Newly Generated and Non-Newly Generated “Immature” Neurons in the Mammalian Brain: A Possible Reservoir of Young Cells to Prevent Brain Aging and Disease?
Abstract
1. Introduction
1.1. Stability and Plasticity in the Nervous System
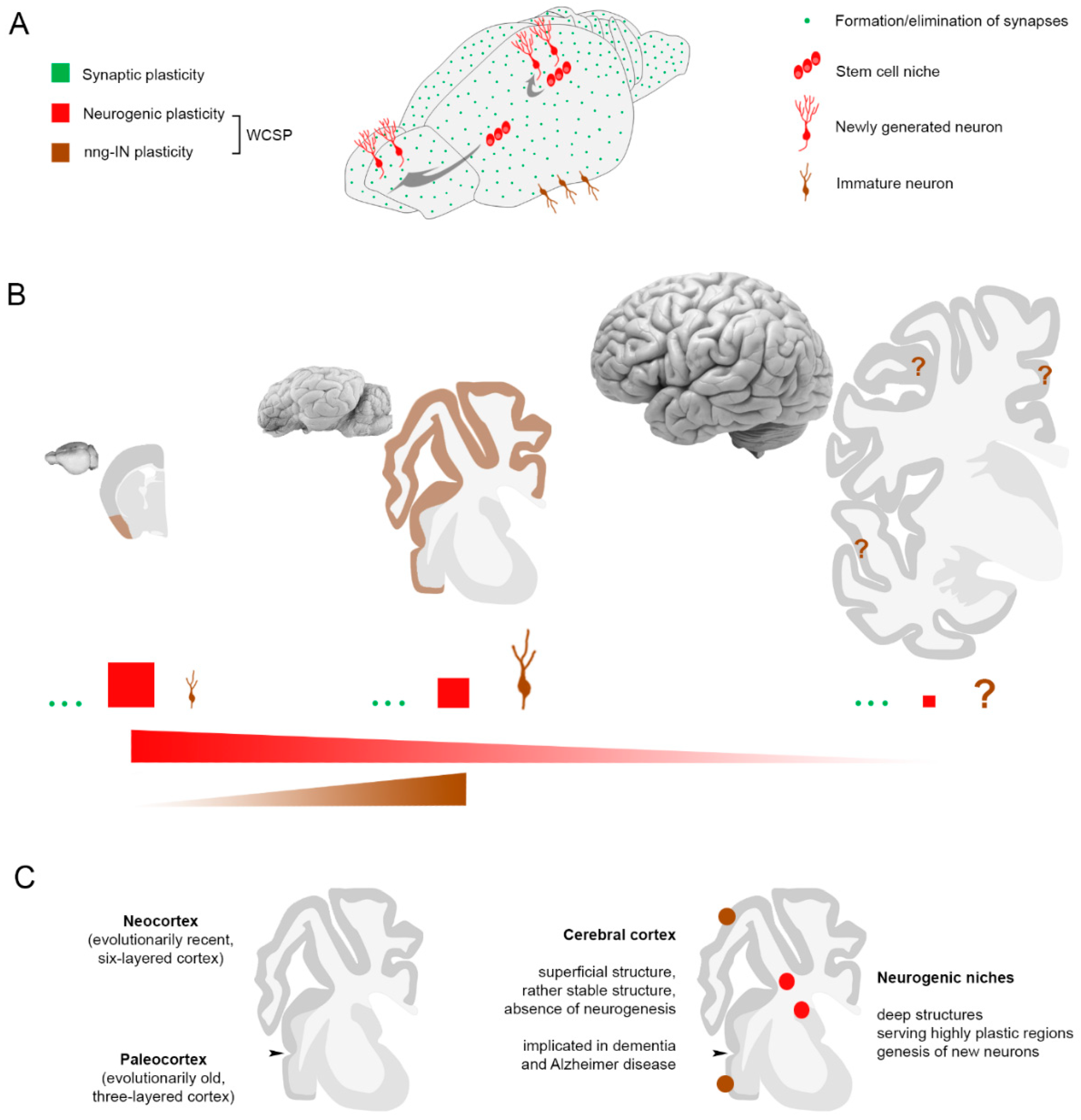
