Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Tissue Samples
2.3. Phosphoprotein Isolation, Pro-Q Diamond and 2-DE
2.4. Quantification of Spot Levels
2.5. Trypsin Digestion and MS Analysis
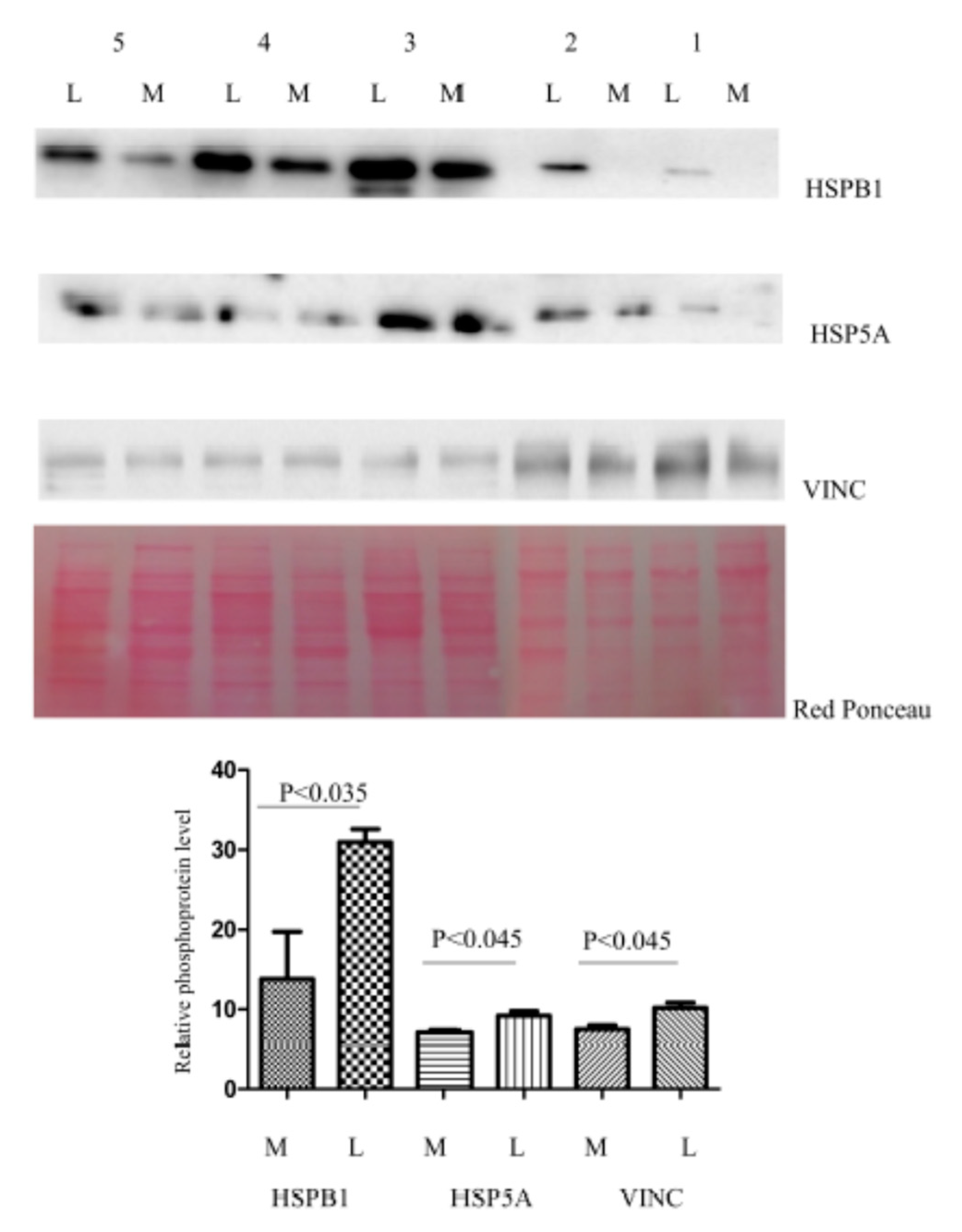
2.6. Western Blotting
2.7. Ingenuity Pathway (IPA) and PANTHER Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Differentially Phosphorylated Proteins with Pro-Q Diamond Gel Stain
3.2. Enrichment of Immobilized Metal Affinity Chromatography (IMAC)
3.3. Functional Analysis of the Leiomyoma Phosphoproteome
3.4. Immunohistochemical Study of Altered Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kim, J.J.; Sefton, E.C. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol. Cell. Endocrinol. 2012, 358, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhu, X.; Dong, K.; Lin, Y.; Hu, Y.; Zhu, C. Reduced expression of 14-3-3 γ in uterine leiomyoma as identified by proteomics. Fertil. Steril. 2008, 90, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, J.S.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.J.; et al. Ovarian steroids, stem cells and uterine leiomyoma: Therapeutic implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef]
- Ura, B.; Di Lorenzo, G.; Romano, F.; Monasta, L.; Mirenda, G.; Scrimin, F.; Ricci, G. Interstitial Fluid in Gynecologic Tumors and Its Possible Application in the Clinical Practice. Int. J. Mol. Sci. 2018, 19, 4018. [Google Scholar] [CrossRef]
- Chen, I.H.; Xue, L.; Hsu, C.C.; Paez, J.S.; Pan, L.; Andaluz, H.; Wendt, M.K.; Iliuk, A.B.; Zhu, J.K.; Tao, W.A. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Fuhs, S.R.; Meisenhelder, J.; Aslanian, A.; Ma, L.; Zagorska, A.; Stankova, M.; Binnie, A.; Al-Obeidi, F.; Mauger, J.; Lemke, G.; et al. Monoclonal 1- and 3-Phosphohistidine Antibodies: New Tools to Study Histidine Phosphorylation. Cell 2015, 162, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Iliuk, A.B.; Arrington, J.V.; Tao, W.A. Analytical challenges translating mass spectrometry-based phosphoproteomics from discovery to clinical applications. Electrophoresis 2014, 35, 3430–3440. [Google Scholar] [CrossRef]
- Hermon, T.L.; Moore, A.B.; Yu, L.; Kissling, G.E.; Castora, F.J.; Dixon, D. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008, 453, 557–569. [Google Scholar] [CrossRef][Green Version]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef]
