K.; Leonardi, M.; Paydak, H.; Mheta, J.L. Atrial fibrillation following autologous stem cell transplantation in patients with multiple myeloma: Incidence and risk factors. Ther. Adv. Cardiovasc. Dis. 2012, 6, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Murdych, T.; Weisdorf, D. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transpl. 2001, 28, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Antin, J.H.; Guinan, E.C.; Rappeport, J.M. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood 1986, 68, 1114–1118. [Google Scholar]
- Steinherz, L.J.; Steinherz, P.G.; Mangiacasale, D.; O’Reilly, R.; Allen, J.; Sorell, M.; Miller, D.R. Cardiac changes with cyclophosphamide. Med. Pediatr. Oncol. 1981, 9, 417–422. [Google Scholar] [CrossRef]
- Armenian, S.H.; Chow, E.J. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer 2014, 120, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Tichelli, A.; Rovó, A.; Passweg, J.; Schwarze, C.P.; Van Lint, M.T.; Arat, M.; Socie, G. Late complications after hematopoietic stem cell transplantation. Expert Rev. Hematol. 2009, 2, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-L.; Francisco, L.; Kawashima, T.; Leisenring, W.; Robison, L.L.; Baker, K.S.; Weisdorf, D.J.; Forman, S.J.; Bhatia, S. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: A report from the bone marrow transplant survivor study. Blood 2010, 116, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.D.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann. Intern. Med. 2011, 155, 21. [Google Scholar] [CrossRef]
- Scott, J.M.; Armenian, S.; Giralt, S.; Moslehi, J.; Wang, T.; Jones, L.W. Cardiovascular disease following hematopoietic stem cell transplantation: Pathogenesis, detection, and the cardioprotective role of aerobic training. Crit. Rev. Oncol. Hematol. 2016, 98, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Scott Baker, K.; Ness, K.K.; Steinberger, J.; Carter, A.; Francisco, L.; Burns, L.J.; Sklar, C.; Forman, S.; Weisdorf, D.; Gurney, J.G.; et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood 2007, 109, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Tichelli, A.; Bucher, C.; Rovó, A.; Stussi, G.; Stern, M.; Paulussen, M.; Halter, J.; Meyer-Monard, S.; Heim, D.; Tsakiris, D.A.; et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood 2007, 110, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Khayata, M.; Al-Kindi, S.; Njoroge, L.; De Lima, M.J.G.; Oliveira, G.H. Preexisting cardiovascular disease in patients undergoing hematopoietic stem cell transplantation. J. Clin. Oncol. 2018, 36, e19501. [Google Scholar] [CrossRef]
- Tichelli, A.; Bhatia, S.; Socié, G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br. J. Haematol. 2008, 142, 11–26. [Google Scholar] [CrossRef]
- Rhodes, M.; Lautz, T.; Kavanaugh-Mchugh, A.; Manes, B.; Calder, C.; Koyama, T.; Liske, M.; Parra, D.; Frangoul, H. Pericardial effusion and cardiac tamponade in pediatric stem cell transplant recipients. Bone Marrow Transpl. 2005, 36, 139–144. [Google Scholar] [CrossRef]
- Angelucci, E.; Mariotti, E.; Lucarelli, G.; Baronciani, D.; Cesaroni, P.; Durazzi, S.M.; Galimberti, M.; Giardini, C.; Muertto, P.; Polchi, P. Sudden cardiac tamponade after chemotherapy for marrow transplantation in thalassaemia. Lancet 1992, 339, 287–289. [Google Scholar] [CrossRef]
- Okamoto, S. Current indication for hematopoietic cell transplantation in adults. Hematol. Oncol. Stem Cell Ther. 2017, 10, 178–183. [Google Scholar] [CrossRef]
- Qazilbash, M.H.; Amjad, A.I.; Qureshi, S.; Qureshi, S.R.; Saliba, R.M.; Khan, Z.U.; Hosing, C.; Giralt, SA.; De Lima, M.J.; Popat, U.R.; et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients with low left ventricular ejection fraction. Biol. Blood Marrow Transpl. 2009, 15, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.H.; Popp, R.L.; Weyman, A.E. Assessment of left ventricular function by echocardiography: A technique in evolution. J. Am. Soc. Echocardiogr. 2008, 21, 14–21. [Google Scholar] [CrossRef]
