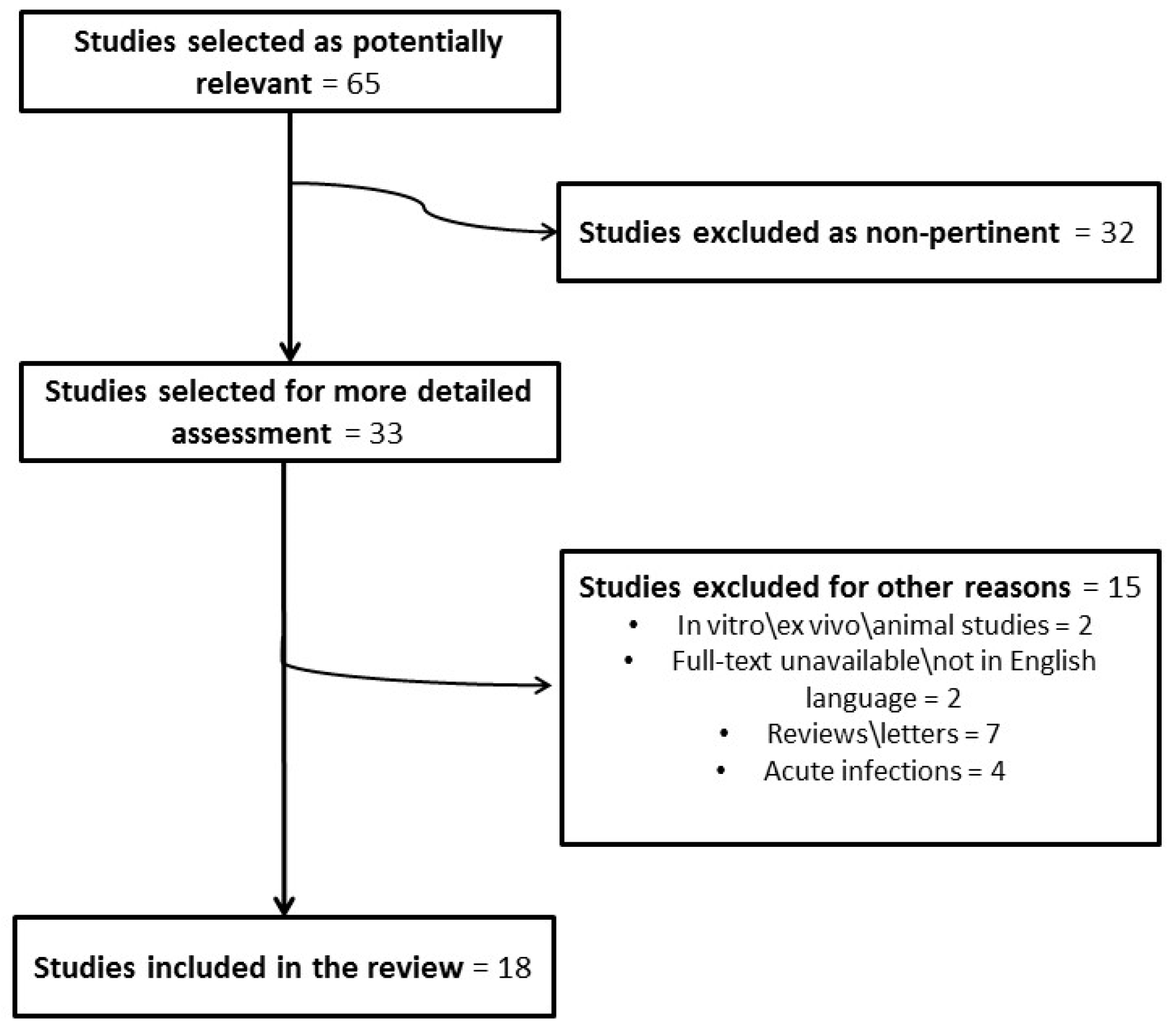
Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease
Abstract
1. Introduction
2. Bacterial Biofilms in Chronic Adenoiditis and Sampling Procedures
3. Nasopharyngeal Bacterial Biofilm: Is There A Correlation between Adenoiditis and Recurrent/Chronic Middle Ear Infections?
4. Topographic Distribution of Bacterial Biofilms in the Nasopharynx.
5. Conclusions
6. Key Concepts
- Chronic adenoiditis frequently occurs in paediatric patients and is often complicated by the subsequent development of recurrent or chronic middle ear diseases.
- The adenoids and surrounding nasopharynx may be sources of otopathogenic biofilms that periodically release planktonic species that are capable of colonising middle ear mucosa through an impaired Eustachian tube, thus predisposing individuals to the development of chronic nasopharyngeal and middle ear infections.