- Yu, L.; Moore, A.B.; Dixon, D. Receptor tyrosine kinases and their hormonal regulation in uterine leiomyoma. Semin. Reprod. Med. 2010, 28, 250–259. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Chegini, N.; Luo, X.; Ding, L.; Ripley, D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol. Cell. Endocrinol. 2003, 209, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Karra, L.; Shushan, A.; Ben-Meir, A.; Rojansky, N.; Klein, B.Y.; Shveiky, D.; Levitzki, R.; Ben-Bassat, H. Changes related to phosphatidylinositol 3-kinase/Akt signaling in leiomyomas: Possible involvement of glycogen synthase kinase 3alpha and cyclin D2 in the pathophysiology. Fertil. Steril. 2010, 93, 2646–2651. [Google Scholar] [CrossRef]
- Nierth-Simpson, E.N.; Martin, M.M.; Chiang, T.C.; Melnik, L.I.; Rhodes, L.V.; Muir, S.E.; Burow, M.E.; McLachlan, J.A. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: Implications for proliferation. Endocrinology 2009, 150, 2436–2445. [Google Scholar] [CrossRef]
- Yu, L.; Saile, K.; Swartz, C.D.; He, H.; Zheng, X.; Kissling, G.E.; Di, X.; Lucas, S.; Robboy, S.J.; Dixon, D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol. Med. 2008, 14, 264–275. [Google Scholar] [CrossRef]
- Ura, B.; Scrimin, F.; Arrigoni, G.; Franchin, C.; Monasta, L.; Ricci, G. A Proteomic Approach for the Identification of Up-Regulated Proteins Involved in the Metabolic Process of the Leiomyoma. Int. J. Mol. Sci. 2016, 17, 540. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.F.B.; Nahar, P.; Tanwar, P.S. Proteomic Characterization of the Extracellular Matrix of Human Uterine Fibroids. Endocrinology 2018, 159, 2656–2669. [Google Scholar] [CrossRef]
- Ura, B.; Scrimin, F.; Arrigoni, G.; Athanasakis, E.; Aloisio, M.; Monasta, L.; Ricci, G. Abnormal expression of leiomyoma cytoskeletal proteins involved in cell migration. Oncol. Rep. 2016, 35, 3094–3100. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Li, Z.; Shen, Q.; Zhang, D. Comparative analysis of muscle phosphoproteome induced by salt curing. Meat. Sci. 2017, 133, 19–25. [Google Scholar] [CrossRef]
- Ura, B.; Monasta, L.; Arrigoni, G.; Franchin, C.; Radillo, O.; Peterlunger, I.; Ricci, G.; Scrimin, F. A proteomic approach for the identification of biomarkers in endometrial cancer uterine aspirate. Oncotarget 2017, 8, 109536–109545. [Google Scholar] [CrossRef]