1.2. Different Types of Brain Structural Plasticity
Functional and structural plasticity
1.3. Brain Structural Plasticity: Roles in Brain Repair and Preventive Strategies
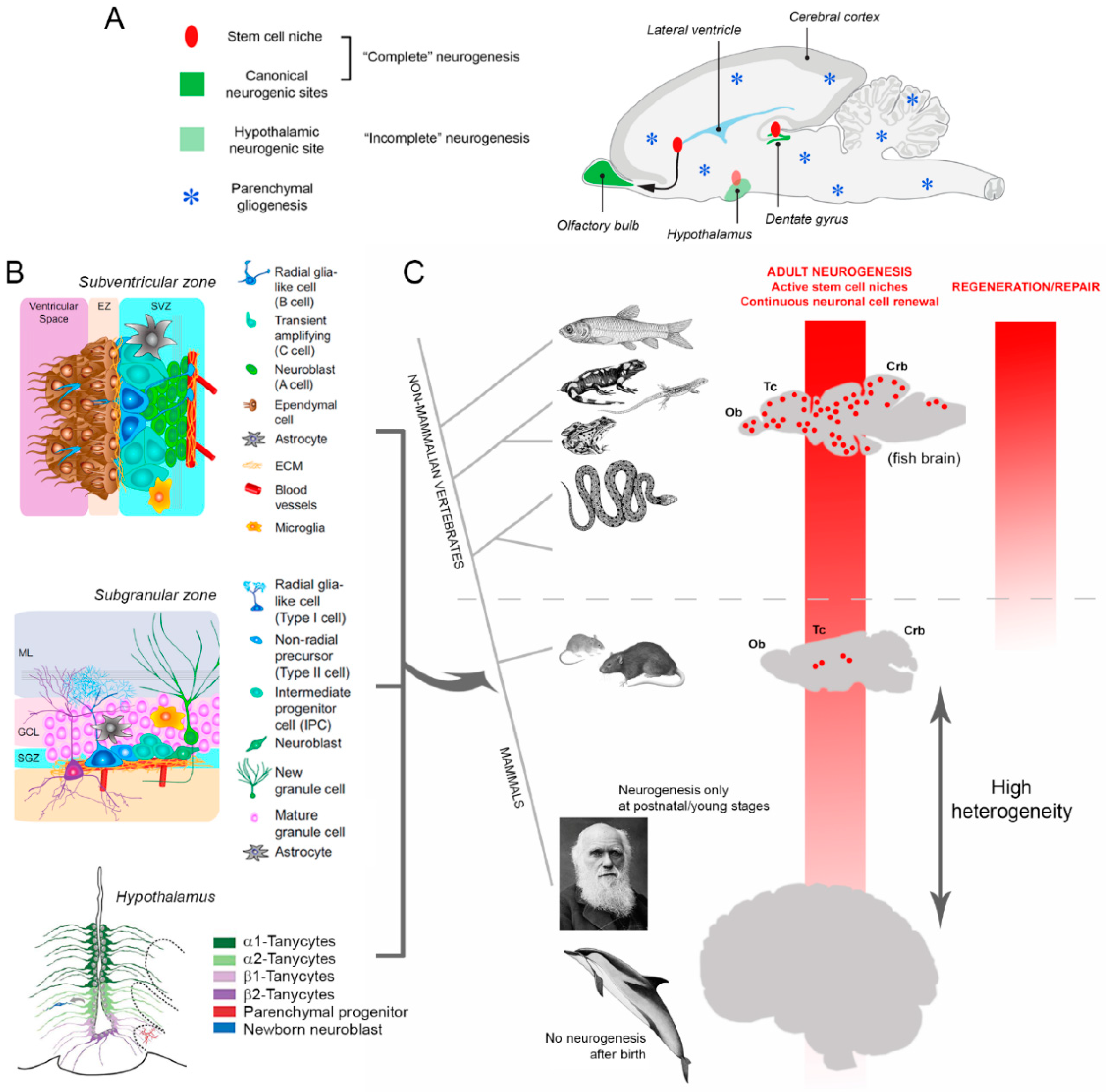
1.4. Neurogenic Plasticity: The Dream of Brain Regeneration Facing Reality
