The Association between Habitual Sleep Duration and Sleep Quality with Glycemic Traits: Assessment by Cross-Sectional and Mendelian Randomization Analyses
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Study Population
2.2. Sleep Characteristics
2.3. Glycemic Traits
2.4. Covariates
2.5. Statistical Analysis
2.6. Mendelian Randomization Analysis
3. Results
3.1. Baseline Characteristics
3.2. Sleep Duration and Glycemic Traits
3.3. Sleep Quality and Glycemic Traits
3.4. Genetically-Determined Habitual Sleep Duration and Glycemic Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gregg, E.W.; Shaw, J.E. Global Health Effects of Overweight and Obesity. N. Engl. J. Med. 2017, 377, 80–81. [Google Scholar] [CrossRef]
- Wong, P.M.; Manuck, S.B.; Dinardo, M.M.; Korytkowski, M.; Muldoon, M.F. Shorter Sleep Duration is Associated with Decreased Insulin Sensitivity in Healthy White Men. Sleep 2015, 38, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Van Cauter, E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 151–173. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.-B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Liu, A.; Kushida, C.A.; Reaven, G.M. Habitual Shortened Sleep and Insulin Resistance: An Independent Relationship in Obese Individuals. Metab. Clin. Exp. 2013, 62, 1553–1556. [Google Scholar] [CrossRef]
- Rutters, F.; Besson, H.; Walker, M.; Mari, A.; Konrad, T.; Nilsson, P.M.; Balkau, B.; Dekker, J.M. The Association Between Sleep Duration, Insulin Sensitivity, and β-Cell Function: The EGIR-RISC Study. J. Clin. Endocrinol. Metab. 2016, 101, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, D.; Balkau, B.; Segrestin, B.; Gottsäter, M.; Gabriel, R.; Hatunic, M.; Mari, A.; Dekker, J.M.; Rutters, F.; on behalf of the EGIR-RISC Study Group. Associations between sleep duration and sleep debt with insulin sensitivity and insulin secretion in the EGIR-RISC Study. Diabetes Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brady, E.; Bodicoat, D.; Hall, A.; Khunti, K.; Yates, T.; Edwardson, C.; Davies, M. Sleep duration, obesity and insulin resistance in a multi-ethnic UK population at high risk of diabetes. Diabetes Res. Clin. Pract. 2018, 139, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; He, D.; Zhang, M.; Xue, J.; Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med. Rev. 2014, 18, 293–297. [Google Scholar] [CrossRef] [PubMed]
- A Schoenborn, C.; Adams, P.F.; A Peregoy, J. Health behaviors of adults: United States, 2008–2010. Health Stat. Ser. 10 2013, 10, 1–184. [Google Scholar]