- Khang, R.; Park, C.; Shin, J.H. The biguanide metformin alters phosphoproteomic profiling in mouse brain. Neurosci. Lett. 2014, 579, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, A.; Petillo, O.; Di Lieto, A.; Melone, M.A.; Margarucci, S.; Cannas, M.; Peluso, G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: Evidence for a stimulation of protein kinase-dependent pathway. J. Cell Physiol. 2001, 186, 414–424. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, D.; Sheng, J.; Luo, L.; Zhang, W. Identification of TRADD as a potential biomarker in human uterine leiomyoma through iTRAQ based proteomic profiling. Mol. Cell. Probes 2017, 36, 15–20. [Google Scholar] [CrossRef]
- Krammer, P.H. CD95’s deadly mission in the immune system. Nature 2000, 407, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Balomenos, D.; Shokri, R.; Daszkiewicz, L.; Vázquez-Mateo, C.; Martínez-A, C. On How Fas Apoptosis-Independent Pathways Drive T Cell Hyperproliferation and Lymphadenopathy in lpr Mice. Front. Immunol. 2017, 8, 237. [Google Scholar] [CrossRef]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Foster, C.R.; Przyborski, S.A.; Wilson, R.G.; Hutchison, C.J. Lamins as cancer biomarkers. Biochem. Soc. Trans. 2010, 38, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Cross, M.J.; Dixelius, J.; Matsumoto, T.; Claesson-Welsh, L. VEGF-receptor signal transduction. Trends Biochem. Sci. 2003, 28, 488–494. [Google Scholar] [CrossRef]
- Hassan, M.H.; Eyzaguirre, E.; Arafa, H.M.; Hamada, F.M.; Salama, S.A.; Al-Hendy, A. Memy I: A novel murine model for uterine leiomyoma using adenovirus-enhanced human fibroid explants in severe combined immune deficiency mice. Am. J. Obstet. Gynecol. 2008, 199, 156.e1. [Google Scholar] [CrossRef]
- Kostenko, S.; Johannessen, M.; Moens, U. PKA-induced F-actin rearrangement requires phosphorylation of Hsp27 by the MAPKAP kinase MK5. Cell Signal. 2009, 21, 712–718. [Google Scholar] [CrossRef]
- Shiota, M.; Bishop, J.L.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef]
- Ura, B.; Scrimin, F.; Arrigoni, G.; Aloisio, M.; Monasta, L.; Ricci, G. Dysregulated chaperones associated with cell proliferation and negative apoptosis regulation in the uterine leiomyoma. Oncol. Lett. 2018, 15, 8005–8010. [Google Scholar] [CrossRef]