1.5. Variations in Adult Neurogenesis among Mammals and its Overall Reduction in Large-Brained Species
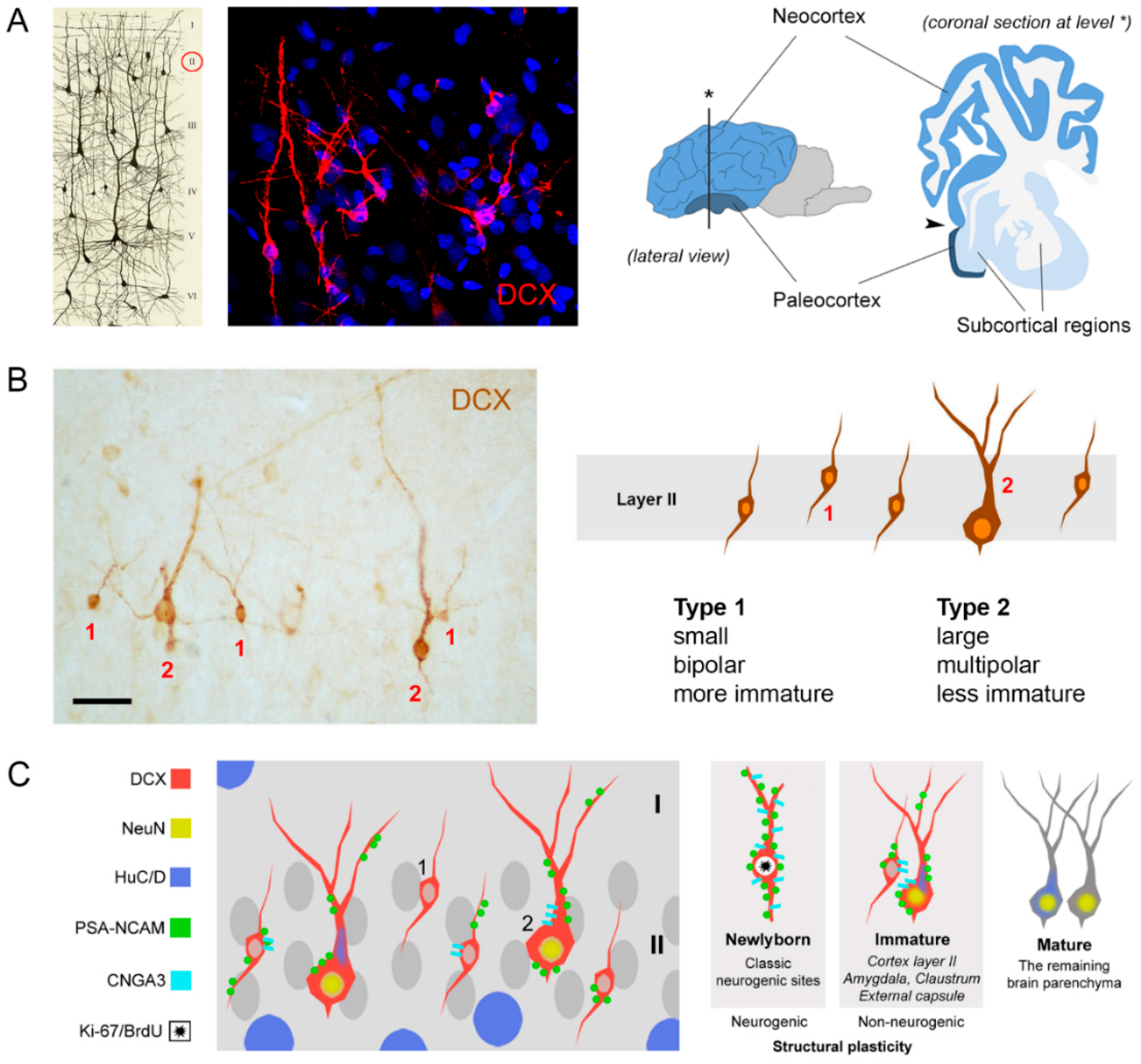
2. Immature Neurons: A New Story in Brain Plasticity?
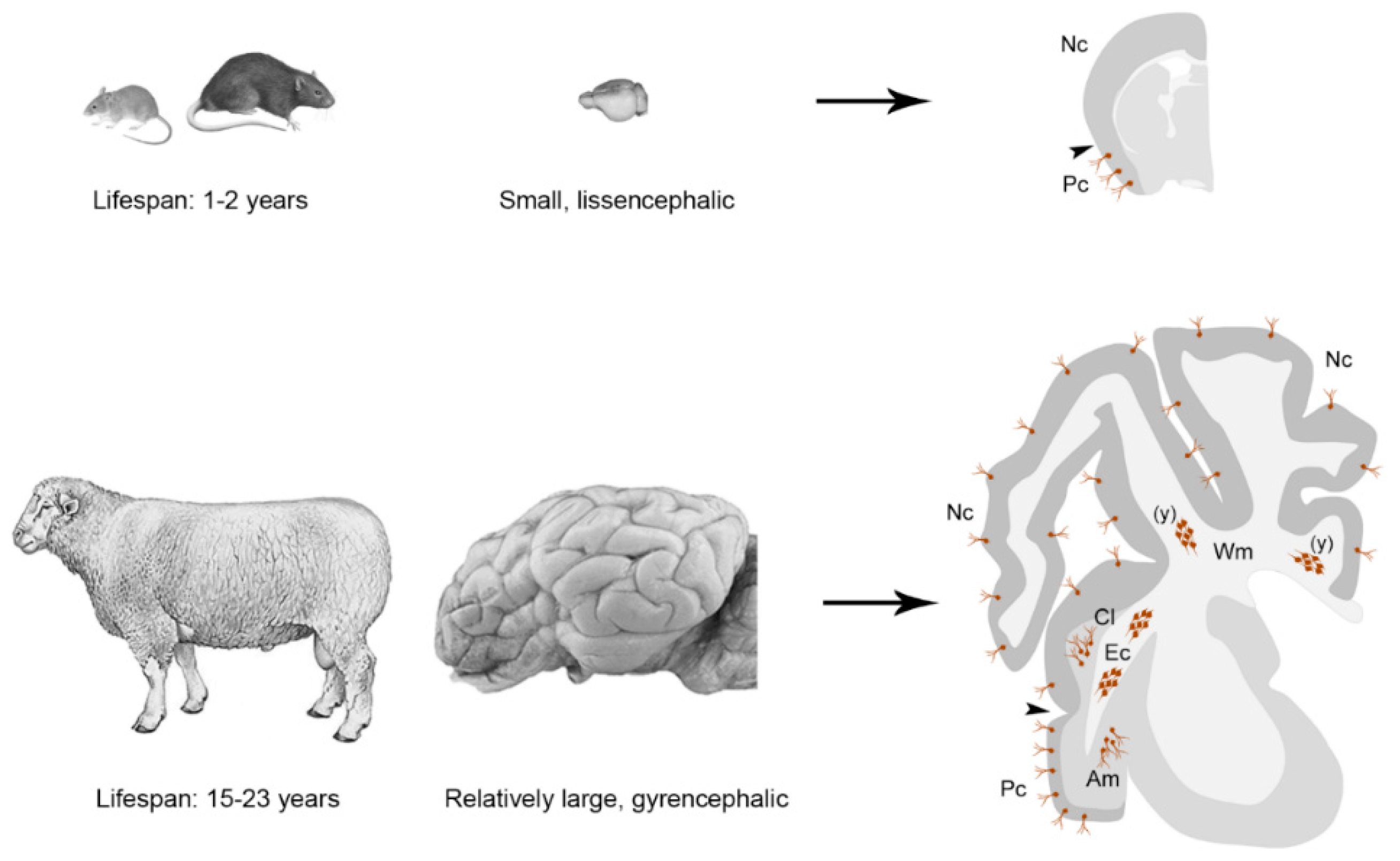
Non-Newly Generated Immature Neurons: Young Cells for Large-Brained Mammals?