- Donga, E.; Van Dijk, M.; Van Dijk, J.G.; Biermasz, N.R.; Lammers, G.-J.; Van Kralingen, K.W.; Corssmit, E.P.M.; Romijn, J.A. A Single Night of Partial Sleep Deprivation Induces Insulin Resistance in Multiple Metabolic Pathways in Healthy Subjects. J. Clin. Endocrinol. Metab. 2010, 95, 2963–2968. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A.; Congrete, S.; Romphothong, K. Sleep duration and insulin resistance in individuals without diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2015, 109, e11–e12. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nat. Cell Boil. 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.E.; Fatemifar, G.; Palmer, T.M.; White, J.; Prieto-Merino, D.; Zabaneh, D.; Engmann, J.E.L.; Shah, T.; Wong, A.; Warren, H.R.; et al. Causal Associations of Adiposity and Body Fat Distribution with Coronary Heart Disease, Stroke Subtypes and Type 2 Diabetes: A Mendelian Randomization Analysis. Circulation 2017, 135, 2373–2388. [Google Scholar] [CrossRef]
- Dekker, S.A.; Noordam, R.; Biermasz, N.R.; Roos, A.; Lamb, H.J.; Rosendaal, F.R.; Rensen, P.C.; Heemst, D.; Mutsert, R.; De Roos, A.; et al. Habitual Sleep Measures are Associated with Overall Body Fat, and not Specifically with Visceral Fat, in Men and Women. Obesity 2018, 26, 1651–1658. [Google Scholar] [CrossRef]
- Javaheri, S.; Storfer-Isser, A.; Rosen, C.L.; Redline, S. Association of short and long sleep durations with insulin sensitivity in adolescents. J. Pediatr. 2011, 158, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Wang, J.; Beatty, N.; Batemarco, T.; Sica, A.L.; Greenberg, H. Obstructive sleep apnea: An unexpected cause of insulin resistance and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 187–204. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Berg, J.F.V.D.; Miedema, H.M.E.; Tulen, J.H.M.; Neven, A.K.; Hofman, A.; Witteman, J.C.M.; Tiemeier, H. Long Sleep Duration is Associated With Serum Cholesterol in the Elderly: The Rotterdam Study. Psychosom. Med. 2008, 70, 1005–1011. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kang, G.; Kim, S.-W.; Kim, J.-M.; Yoon, J.-S.; Shin, I.-S. Associations between sleep duration and abnormal serum lipid levels: data from the Korean National Health and Nutrition Examination Survey (KNHANES). Sleep Med. 2016, 24, 119–123. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Hall, M.; Jennings, J.R.; Buysse, D.J.; Manuck, S.B. Self-reported Sleep Quality is Associated With the Metabolic Syndrome. Sleep 2007, 30, 219–223. [Google Scholar]