- Paul, C.; Simon, S.; Gibert, B.; Virot, S.; Manero, F.; Arrigo, A.P. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27). Exp. Cell Res. 2010, 316, 1535–1552. [Google Scholar] [CrossRef]
- Evensen, N.A.; Kuscu, C.; Nguyen, H.L.; Zarrabi, K.; Dufour, A.; Kadam, P.; Hu, Y.J.; Pulkoski-Gross, A.; Bahou, W.F.; Zucker, S.; et al. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration. J. Natl. Cancer Inst. 2013, 105, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.T.; Watowich, S.S.; Lamb, R.A. Analysis in vivo of GRP78-BiP/substrate interactions and their role in induction of the GRP78-BiP gene. Mol. Biol. Cell 1992, 3, 143–155. [Google Scholar] [CrossRef]
- Dutta, A.; Girotra, M.; Merchant, N.; Nair, P.; Dutta, S.K. Evidence of multimeric forms of HSP70 with phosphorylation on serine and tyrosine residues—Implications for roles of HSP70 in detection of GI cancers. Asian Pac. J. Cancer Prev. 2013, 14, 5741–5745. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Wong, C.Y.; Ooi, L.L.; Druker, B.J.; Epstein, R.J. Selective tyrosine hyperphosphorylation of cytoskeletal and stress proteins in primary human breast cancers: Implications for adjuvant use of kinase-inhibitory drugs. Clin. Cancer Res. 2004, 10, 3980–3987. [Google Scholar] [CrossRef]
- Viitanen, P.V.; Lorimer, G.H.; Seetharam, R.; Gupta, R.S.; Oppenheim, J.; Thomas, J.O.; Cowan, N.J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J. Biol. Chem. 1992, 267, 695–698. [Google Scholar]
- Levy-Rimler, G.; Viitanen, P.; Weiss, C.; Sharkia, R.; Greenberg, A.; Niv, A.; Lustig, A.; Delarea, Y.; Azem, A. The effect of nucleotides and mitochondrial chaperonin 10 on the structure and chaperone activity of mitochondrial chaperonin 60. Eur. J. Biochem. 2001, 268, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.H.; Vong, Q.P.; Lin, W.; Bouck, D.; Wendt, S.; Sullivan, E.; Li, Y.; Bari, R.; Chen, T.; Leung, W. PRL-3 mediates the protein maturation of ULBP2 by regulating the tyrosine phosphorylation of HSP60. J. Immunol. 2015, 194, 2930–2941. [Google Scholar] [CrossRef]
- Wang, R.; Sun, P.D. Natural killer cell-mediated shedding of ULBP2. PLoS ONE 2014, 9, e91133. [Google Scholar] [CrossRef]