3. The Concept of “Cognitive Reserve” and the Missing Substrate(s)
3.1. Brain Reserve and Cognitive Reserve
3.2. Which are the Neuroanatomical Substrates of Brain/Cognitive Reserve?
4. Immature Neurons: A Reservoir of Young Cells for the Adult/Aging Brain?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chklovskii, D.B.; Mel, B.W.; Svoboda, K. Cortical rewiring and information storage. Nature 2004, 431, 782–788. [Google Scholar] [CrossRef]
- Frotscher, M. Specificity of interneuronal connections. Ann. Anat. 1992, 174, 377–382. [Google Scholar] [CrossRef]
- Edelman, G.M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu. Rev. Cell. Biol. 1986, 2, 81–116. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Caroni, P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays 1997, 19, 767–775. [Google Scholar] [CrossRef]
- Bonfanti, L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006, 80, 129–164. [Google Scholar] [CrossRef]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.; Miquel, M. Casting a wide net: Role of perineuronal nets in neural plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef]
- Bonfanti, L. The (real) neurogenic/gliogenic potential of the postnatal and adult brain parenchyma. ISRN Neurosci. 2013, 2013, 354136. [Google Scholar] [CrossRef]
- Götz, M.; Nakafuku, M.; Petrik, D. Neurogenesis in the developing and adult brain-similarities and key differences. Cold Spring Harb. Perspect. Biol. 2016, 8, a018853. [Google Scholar] [CrossRef]
- Bonfanti, L.; Peretto, P. Adult neurogenesis in mammals: A theme with many variations. Eur. J. Neurosci. 2011, 34, 930–950. [Google Scholar] [CrossRef]
- Bonfanti, L. From hydra regeneration to human brain structural plasticity: A long trip through narrowing roads. ScientificWorldJournal. 2011, 11, 1270–1299. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; McEwen, B.S. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat. Neurosci. 2012, 15, 689–695. [Google Scholar] [CrossRef]
- Bonfanti, L.; Nácher, J. New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: The case of cortical layer II immature neurons. Prog. Neurobiol. 2012, 98, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Gould, E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J. Neurosci. 2010, 30, 13499–13503. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends. Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; van Praag, H. Running changes the brain: The long and the short of it. Physiology (Bethesda) 2017, 32, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.P.; Bonfanti, L. Adult neurogenesis in mammals: Variations and confusion. Brain. Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Faykoo-Martinez, M.; Toor, I.; Holmes, M.M. Solving the neurogenesis puzzle: Looking for pieces outside the traditional box. Front. Neurosci. 2017, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, R.W. Adult hippocampal neurogenesis in mammals (and humans): The death of a central dogma in neuroscience and its replacement by a new dogma. Dev. Neurobiol. 2019, 79, 268–280. [Google Scholar] [CrossRef]
- Snyder, J.S. Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 2019, 42, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, A.; Blanpied, T.A. Subsynaptic spatial organization as a regulator of synaptic strength and plasticity. Curr. Opin. Neurobiol. 2018, 51, 147–153. [Google Scholar] [CrossRef]
- Holtmaat, A.; Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009, 10, 759. [Google Scholar] [CrossRef]
- Chen, J.L.; Nedivi, E. Neuronal structural remodeling: Is it all about access? Curr. Opin. Neurobiol. 2010, 20, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Toni, N.; Buchs, P.A.; Nikonenko, I.; Bron, C.R.; Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999, 402, 421–425. [Google Scholar] [CrossRef] [PubMed]
- De Roo, M.; Klauser, P.; Muller, D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008, 6, e219. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Rathipriya, A.G.; Bolla, S.R.; Bhat, A.; Ray, B.; Mahalakshmi, A.M.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Guillemin, G.J.; et al. Dendritic spines: Revisiting the physiological role. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019, 92, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, I.; Jourdain, P.; Alberi, S.; Toni, N.; Muller, D. Activity-induced changes of spine morphology. Hippocampus 2002, 12, 585–591. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Poulain, D.A.; Oliet, S.H. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 2008, 88, 983–1008. [Google Scholar] [CrossRef]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Gascòn, S.; Ortega, F.; Götz, M. Transiet CREB-mediated transcription is key in direct neuronal reprogramming. Neurogenesis (Austin) 2017, 4, e1285383. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Götz, M.; Parmar, M. New approaches for brain repair-from rescue to reprogramming. Nature 2018, 557, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Climent, M.A.; Castillo-Gómez, E.