- Koren, D.; Dumin, M.; Gozal, D. Role of sleep quality in the metabolic syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 281–310. [Google Scholar] [CrossRef]
- Tang, Y.; Meng, L.; Li, D.; Yang, M.; Zhu, Y.; Li, C.; Jiang, Z.; Yu, P.; Li, Z.; Song, H.; et al. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chin. Med. J. 2014, 127, 3543–3547. [Google Scholar] [PubMed]
- Tasali, E.; Leproult, R.; Ehrmann, D.A.; Van Cauter, E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1044–1049. [Google Scholar] [CrossRef]
- Byberg, S.; Hansen, A.-L.S.; Christensen, D.L.; Vistisen, D.; Aadahl, M.; Linneberg, A.; Witte, D. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabet. Med. 2012, 29, e354–e360. [Google Scholar] [CrossRef]
- Engeda, J.; Mezuk, B.; Ratliff, S.; Ning, Y. Association between duration and quality of sleep and the risk of pre-diabetes: Evidence from NHANES. Diabet. Med. 2013, 30, 676–680. [Google Scholar] [CrossRef] [PubMed]
- De Mutsert, R.; Den heijer, M.; Rabelink, T.J.; Smit, J.W.; Romijn, J.A.; Jukema, J.W.; de Roos, A.; Cobbaert, C.M.; Kloppenburg, M.; le Cessie, S.; et al. The Netherlands Epidemiology of Obesity (NEO) study: Study design and data collection. Eur. J. Epidemiol. 2013, 28, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Noordam, R.; Bos, M.M.; Wang, H.; Winkler, T.W.; Bentley, A.R.; Kilpeläinen, T.; De Vries, P.S.; Sung, Y.J.; Schwander, K.; Cabe, B.E.; et al. Multi-ancestry analysis of gene-sleep interactions in 126,926 individuals identifies multiple novel blood lipid loci that contribute to our understanding of sleep-associated adverse blood lipid profile. bioRxiv 2019. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shen, S.; Hanley, A.J.; Vuksan, V.; Hamilton, J.K.; Zinman, B. Hyperbolic Relationship Between Insulin Secretion and Sensitivity on Oral Glucose Tolerance Test. Obesity 2008, 16, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.L.; Graubard, I.B. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am. J. Public Health 1991, 81, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Redline, S.; Saxena, R. Polygenic risk score identifies associations between sleep duration and diseases determined from an electronic medical record biobank. Sleep 2018, 42. [Google Scholar] [CrossRef]