- Foley, T.A.; Mankad, S.V.; Anavekar, N.S.; Bonnichsen, C.R.; Morris, M.F.; Miller, T.D.; Araoz, P.A. Measuring left ventricular ejection fraction—Techniques and potential pitfalls. Eur. Cardiol. Rev. 2012, 8, 108. [Google Scholar] [CrossRef]
- Lane, C.; Dorian, P.; Ghosh, N.; Radina, M.; O’Donnell, S.; Thorpe, K.; Mangat, I.; Korley, V.; Pinter, A. Limitations in the current screening practice of assessing left ventricular ejection fraction for a primary prophylactic implantable defibrillator in southern Ontario. Can. J. Cardiol. 2010, 26, e118–e124. [Google Scholar] [CrossRef]
- Khouri, M.G.; Douglas, P.S.; Mackey, J.R.; Martin, M.; Scott, J.M.; Scherrer-Crosbie, M.; Jones, L.W. Cancer therapy—Induced cardiac toxicity in early breast cancer. Circulation 2012, 126, 2749–2763. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Hunt, S.A.; Carlson, R.W.; Guardino, A.E. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J. Clin. Oncol. 2007, 25, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Venkateshvaran, A.; Alenezi, F. Speckle tracking strain echocardiography: What sonographers need to know! J. Indian Acad. Echocardiogr. Cardiovasc. Imaging 2017, 1, 133. [Google Scholar]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Carlos Plana, J.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging from the Cleveland Clinic 2014. Available online: http://dx.doi.org/10.1016/j.echo.2014.07.012 (accessed on 22 April 2019).
- Baratta, S.; Damiano, M.A.; Marchese, M.L.; Trucco, J.I.; Rizzo, M.M.; Bernok, F.; Cheitman, D.; Olano, D.; Rojas, M.; Hita, A. Serum markers, conventional doppler echocardiography and two-dimensional systolic strain in the diagnosis of chemotherapy-induced myocardial toxicity. Argent. J. Cardiol. 2013, 81, 133–138. [Google Scholar]
- Thavendiranathan, P.; Poulin, F.; Lim, K.-D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef]
- Sengeløv, M.; Jørgensen, P.G.; Skov Jensen, J.; Bruun, N.E.; Olsen, F.J.; Fritz-Hansen, T.; Nochioka, K.; Biering-Sorensen, T. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure with Reduced Ejection Fraction 2015. Available online: http://imaging.onlinejacc.org/content/jimg/8/12/1351.full.pdf (accessed on 23 February 2019).
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Kim, H.J.; Lee, E.-J.; Moon, S.; Lee, J.Y.; Lee, J.W.; Chung, N.G.; Cho, B.; Kim, H.K. Early left ventricular dysfunction in children after hematopoietic stem cell transplantation for acute leukemia: a case control study using speckle tracking echocardiography. Korean Circ. J. 2015, 45, 51–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Risum, N.; Ali, S.; Olsen, N.T.; Jons, C.; Khouri, M.G.; Lauridsen, T.K.; Samad, Z.; Velazquez, E.J.; Sogaard, P.; Kisslo, J. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J. Am. Soc. Echocardiogr. 2012, 25, 1195–1203. [Google Scholar] [CrossRef]
- Sandner, T.A.; Houck, P.; Runge, V.M.; Sincleair, S.; Huber, A.M.; Theisen, D.; Reiser, M.F.; Wintersperger, B.J. Accuracy of accelerated cine MR imaging at 3 Tesla in longitudinal follow-up of cardiac function. Eur. Radiol. 2008, 18, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Wassmuth, R.; Lentzsch, S.; Erdbruegger, U.; Schulz-Menger, J.; Doerken, B.; Dietz, R.; Friedrich, M.G. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging—A pilot study. Am. Heart J. 2001, 141, 1007–1113. [Google Scholar] [CrossRef] [PubMed]
- Ylänen, K.; Poutanen, T.; Savikurki-Heikkilä, P.; Rinta-Kiikka, I.; Eerola, A.; Vettenranta, K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J. Am. Coll. Cardiol. 2013, 61, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Najjar, S.S.; Boudreau, R.M.; Venkitachalam, L.; Kupelian, V.; Simonsick, E.M.; Havlik, R.; Lakatta, E.G.; Spurgeon, H.; Kritchevsky, S.; et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005, 111, 3384–3390. [Google Scholar] [CrossRef]
- Chaosuwannakit, N.; D’Agostino, R.; Hamilton, C.A.; Lane, K.S.; Ntim, W.O.; Lawrence, J.; Melin, S.A.; Ellis, L.R.; Torti, F.M.; Little, W.C.; et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J. Clin. Oncol. 2010, 28, 166–172. [Google Scholar] [CrossRef]