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Martínez-Guijarro, F.J.; Nácher, J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex. 2008, 18, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Piumatti, M.; Palazzo, O.; La Rosa, C.; Crociara, P.; Parolisi, R.; Luzzati, F.; Lévy, F.; Bonfanti, L. Non-newly generated “immature” neurons in the sheep brain are not restricted to cerebral cortex. J. Neurosci. 2018, 38, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Weil, Z.M.; Norman, G.J.; DeVries, A.C.; Nelson, R.J. The injured nervous system: A Darwinian perspective. Prog. Neurobiol. 2008, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Pluchino, S.; Bonfanti, L.; Schwartz, M. Brain regeneration in physiology and pathology: The immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011, 91, 1281–1304. [Google Scholar] [CrossRef]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef]
- Thakur, K.T.; Albanese, E.; Giannakopoulos, P.; Jette, N.; Linde, M.; Prince, M.J.; Steiner, T.J.; Dua, T. Neurological disorders. In Mental, neurological, and substance use disorders: Disease control prioritie (Chapter 5), 3rd ed.; Patel, V., Chisholm, D., Dua, T., Laxminarayan, R., Medina-Mora, M.E., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Vaupel, J.W. Biodemography of human ageing. Nature 2010, 464, 536–542. [Google Scholar] [CrossRef]
- World Health Organization. Towards a Dementia Plan: A WHO Guide. Available online: https://www.who.int/mental_health/neurology/dementia/en/ (accessed on 11 April 2019).
- Ganz, J.; Brand, M. Adult neurogenesis in fish. Cold Spring Harb. Perspect. Biol. 2016, 8, a019018. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Segal, M. Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 2014, 94, 141–188. [Google Scholar] [CrossRef] [PubMed]
- Peretto, P.; Bonfanti, L. Major unsolved points in adult neurogenesis: Doors open on a translational future? Front. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Ming, G.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Song, J. Treating brain disorders by targeting adult neural stem cells. Trends. Mol. Med. 2018, 24, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Palma-Tortosa, S.; García-Culebras, A.; Moraga, A.; Hurtado, O.; Perez-Ruiz, A.; Durán-Laforet, V.; Parra, J.; Cuartero, M.I.; Pradillo, J.M.; Moro, M.A.; et al. Specific features of SVZ neurogenesis after cortical ischemia: A longitudinal study. Sci. Rep. 2017, 7, 16343. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Göritz, C.; Tatarishvili, J.; Dias, D.O.; Smith, E.N.; Lindvall, O.; Kokaia, Z.; Frisén, J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 2014, 346, 237–241. [Google Scholar] [CrossRef]
- Nato, G.; Caramello, A.; Trova, S.; Avataneo, V.; Rolando, C.; Taylor, V.; Buffo, A.; Peretto, P.; Luzzati, F. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington’s disease. Development 2015, 142, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on extracellular vescicles: Physiological role and signaling properties of extracellular membrane vescicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Stem cells in human neurodegenerative disorders-time for clinical translation? J. Clin. Invest. 2010, 120, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef]
- Donegà, M.; Giusto, E.; Cossetti, C.; Pluchino, S. Systemic neural stem cell-based therapeutic interventions for inflammatory CNS disorders. In Neural Stem Cells: New Perspectives; Bonfanti, L., Ed.; INTECH: Rijeka, Croatia, 2013; pp. 287–347. [Google Scholar]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.A.; Nottebohm, F.N. Neurons generated in the adult brain are recruited into functional circuits. Science 1984, 225, 1046–1048. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; García-Verdugo, J.M.; Tramontin, A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Peretto, P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog. Neurobiol. 2007, 83, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Tong, C.K.; Alvarez-Buylla, A. Restricted nature of adult neural stem cells: Re-evaluation of their potential for brain repair. Front. Neurosci. 2014, 8, 162. [Google Scholar] [CrossRef]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [PubMed]
- Urbán, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef]
- Berg, D.A.; Su, Y.; Jimenez-Cyrus, D.; Patel, A.; Huang, N.; Morizet, D.; Lee, S.; Shah, R.; Ringeling, F.R.; Jain, R.; et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell 2019, 177, 654–668. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.A.; Xu, A.W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. 2010, 30, 723–730. [Google Scholar] [CrossRef]
- Maggi, R.; Zasso, J.; Conti, L. Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus. Front. Cell Neurosci. 2015, 8, 440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- La Rosa, C.; Bonfanti, L. Brain plasticity in mammals: An example for the role of comparative medicine in the neurosciences. Front. Vet. Sci. 2018, 5, 274. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef]
- Luzzati, F.; De Marchis, S.; Fasolo, A.; Peretto, P. Neurogenesis in the caudate nucleus of the adult rabbit. J. Neurosci. 2006, 26, 609–621. [Google Scholar] [CrossRef]