- Kang, S.W.; Chae, H.Z.; Seo, M.S.; Kim, K.; Baines, I.C.; Rhee, S.G. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 1998, 273, 6297–6302. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Fu, Z.; Wang, H.; Feng, J.; Wei, J.; Guo, J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells’ survival by protecting cells from oxidative stress. Mol. Cell Biochem. 2014, 387, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Rashidian, J.; Mount, M.P.; Aleyasin, H.; Parsanejad, M.; Lira, A.; Haque, E.; Zhang, Y.; Callaghan, S.; Daigle, M.; et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron 2007, 55, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Melling, C.W.; Thorp, D.B.; Milne, K.J.; Noble, E.G. Myocardial Hsp70 phosphorylation and PKC-mediated cardioprotection following exercise. Cell Stress Chaperones 2009, 14, 141–150. [Google Scholar] [CrossRef]
- Majumder, P.K.; Mishra, N.C.; Sun, X.; Bharti, A.; Kharbanda, S.; Saxena, S.; Kufe, D. Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Diff. 2001, 12, 465–470. [Google Scholar]
- Shushan, A.; Rojansky, N.; Laufer, N.; Klein, B.Y.; Shlomai, Z.; Levitzki, R.; Hartzstark, Z.; Ben-Bassat, H. The AG1478 tyrosine kinase inhibitor is an effective suppressor of leiomyoma cell growth. Hum. Reprod. 2004, 19, 1957–1967. [Google Scholar] [CrossRef][Green Version]
- Shushan, A.; Ben-Bassat, H.; Mishani, E.; Laufer, N.; Klein, B.Y.; Rojansky, N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil. Steril. 2007, 87, 127–135. [Google Scholar] [CrossRef]
- Lattuada, D.; Viganó, P.; Mangioni, S.; Sassone, J.; Di Francesco, S.; Vignali, M.; Di Blasio, A.M. Accumulation of retinoid X receptor-alpha in uterine leiomyomas is associated with a delayed ligand-dependent proteasome-mediated degradation and an alteration of its transcriptional activity. Mol. Endocrinol. 2007, 21, 602–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayusawa, D.; Kaneda, S.; Itoh, Y.; Yasuda, H.; Murakami, Y.; Sugasawa, K.; Hanaoka, F.; Seno, T. Complementation by a cloned human ubiquitin-activating enzyme E1 of the S-phase-arrested mouse FM3A cell mutant with thermolabile E1. Cell Struct. Funct. 1992, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Moudry, P.; Lukas, C.; Macurek, L.; Hanzlikova, H.; Hodny, Z.; Lukas, J.; Bartek, J. Ubiquitin-activating enzyme UBA1 is required for cellular response to DNA damage. Cell Cycle 2012, 11, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lukkarila, J.L.; da Silva, S.R.; Paiva, S.L.; Gunning, P.T.; Schimmer, A.D. Targeting the ubiquitin E1 as a novel anti-cancer strategy. Curr. Pharm. Des. 2013, 19, 3201–3209. [Google Scholar] [CrossRef]