- Drafts, B.C.; Twomley, K.M.; D’Agostino, R.; Lawrence, J.; Avis, N.; Ellis, L.R.; Thohan, V.; Jordan, J.; Melin, S.A.; Torti, F.M.; et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc. Imaging 2013, 6, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Fonarow, G.C.; Bonow, R.O.; Butler, J.; Gheorghiade, M. Therapeutic targets in heart failure. J. Am. Coll. Cardiol. 2014, 63, 2188–2198. [Google Scholar] [CrossRef]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef]
- Jordan, J.H.; Vasu, S.; Morgan, T.M.; D’Agostino, R.B.; Meléndez, G.C.; Hamilton, C.A.; Arai, A.E.; Liu, S.; Liu, C.Y.; Lima, J.A.; et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ. Cardiovasc. Imaging 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Dekker, D.L.; Piziali, R.L.; Dong, E. A system for ultrasonically imaging the human heart in three dimensions. Comput. Biomed. Res. 1974, 7, 544–553. [Google Scholar] [CrossRef]
- Gopal, A.S.; Keller, A.M.; Rigling, R.; King, D.L.; King, D.L. Left ventricular volume and endocardial surface area by three-dimensional echocardiography: Comparison with two-dimensional echocardiography and nuclear magnetic resonance imaging in normal subjects. J. Am. Coll. Cardiol. 1993, 22, 258–270. [Google Scholar] [CrossRef]
- Wang, X.-F.; Deng, Y.-B.; Nanda, N.C.; Deng, J.; Miller, A.P.; Xie, M.-X. Live three-dimensional echocardiography: imaging principles and clinical application. Echocardiography 2003, 20, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Bricknell, K.; Chan, J.; Hanekom, L.; Marwick, T.H. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am. J. Cardiol. 2007, 99, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Caiani, E.G.; Corsi, C.; Sugeng, L.; MacEneaney, P.; Weinert, L.; Mor-Avi, V.; Lang, R.M. Improved quantification of left ventricular mass based on endocardial and epicardial surface detection with real time three dimensional echocardiography. Heart 2006, 92, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hare, J.L.; Jenkins, C.; Nakatani, S.; Ogawa, A.; Yu, C.-M.; Marwick, T.H. Feasibility and clinical decision-making with 3D echocardiography in routine practice. Heart 2007, 94, 440–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thavendiranathan, P.; Grant, A.D.; Negishi, T.; Plana, J.C.; Popović, Z.B.; Marwick, T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes. J. Am. Coll. Cardiol. 2013, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Rischpler, C. Cardiovascular imaging in cardio-oncology. J. Thorac. Dis. 2018, 10, S4351–S4366. [Google Scholar] [CrossRef] [PubMed]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.; Hines, H.H.; Grant, G.; Taylor, K.; Ryals, C. Automated quantification of myocardial ischemia and wall motion defects by use of cardiac SPECT polar mapping and 4-dimensional surface rendering. J. Nucl. Med. Technol. 2006, 34, 3–17. [Google Scholar]
- Gayed, I.W.; Liu, H.H.; Yusuf, S.W.; Komaki, R.; Wei, X.; Wang, X.; Chang, J.Y.; Swafford, J.; Broemeling, L.; Liao, Z. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J. Nucl. Med. 2006, 47, 1756–1762. [Google Scholar]
- Imbert, L.; Marie, P.-Y. CZT cameras: A technological jump for myocardial perfusion SPECT. J. Nucl. Cardiol. 2016, 23, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr. Cardiol. Rep. 2010, 12, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Kizilbash, M.A. Coronary computed tomography in coronary risk assessment. J. Cardiopulm. Rehabil. 2005, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Elias-Smale, S.E.; Proença, R.V.; Koller, M.T.; Kavousi, M.; van Rooij, F.J.A.; Hunink, M.G.; Steyerberg, E.W.; Hofman, A.; Oudkerk, M.; Witteman, J.C. Coronary Calcium score improves classification of coronary heart disease risk in the elderly. J. Am. Coll. Cardiol. 2010, 56, 1407–1414. [Google Scholar] [CrossRef]