- Ponti, G.; Peretto, P.; Bonfanti, L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS ONE 2008, 3, e2366. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Peretto, P.; Bonfanti, L. A subpial, transitory germinal zone forms chains of neuronal precursors in the rabbit cerebellum. Dev. Biol. 2006, 294, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Horner, P.J.; Gage, F.H. Regenerating the damaged central nervous system. Nature 2000, 407, 963–970. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Peretto, P.; Bonfanti, L. Adult neurogenesis 20 years later: Physiological function vs. brain repair. Front. Neurosci. 2015, 9, 71. [Google Scholar] [CrossRef][Green Version]
- Amrein, I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a021295. [Google Scholar] [CrossRef] [PubMed]
- Brockes, J.P.; Kumar, A. Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 2008, 24, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Amrein, I.; Dechmann, D.K.; Winter, Y.; Lipp, H.P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera). PLoS ONE 2007, 2, e455. [Google Scholar] [CrossRef]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Non-neurogenic SVZ-like niche in dolphins, mammals devoid of olfaction. Brain Struct. Funct. 2017, 222, 2625–2639. [Google Scholar] [CrossRef]
- Parolisi, R.; Cozzi, B.; Bonfanti, L. Humans and dolphins: Decline and fall of adult neurogenesis. Front. Neurosci. 2018, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Patzke, N.; Spocter, M.A.; Karlsson, K.Æ.; Bertelsen, M.F.; Haagensen, M.; Chawana, R.; Streicher, S.; Kaswera, C.; Gilissen, E.; Alagaili, A.N.; et al. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct. Funct. 2015, 220, 361–383. [Google Scholar] [CrossRef]
- Bonfanti, L.; Amrein, I. Editorial: Adult neurogenesis: Beyond rats and mice. Front. Neurosci. 2018, 12, 904. [Google Scholar] [CrossRef]
- Van Dijk, R.M.; Huang, S.H.; Slomianka, L.; Amrein, I. Taxonomic separation of hippocampal networks: Principal cells populations and adult neurogenesis. Front. Neuroanat. 2016, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.L.; Darlington, R.B. Linked regularities in the development and evolution of mammalian brains. Science 1995, 268, 1578–1584. [Google Scholar] [CrossRef]
- Workman, A.D.; Charvet, C.J.; Clancy, B.; Darlington, R.B.; Finlay, B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013, 33, 7368–7383. [Google Scholar] [CrossRef] [PubMed]
- Charvet, C.J.; Finlay, B.L. Comparing Adult Hippocampal Neurogenesis Across Species: Translating Time to Predict the Tempo in Humans. Front. Neurosci. 2018, 12, 706. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblast in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef]
- Conover, J.C.; Todd, K.L. Development and aging of a brain neural stem cell niche. Exp. Gerontol. 2017, 94, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Obernier, K.; Guinto, C.; Jose, L.; Bonfanti, L.; Alvarez-Buylla, A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. USA 2013, 110, E1045–E1054. [Google Scholar] [CrossRef] [PubMed]
- Bordiuk, O.L.; Smith, K.; Morin, P.J.; Semenov, M.V. Cell proliferation and neurogenesis in adult mouse brain. PLoS ONE 2014, 9, e111453. [Google Scholar] [CrossRef] [PubMed]
- Shook, B.A.; Manz, D.H.; Peters, J.J.; Kang, S.; Conover, J.C. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J. Neurosci. 2012, 32, 6947–6956. [Google Scholar] [CrossRef]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodas Rodriguez, J.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell 2018, 22, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, N.M.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging. 2010, 31, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Gutierrez Martin, A.J.; et al. Does adult neurogenesis persist in the human hippocampus? Cell Stem Cell 2018, 23, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Tartt, A.N.; Fulmore, C.A.; Liu, Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Hen, R.; Mann, J.J.; Boldrini, M. Considerations for assessing the extent of hippocampal neurogenesis in the adult aging human brain. Cell Stem Cell 2018, 23, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Sorrels, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adult. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.L.; Milenkovic, I.; Rasika, S.; et al. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer’s disease adults. Cereb. Cortex. 2018, 28, 2458–2478. [Google Scholar] [CrossRef]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef]
- Moreno-Jimenéz, E.P.; Flor-Garcia, M.; Terreros-Roncal, J.; Rabano, A.; Cafini, F.; Pallas-Bazarra, N.; Avila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Penz, O.K.; Fuzik, J.; Kurek, A.B.; Romanov, R.; Larson, J.; Park, T.J.; Harkany, T.; Keimpema, E. Protracted brain development in a rodent model of extreme longevity. Sci. Rep. 2015, 5, 11592. [Google Scholar] [CrossRef]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef]
- Palazzo, O.; La Rosa, C.; Piumatti, M.; Bonfanti, L. Do large brains of long-living mammals prefer non-newly generated, immature neurons? Neural Regen Res. 2018, 13, 633–634. [Google Scholar] [PubMed]