- Manning, A.K.; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium; Hivert, M.-F.; Scott, R.A.; Grimsby, J.L.; Bouatia-Naji, N.; Chen, H.; Rybin, D.; Liu, C.-T.; Bielak, L.F.; et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 2012, 44, 659–669. [Google Scholar] [CrossRef]
- Upuis, J.; DIAGRAM Consortium; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Bos, M.M.; Smit, R.A.J.; Trompet, S.; Van Heemst, D.; Noordam, R. Thyroid Signaling, Insulin Resistance, and 2 Diabetes Mellitus: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2017, 102, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Smith, G.D.; Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Lane, J.M.; Liang, J.; Vlasac, I.; Anderson, S.G.; Bechtold, D.A.; Bowden, J.; Emsley, R.; Gill, S.; Little, M.A.; Luik, A.I.; et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat. Genet. 2017, 49, 274–281. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Lanfranco, F.; Gianotti, L.; Pivetti, S.; Navone, F.; Rossetto, R.; Grottoli, S.; Gai, V.; Ghigo, E.; Maccario, M. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin. Endocrinol. 2003, 59, 374–379. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A.; Information, P.E.K.F.C. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Amra, B.; Rahmati, B.; Soltaninejad, F.; Feizi, A. Screening Questionnaires for Obstructive Sleep Apnea: An Updated Systematic Review. Oman Med. J. 2018, 33, 184–192. [Google Scholar] [CrossRef] [PubMed]
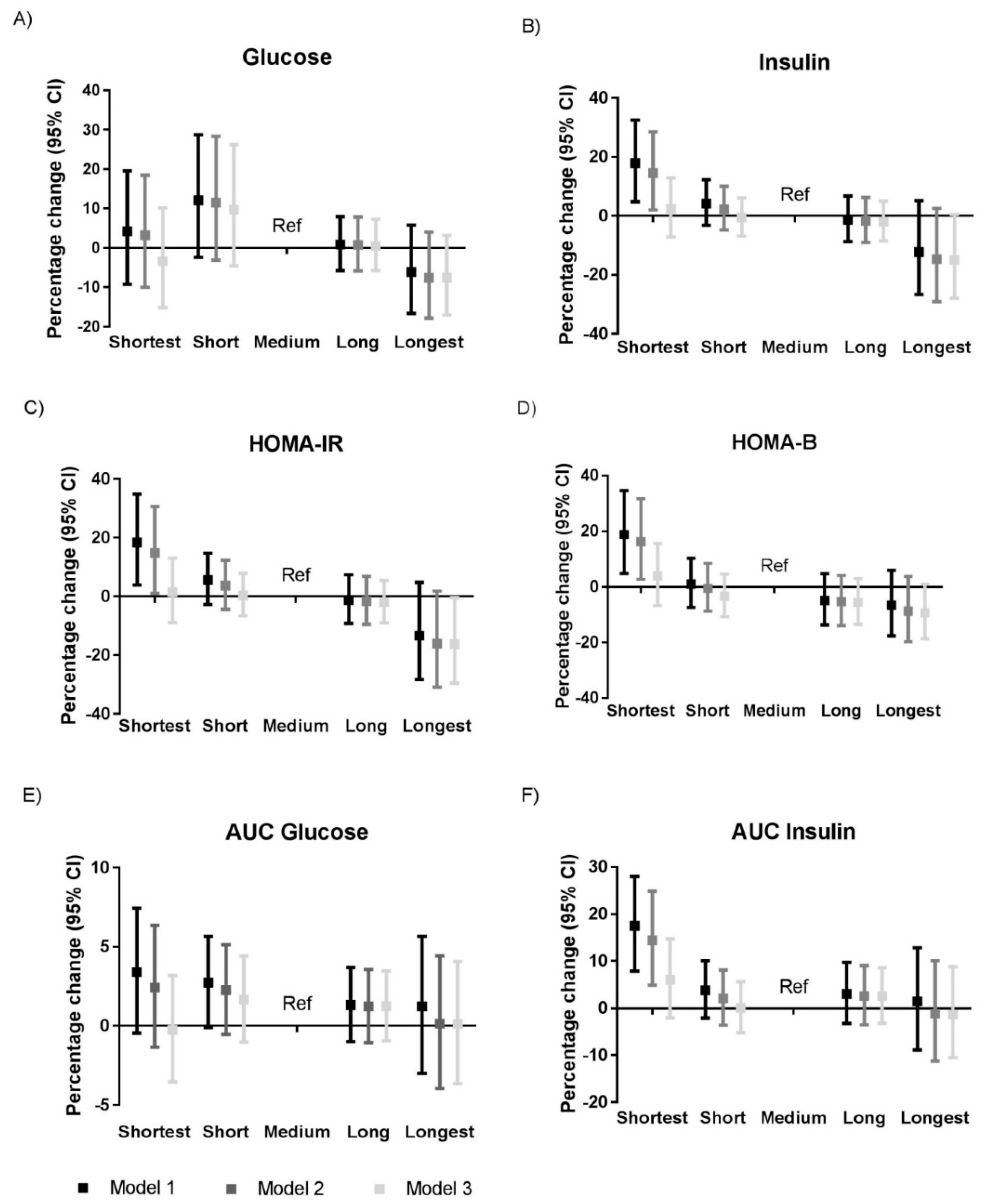
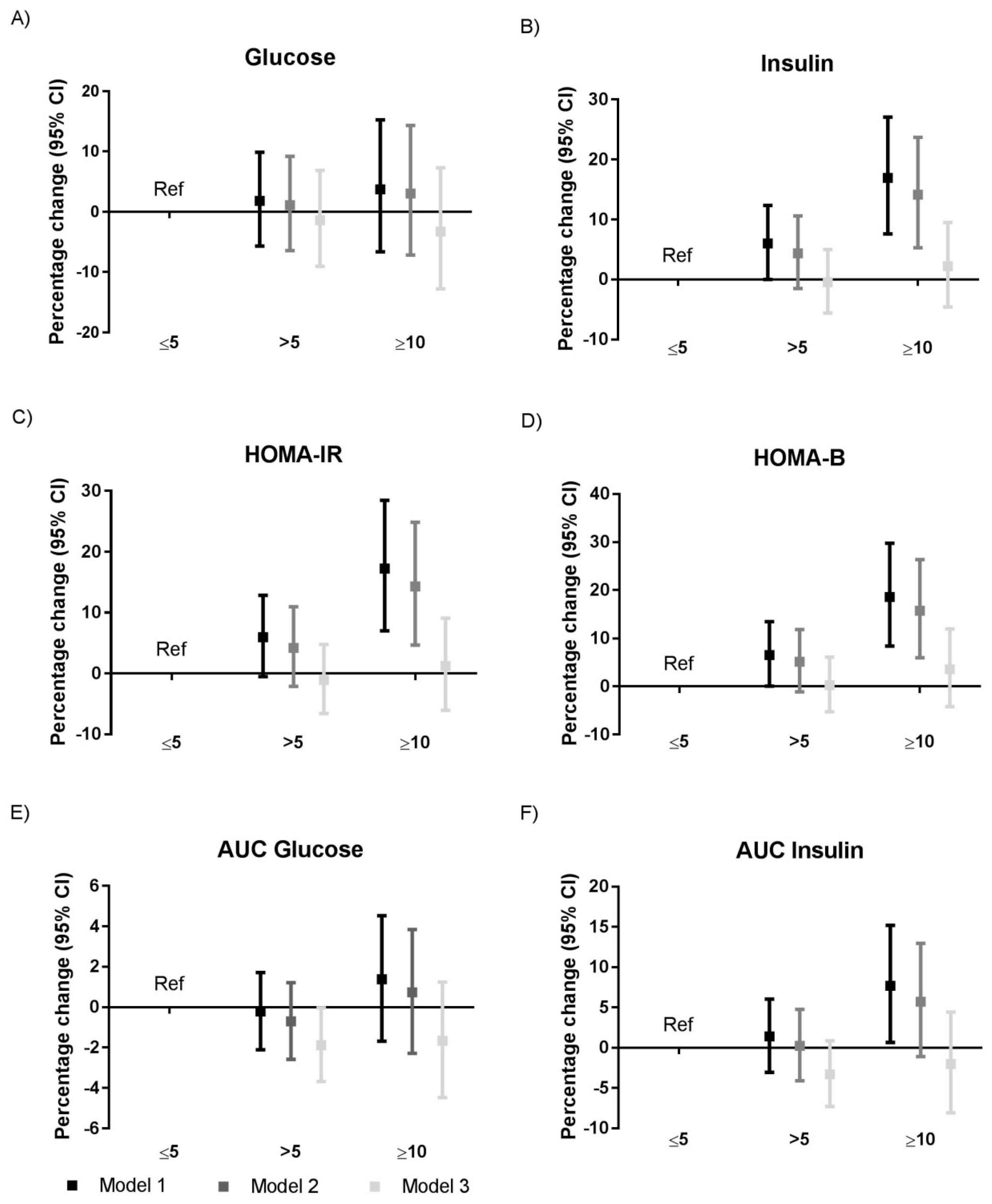
| Sleep Duration | Shortest | Short | Medium | Long | Longest |
|---|---|---|---|---|---|
| 0–5% | 5–20% | 20–80% | 80–95% | 95–100% | |
| Age (years) | 57 (5) | 58 (5) | 55 (6) | 54 (6) | 58 (6) |
| Sex (% men) | 45 | 49 | 42 | 43 | 53 |
| BMI (kg/m2) | 27 (5) | 26 (4) | 26 (4) | 26 (4) | 26 (5) |
| Ethnicity (% white) | 90 | 94 | 96 | 96 | 94 |
| Education (% high) | 39 | 41 | 50 | 48 | 36 |
| Smoking (%current) | 18 | 14 | 16 | 16 | 20 |
| Sleep medication (%) | 14 | 10 | 4 | 5 | 7 |
| Alcohol consumption (g/day) | 12 (3; 22) | 11 (3; 22) | 10 (3; 21) | 9 (2; 21) | 11 (1; 23) |
| Physical activity (MET/h/week) | 25 (12; 44) | 30 (16;50) | 31 (17; 51) | 32 (15; 52) | 30 (16; 49) |
| Sleep duration (h/day) | 5 (4; 5) | 6 (6;6) | 7 (7; 8) | 8 (8; 8) | 9 (9; 9) |
| PSQI (total score) | 11 (9; 13) | 7 (5; 9) | 4 (3; 6) | 3 (1; 4) | 3 (1; 5) |
| Sleep apnea (%) | 35 | 25 | 17 | 17 | 24 |
| Fasting glucose (mmol/L) | 6 (1) | 6 (2) | 6 (1) | 6 (1) | 6 (2) |