- Erbel, R.; Möhlenkamp, S.; Moebus, S.; Schmermund, A.; Lehmann, N.; Stang, A.; Dragano, N.; Grönemeyer, D.; Seibel, R.; Kälsch, H.; et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis. J. Am. Coll. Cardiol. 2010, 56, 1397–1406. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef]
- Budoff, M.J.; Young, R.; Burke, G.; Jeffrey Carr, J.; Detrano, R.C.; Folsom, A.R.; Kronmal, R.; Lima, J.A.C.; Liu, K.J.; McClelland, R.L.; et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: The multi-ethnic study of atherosclerosis (MESA). Eur. Heart J. 2018, 39, 2401–2408. [Google Scholar] [CrossRef]
- Jain, N.A.; Chen, M.Y.; Shanbhag, S.; Lu, K.; Pophali, P.A.; Ito, S.; Koklanaris, E.; Hourigan, C.S.; Barrett, J.A.; Battiwalla, M. Contrast enhanced cardiac CT reveals coronary artery disease in 45% of asymptomatic allo-SCT long-term survivors. Bone Marrow Transpl. 2014, 49, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Andersen, R.; Wethal, T.; Günther, A.; Fosså, A.; Edvardsen, T.; Fosså, S.D.; Kjekshus, J. Relation of coronary artery calcium score to premature coronary artery disease in survivors> 15 years of Hodgkin’s lymphoma. Am. J. Cardiol. 2010, 105, 149–152. [Google Scholar] [CrossRef]
- Iwasaki, K.; Matsumoto, T. Relationship between coronary calcium score and high-risk plaque/significant stenosis. World J. Cardiol. 2016, 8, 481–487. [Google Scholar] [CrossRef]
- Budoff, M.J.; Nasir, K.; McClelland, R.L.; Detrano, R.; Wong, N.; Blumenthal, R.S.; Kondos, G.; Kronmal, R.A. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2009, 53, 345–352. [Google Scholar] [CrossRef]
- Yeboah, J.; Delaney, J.A.; Nance, R.; McClelland, R.L.; Polak, J.F.; Sibley, C.T.; Bertoni, A.; Burke, G.L.; Carr, J.J.; Herrington, D.M. Mediation of cardiovascular risk factor effects through subclinical vascular disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.A.; Simons, D.B.; Fitzpatrick, L.A.; Sheedy, P.F.; Schwartz, R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. Circulation 1995, 92, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.; Rader, D.J.; Rubin, G.D.; et al. Assessment of coronary artery disease by cardiac computed tomography. Circulation 2006, 114, 1761–1791. [Google Scholar] [CrossRef]
- Carità, P.; Guaricci, A.I.; Muscogiuri, G.; Carrabba, N.; Pontone, G. Prognostic value and therapeutic perspectives of coronary CT angiography: A literature review. BioMed Res. Int. 2018, 2018, 6528238. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary plaque disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Flores, F.; French, W.J.; Mao, S.S.; Shavelle, D.; Ebrahimi, R.; Budoff, M. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am. J. Cardiol. 2011, 107, 10–16. [Google Scholar] [CrossRef]
- Patel, M.R.; Dai, D.; Hernandez, A.F.; Douglas, P.S.; Messenger, J.; Garratt, K.N.; Maddox, T.M.; Peterson, E.D.; Roe, M.T. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am. Heart J. 2014, 167, 846–852. [Google Scholar] [CrossRef]
- Rademaker, J.; Schöder, H.; Ariaratnam, N.S.; Strauss, H.W.; Yahalom, J.; Steingart, R.; Oeffinger, K.C. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: Coronary CT angiography findings and calcium scores in nine asymptomatic patients. Am. J. Roentgenol. 2008, 191, 32–37. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemu, M.; Zimmerman, A.; Kalra, D.; Okwuosa, T. Pretransplant Cardiac Evaluation Using Novel Technology. J. Clin. Med. 2019, 8, 690. https://doi.org/10.3390/jcm8050690
Hemu M, Zimmerman A, Kalra D, Okwuosa T. Pretransplant Cardiac Evaluation Using Novel Technology. Journal of Clinical Medicine. 2019; 8(5):690. https://doi.org/10.3390/jcm8050690
Chicago/Turabian StyleHemu, Mohamad, Allison Zimmerman, Dinesh Kalra, and Tochukwu Okwuosa. 2019. "Pretransplant Cardiac Evaluation Using Novel Technology" Journal of Clinical Medicine 8, no. 5: 690. https://doi.org/10.3390/jcm8050690
APA StyleHemu, M., Zimmerman, A., Kalra, D., & Okwuosa, T. (2019). Pretransplant Cardiac Evaluation Using Novel Technology. Journal of Clinical Medicine, 8(5), 690. https://doi.org/10.3390/jcm8050690