- Luzzati, F.; Bonfanti, L.; Fasolo, A.; Peretto, P. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb. Cortex. 2009, 19, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Benedetti, B.; Rotheneichner, P.; O’ Sullivan, A.; Kreutzer, C.; Belles, M.; Nacher, J.; Weiger, T.M.; Aigner, L.; Couillard-Després, S. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: A local reservoir for adult cortical neuroplasticity? Front. Biol. 2016, 11, 193–213. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. The persistent expression of a highly polysialylated NCAM in the dentate gyrus of the adult rat. Neurosci. Res. 1991, 12, 503–513. [Google Scholar] [CrossRef]
- Bonfanti, L.; Olive, S.; Poulain, D.A.; Theodosis, D.T. Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: An immunohistochemical study. Neuroscience 1992, 49, 419–436. [Google Scholar] [CrossRef]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef]
- Nacher, J.; Alonso-Llosa, G.; Rosell, D.; McEwen, B. PSA-NCAM expression in the piriform cortex of the adult rat. Modulation by NMDA receptor antagonist administration. Brain Res. 2002, 927, 111–121. [Google Scholar] [CrossRef]
- Abrous, D.N.; Montaron, M.F.; Petry, K.G.; Rougon, G.; Darnaudéry, M.; Le Moal, M.; Mayo, W. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res. 1997, 744, 285–292. [Google Scholar] [CrossRef]
- Murphy, K.J.; Fox, G.B.; Foley, A.G.; Gallagher, H.C.; O’Connell, A.; Griffin, A.M.; Nau, H.; Regan, C.M. Pentyl-4-yn-valproic acid enhances both spatial and avoidance learning, and attenuates age-related NCAM-mediated neuroplastic decline within the rat medial temporal lobe. J. Neurochem. 2001, 78, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Varea, E.; Castillo-Gómez, E.; Gómez-Climent, M.A.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Martínez-Guijarro, F.J.; Nácher, J. Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol. Aging. 2009, 30, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Luo, D.W.; Patrylo, P.R.; Luo, X.G.; Struble, R.G.; Clough, R.W.; Yan, X.X. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: Partial colocalization with mature interneuron markers. Exp. Neurol. 2008, 211, 271–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Y.; Xiong, K.; Chu, Y.; Luo, D.W.; Luo, X.G.; Yuan, X.Y.; Struble, R.G.; Clough, R.W.; Spencer, D.D.; Williamson, A.; et al. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp. Neurol. 2009, 216, 342–356. [Google Scholar] [CrossRef]
- Zhang, X.M.; Cai, Y.; Chu, Y.; Chen, E.Y.; Feng, J.C.; Luo, X.G.; Xiong, K.; Struble, R.G.; Clough, R.W.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef]
- Rotheneichner, P.; Belles, M.; Benedetti, B.; König, R.; Dannehl, D.; Kreutzer, C.; Zaunmair, P.; Engelhardt, M.; Aigner, L.; Nacher, J.; et al. Cellular plasticity in the adult murine piriform cortex: Continuous maturation of dormant precursors into excitatory neurons. Cereb. Cortex. 2018, 28, 2610–2621. [Google Scholar] [CrossRef]
- LaBar, K.S.; Gatenby, J.C.; Gore, J.C.; LeDoux, J.E.; Phelps, E.A. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 1998, 20, 937–945. [Google Scholar] [CrossRef]
- Crick, F.C.; Koch, C. What is the function of the claustrum? Philos Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 1271–1279. [Google Scholar] [CrossRef]
- Arellano, J.I.; Harding, B.; Thomas, J.L. Adult human hippocampus: No new neurons in sight. Cereb. Cortex. 2018, 28, 2479–2481. [Google Scholar] [CrossRef]
- Luo, H.; Hasegawa, K.; Liu, M.; Song, W.J. Comparison of the upper marginal neurons of cortical layer 2 with layer 2/3 pyramidal neurons in mouse temporal cortex. Front. Neuroanat. 2017, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Striedter, G.F. Principles of brain evolution; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Pandya, D.N.; Seltzer, B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 1982, 204, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- Stern, Y. An approach to studying the neural correlates of reserve. Brain Imaging Behav. 2017, 11, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve. Neuropsychologia. 2009, 47, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y. Cognitive reserve and lifestyle. J. Clin. Exp. Neuropsychol. 2003, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Darwish, H.; Farran, N.; Assaad, S.; Chaaya, M. Cognitive Reserve Factors in a Developing Country: Education and Occupational Attainment Lower the Risk of Dementia in a Sample of Lebanese Older Adults. Front. Aging Neurosci. 2018, 10, 277. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Kemper, S.J.; Mortimer, J.A.; Greiner, L.H.; Wekstein, D.R.; Markesbery, W.R. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA 1996, 275, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Gazes, Y.; Razlighi, Q.; Steffener, J.; Habeck, C. A task-invariant cognitive reserve network. Neuroimage 2018, 178, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Erten-Lyons, D.; Woltjer, R.L.; Dodge, H.; Nixon, R.; Vorobik, R.; Calvert, J.F.; Leahy, M.; Montine, T.; Kaye, J. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology 2009, 72, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.L.; Xu, H.; Woltjer, R.L.; Westaway, S.K.; Clark, D.; Erten-Lyons, D.; Kaye, J.A.; Welsh-Bohmer, K.A.; Troncoso, J.C.; Markesbery, W.R.; et al. Alzheimer disease pathology in cognitively healthy elderly: A genome-wide study. Neurobiol. Aging 2011, 32, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Zolochevska, O.; Taglialatela, G. Non-demented individuals with Alzheimer’s disease neuropathology: Resistance to cognitive decline may reveal new treatment strategies. Curr. Pharm. Des. 2016, 22, 4063–4068. [Google Scholar] [CrossRef] [PubMed]