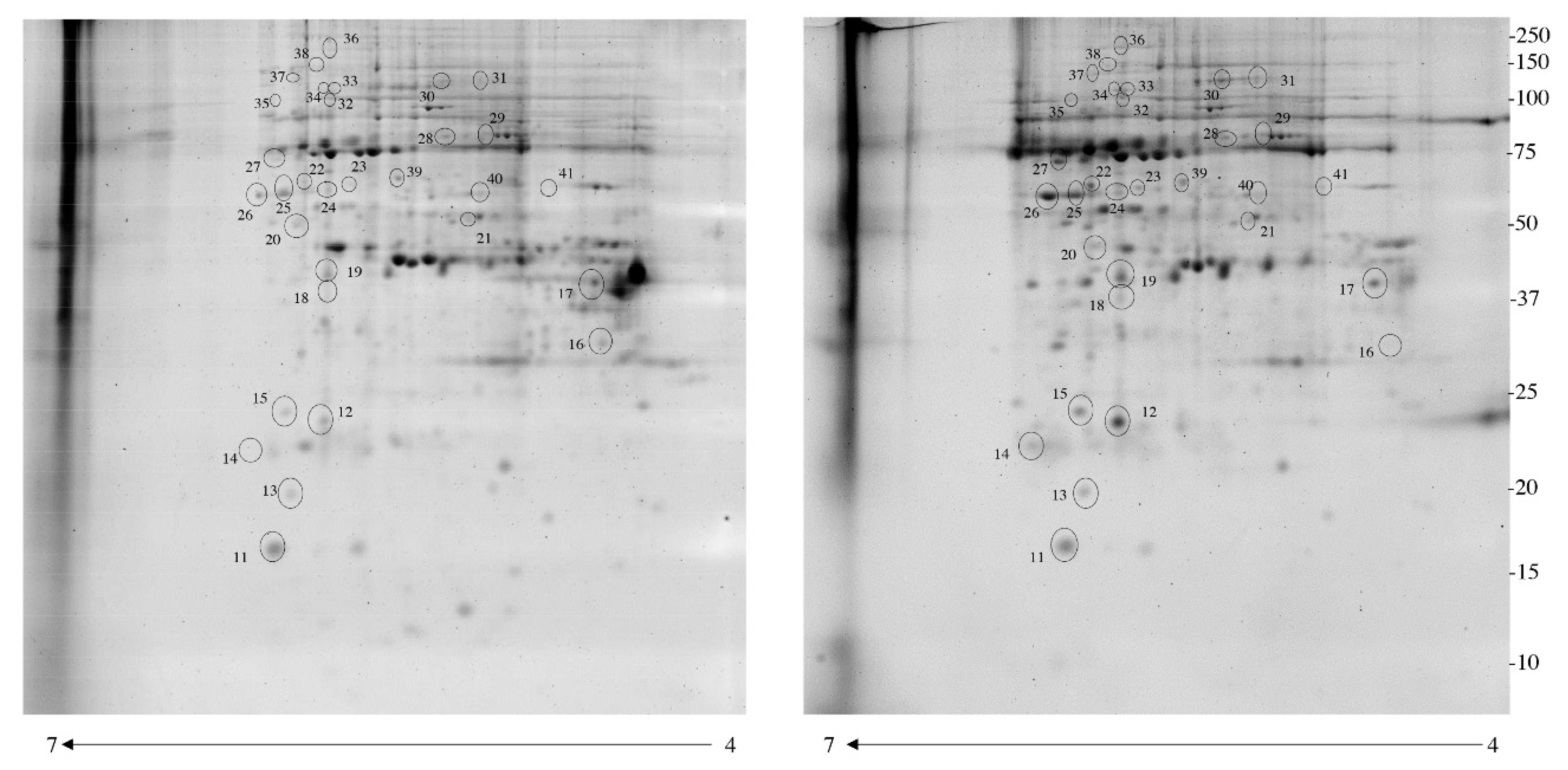
| Accession No | Spot No | Protein Description | Gene Symbol | Protein Score | Fold Change * | P-Value |
|---|---|---|---|---|---|---|
| P30101 | 22 | Protein disulfide-isomerase A3 | PDIA3 | 295 | 5.2 | 0.0277 |
| H9KV75 | 25 | Alpha-actinin-1 | ACTN1 | 93 | 4.9 | 0.0431 |
| P11021 | 29 | 78 kDa glucose-regulated protein | HSPA5 | 675 | 4.6 | 0.045 |
| P05387 | 7 | 60S acidic ribosomal protein P2 | RPLP2 | 121 | 4.5 | 0.0422 |
| H0YJW3 | 32 | Alpha-actinin-1 (Fragment) | ACTN1 | 141 | 3.8 | 0.0273 |
| Q5JRR6 | 34 | Ubiquitin-like modifier-activating enzyme 1 | UBA1 | 64 | 3.8 | 0.0277 |
| A0A087WU08 | 18 | Haptoglobin | HP | 159 | 3.4 | 0.0180 |
| A0A087WU08 | 19 | Haptoglobin | HP | 50.8 | 3.3 | 0.0180 |
| E9PFZ2 | 30 | Ceruloplasmin | CP | 154 | 3.2 | 0.0431 |
| H7BZ94 | 41 | Protein disulfide-isomerase | P4HB | 272 | 3.2 | 0.0431 |
| A0A0C4DGB6 | 26 | Serum albumin | ALB | 43 | 3 | 0.0220 |
| H7C3T4 | 15 | Peroxiredoxin-4 | PRDX4 | 159 | 2.85 | 0.0273 |
| Q5JRR6 | 33 | Ubiquitin-like modifier-activating enzyme 1 | UBA1 | 128 | 2.7 | 0.0273 |
| P02790 | 27 | Hemopexin | HPX | 69 | 2.56 | 0.0431 |
| Q3BDU5 | 20 | Prelamin-A/C | LMNA | 395 | 2.5 | 0.0277 |
| H7BZ94 | 40 | Protein disulfide-isomerase | P4HB | 72 | 2.3 | 0.0277 |
| P18206-2 | 38 | Isoform 1 of Vinculin | VINC | 198 | 2.25 | 0.0277 |
| E9PFZ2 | 31 | Ceruloplasmin | CP | 237 | 2.25 | 0.0431 |
| P30101 | 25 | Protein disulfide-isomerase A3 | PDIA3 | 808 | 2.23 | 0.0220 |
| P10809 | 39 | 60 kDa heat shock protein | HSPD1 | 234 | 2.2 | 0.0431 |
| P02649 | 6 | Apolipoprotein E | APOE | 230 | 2.15 | 0.0431 |
| P01023 | 37 | Alpha-2-macroglobulin | A2M | 52 | 2.14 | 0.0431 |
| P01023 | 12 | Peroxiredoxin-2 | PRDX2 | 64 | 2.1 | 0.0180 |
| P04792 | 14 | Heat shock protein beta-1 | HSPB1 | 151 | 2 | 0.0277 |
| P01024 | 17 | Complement C3 | CO3 | 171 | 2 | 0.0431 |
| E9PN50 | 21 | 26S protease regulatory subunit 6A (Fragment) | PSMC3 | 191 | 2 | 0.0425 |
| P30101 | 23 | Protein disulfide-isomerase A3 | PDIA3 | 79 | 2 | 0.0220 |