| Fasting insulin (mmol/L) | 9 (6; 14) | 8 (6; 12) | 7 (5; 11) | 7 (5; 11) | 7 (4; 12) |
| HOMA-IR | 2 (1; 4) | 2 (1; 3) | 2 (1; 3) | 2 (1; 3) | 2 (1; 3) |
| HOMA-β | 28 (18; 42) | 26 (18; 40) | 24 (16; 37) | 25 (15; 36) | 25 (13; 37) |
| AUC Glucose * | 6 (1) | 6 (1) | 6 (1) | 6 (1) | 6 (1) |
| AUC Insulin # | 47 (34; 62) | 41 (30; 57) | 38 (29; 53) | 38 (30; 54) | 41 (26; 61) |
| Total Sleep Duration | Short Sleep Duration | Long Sleep Duration | ||||
|---|---|---|---|---|---|---|
| SNPs | Estimate (95% CI) | SNPs | Estimate (95% CI) | SNPs | Estimate (95% CI) | |
| Fasting glucose | 54 | −0.03 (−0.11; 0.06) | 20 | −0.09 (−0.19; 0.01) | 5 | −0.05 (−0.24; 0.13) |
| Fasting insulin | 54 | 0.01 (−0.04; 0.07) | 20 | −0.01 (−0.11; 0.08) | 5 | −0.06 (−0.36; 0.23) |
| HOMA-IR | 53 | 0.07 (−0.01; 0.15) | 20 | 0.04 (−0.08; 0.15) | 5 | −0.01 (−0.47; 0.45) |
| HOMA-β | 53 | 0.08 (0.01; 0.14) | 20 | 0.09 (−0.01; 0.19) | 5 | 0.09 (−0.26; 0.45) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bos, M.M.; van Heemst, D.; Donga, E.; de Mutsert, R.; Rosendaal, F.R.; Blauw, G.J.; Rensen, P.C.N.; Biermasz, N.R.; Noordam, R. The Association between Habitual Sleep Duration and Sleep Quality with Glycemic Traits: Assessment by Cross-Sectional and Mendelian Randomization Analyses. J. Clin. Med. 2019, 8, 682. https://doi.org/10.3390/jcm8050682
Bos MM, van Heemst D, Donga E, de Mutsert R, Rosendaal FR, Blauw GJ, Rensen PCN, Biermasz NR, Noordam R. The Association between Habitual Sleep Duration and Sleep Quality with Glycemic Traits: Assessment by Cross-Sectional and Mendelian Randomization Analyses. Journal of Clinical Medicine. 2019; 8(5):682. https://doi.org/10.3390/jcm8050682
Chicago/Turabian StyleBos, Maxime M., Diana van Heemst, Esther Donga, Renée de Mutsert, Frits R. Rosendaal, Gerard Jan Blauw, Patrick C. N. Rensen, Nienke R. Biermasz, and Raymond Noordam. 2019. "The Association between Habitual Sleep Duration and Sleep Quality with Glycemic Traits: Assessment by Cross-Sectional and Mendelian Randomization Analyses" Journal of Clinical Medicine 8, no. 5: 682. https://doi.org/10.3390/jcm8050682
APA StyleBos, M. M., van Heemst, D., Donga, E., de Mutsert, R., Rosendaal, F. R., Blauw, G. J., Rensen, P. C. N., Biermasz, N. R., & Noordam, R. (2019). The Association between Habitual Sleep Duration and Sleep Quality with Glycemic Traits: Assessment by Cross-Sectional and Mendelian Randomization Analyses. Journal of Clinical Medicine, 8(5), 682. https://doi.org/10.3390/jcm8050682