- Giovacchini, G.; Giovannini, E.; Borsò, E.; Lazzeri, P.; Riondato, M.; Leoncini, R.; Duce, V.; Mansi, L.; Ciarmiello, A. The brain cognitive reserve hypothesis: A review with emphasis on the contribution of nuclear medicine neuroimaging techniques. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, N.L.; Reese, L.C.; Sadagoparamanujam, V.M.; Ghirardi, V.; Woltjer, R.L.; Taglialatela, G. Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer’s disease neuropathology. Mol. Neurodegener. 2012, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Sachdev, P. Assessment of complex mental activity across the lifespan: Development of the Lifetime of Experiences Questionnaire (LEQ). Psychol. Med. 2007, 37, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef]
- Schrott, L.M. Effect of training and environment on brain morphology and behavior. Acta Paediatr. Suppl. 1997, 422, 45–47. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Dayer, A.G.; Cleaver, K.M.; Abouantoun, T.; Cameron, H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005, 168, 415–427. [Google Scholar] [CrossRef]
- Gelfo, F.; Mandolesi, L.; Serra, L.; Sorrentino, G.; Caltagirone, C. The neuroprotective effects of experience on cognitive functions: Evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 2018, 370, 218–235. [Google Scholar] [CrossRef]
- Perani, D.; Abutalebi, J. Bilingualism, dementia, cognitive and neural reserve. Curr. Opin. Neurol. 2015, 28, 618–625. [Google Scholar] [CrossRef]
- Semenov, M.V. Adult hippocampal neurogenesis is a developmental process involved in cognitive development. Front. Neurosci. 2019, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef]
- Kohler, S.J.; Williams, N.I.; Stanton, G.B.; Cameron, J.L.; Greenough, W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA 2011, 108, 10326–10331. [Google Scholar] [CrossRef]
- Brus, M.; Meurisse, M.; Gheusi, G.; Keller, M.; Lledo, P.M.; Levy, F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J. Comp. Neurol. 2013, 521, 169–188. [Google Scholar] [CrossRef]
- Biteau, B.; Hochmuth, C.E.; Jasper, H. Maintaining tissue homeostasis: Dynamic control of somatic stem cell activity. Cell Stem Cell 2011, 9, 402–411. [Google Scholar] [CrossRef]
- Chacón-Martínez, C.A.; Koester, J.; Wickstrom, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145, dev165399. [Google Scholar] [CrossRef]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Guirado, R.; Castrén, E. Pharmacological Manipulation of Critical Period Plasticity. In The Oxford Handbook of Developmental Neural Plasticity; Chao, M.V., Ed.; Oxford University Press: New York, NY, USA, 2018; pp. 1–38. [Google Scholar]
- Micheli, L.; Ceccarelli, M.; D’Andrea, G.; Tirone, F. Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain. Res. Bull. 2018, 143, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Dayer, A.G. New interneurons in the adult neocortex: Small, sparse, but significant? Biol. Psychiatry. 2008, 63, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Sailor, K.A.; Schinder, A.F.; Lledo, P.M. Adult neurogenesis beyond the niche: Its potential for driving brain plasticity. Curr. Opin. Neurobiol. 2017, 42, 111–117. [Google Scholar] [CrossRef]
- Chettih, S.N.; Harvey, C.D. Single-neuron perturbations reveal feature-specific competitition in V1. Nature 2019, 567, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.T. The influence of a single neuron on its network. Nature 2019, 567, 320–321. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, C.; Ghibaudi, M.; Bonfanti, L. Newly Generated and Non-Newly Generated “Immature” Neurons in the Mammalian Brain: A Possible Reservoir of Young Cells to Prevent Brain Aging and Disease? J. Clin. Med. 2019, 8, 685. https://doi.org/10.3390/jcm8050685
La Rosa C, Ghibaudi M, Bonfanti L. Newly Generated and Non-Newly Generated “Immature” Neurons in the Mammalian Brain: A Possible Reservoir of Young Cells to Prevent Brain Aging and Disease? Journal of Clinical Medicine. 2019; 8(5):685. https://doi.org/10.3390/jcm8050685
Chicago/Turabian StyleLa Rosa, Chiara, Marco Ghibaudi, and Luca Bonfanti. 2019. "Newly Generated and Non-Newly Generated “Immature” Neurons in the Mammalian Brain: A Possible Reservoir of Young Cells to Prevent Brain Aging and Disease?" Journal of Clinical Medicine 8, no. 5: 685. https://doi.org/10.3390/jcm8050685
APA StyleLa Rosa, C., Ghibaudi, M., & Bonfanti, L. (2019). Newly Generated and Non-Newly Generated “Immature” Neurons in the Mammalian Brain: A Possible Reservoir of Young Cells to Prevent Brain Aging and Disease? Journal of Clinical Medicine, 8(5), 685. https://doi.org/10.3390/jcm8050685