| B7ZAR1 | 24 | T-complex protein 1 subunit epsilon | CCT5 | 150 | 2 | 0.0277 |
| P28070 | 13 | Proteasome subunit beta type-4 | PSMB4 | 168 | 1.75 | 0.0431 |
| J3KRH2 | 11 | Haptoglobin (Fragment) | HP | 342 | 1.7 | 0.0178 |
| P08603 | 36 | Complement factor H | CFH | 69 | 1.7 | 0.0273 |
| Q13409-2 | 28 | Isoform 2B of Cytoplasmic dynein 1 intermediate chain 2 | DYNC1/2 | 85 | 1.51 | 0.0180 |
| Q6NZI2 | 4 | Caveolae-associated protein 1 | CAV1 | 75 | 0.65 | 0.0277 |
| Q6NZI2 | 2 | Caveolae-associated protein 1 | CAV1 | 161 | 0.43 | 0.03 |
| Q6NZI2 | 3 | Caveolae-associated protein 1 | CAV1 | 127 | 0.37 | 0.0180 |
| G3V5V7 | 5 | Heterogeneous nuclear ribonucleoproteins C1/C2 (Fragment) | HNRNPC | 69 | 0.29 | 0.0180 |
| Q6NZI2-3 | 8 | Isoform 3 of Polymerase I and transcript release factor | PTRF | 238 | 0.11 | 0.0422 |
| C9JF17 | 9 | Apolipoprotein D | APOD | 103 | 0.1 | 0.0422 |
| Q15746-10 | 10 | Isoform 8 of Myosin light chain kinase, smooth muscle | MYLK | 51 | 0.08 | 0.03 |
| P17661 | 16 | Desmin | DES | 787 | 0.06 | 0.0180 |
| Q6NZI2 | 1 | Caveolae-associated protein 1 | CAV1 | 63 | 0.03 | 0.0178 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ura, B.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Peterlunger, I.; Stabile, G.; et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. J. Clin. Med. 2019, 8, 691. https://doi.org/10.3390/jcm8050691
Ura B, Monasta L, Arrigoni G, Battisti I, Licastro D, Di Lorenzo G, Romano F, Aloisio M, Peterlunger I, Stabile G, et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. Journal of Clinical Medicine. 2019; 8(5):691. https://doi.org/10.3390/jcm8050691
Chicago/Turabian StyleUra, Blendi, Lorenzo Monasta, Giorgio Arrigoni, Ilaria Battisti, Danilo Licastro, Giovanni Di Lorenzo, Federico Romano, Michelangelo Aloisio, Isabel Peterlunger, Guglielmo Stabile, and et al. 2019. "Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma" Journal of Clinical Medicine 8, no. 5: 691. https://doi.org/10.3390/jcm8050691
APA StyleUra, B., Monasta, L., Arrigoni, G., Battisti, I., Licastro, D., Di Lorenzo, G., Romano, F., Aloisio, M., Peterlunger, I., Stabile, G., Scrimin, F., & Ricci, G. (2019). Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. Journal of Clinical Medicine, 8(5), 691. https://doi.org/10.3390/jcm8050691