- The main pathogens involved in nasopharyngeal biofilm production are the so-called otopathogens (Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae).
- Given the specific topographic distribution of nasopharyngeal biofilm on adenoidal pads, adenoids should be carefully resected in the case of chronic nasopharyngeal infection, taking particular care to avoid any residues near the ostium of the Eustachian tube in order to avoid biofilm survival in any persistent nasopharyngeal infectious focus.
- As nasopharyngeal biofilm-producing otopathogens seem to be also involved in the development of RAOM in young children without adenoidal hypertrophy, it can be speculated that nasopharyngeal biofilms are independently involved in the development of recurrent middle ear infections, regardless of the presence of adenoidal hypertrophy.
- Future studies should investigate the potential virulence of biofilm-producers in the adenoids of otitis-prone children.
Author Contributions
Conflicts of Interest
References
- Cassano, P.; Gelardi, M.; Cassano, M.; Fiorella, M.L.; Fiorella, R. Adenoid tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: A novel approach to therapeutic management. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 1303–1309. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Poddighe, D.; Caimmi, D.; Marseglia, A.; Caimmi, S.; Ciprandi, G.; Klersy, C.; Pagella, F.; Castellazzi, A.M. Role of adenoids and adenoiditis in children with allergy and otitis media. Curr. Allergy Asthma Rep. 2009, 9, 460–464. [Google Scholar] [CrossRef]
- Gates, G.A.; Klein, J.O.; Lim, D.J.; Mogi, G.; Ogra, P.L.; Pararella, M.M.; Paradise, J.L.; Tos, M. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Ann. Otol. Rhinol. Laryngol. Suppl. 2002, 188, 8–18. [Google Scholar] [PubMed]
- American Academy of Family Physicians, American Academy of Otolaryngology—Head and Neck Surgery and American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics 2004, 113, 1412–1429. [Google Scholar] [CrossRef]
- Jensen, R.G.; Koch, A.; Homøe, P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1530–1535. [Google Scholar] [CrossRef]
- Casey, J.R.; Adlowitz, D.G.; Pichichero, M.E. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 2010, 29, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E.; Pichichero, C.L. Persistent acute otitis media: I. Causative pathogens. Pediatr. Infect. Dis. J. 1995, 14, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Madana, J.; Yolmo, D.; Kalaiarasi, R.; Gopalakrishnan, S.; Sujatha, S. Microbiological profile with antibiotic sensitivity pattern of cholesteatomatous chronic suppurative otitis media among children. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1104–1108. [Google Scholar] [CrossRef]
- Torretta, S.; Drago, L.; Marchisio, P.; Mattina, R.; Clemente, I.A.; Pignataro, L. Diagnostic accuracy of nasopharyngeal swabs in detecting biofilm-producing bacteria in chronic adenoiditis: A preliminary study. Otolaryngol. Head Neck Surg. 2011, 144, 784–788. [Google Scholar] [CrossRef]
- Nazzari, E.; Torretta, S.; Pignataro, L.; Marchisio, P.; Esposito, S. Role of biofilm in children with recurrent upper respiratory tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 421–429. [Google Scholar] [CrossRef]
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS 2014, 122, 1013–1019. [Google Scholar] [CrossRef]
- Torretta, S.; Drago, L.; Marchisio, P.; Gaffuri, M.; Clemente, I.A.; Pignataro, L. Topographic distribution of biofilm-producing bacteria in adenoid subsites of children with chronic or recurrent middle ear infections. Ann. Otol. Rhinol. Laryngol. 2013, 122, 109–113. [Google Scholar] [CrossRef]
- Torretta, S.; Marchisio, P.; Drago, L.; Baggi, E.; De Vecchi, E.; Garavello, W.; Nazzari, E.; Pignataro, L.; Esposito, S. Nasopharyngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol. Head Neck Surg. 2012, 146, 991–996. [Google Scholar] [CrossRef]
- Capaccio, P.; Torretta, S.; Marciante, G.A.; Marchisio, P.; Forti, S.; Pignataro, L. Endoscopic Adenoidectomy in Children With Otitis Media With Effusion and Mild Hearing Loss. Clin. Exp. Otorhinolaryngol. 2016, 9, 33–38. [Google Scholar] [CrossRef]
- Galli, J.; Calò, L.; Ardito, F.; Imperiali, M.; Bassotti, E.; Fadda, G.; Paludetti, G. Biofilm formation by Haemophilus influenzae isolated from adeno-tonsil tissue samples, and its role in recurrent adenotonsillitis. Acta Otorhinolaryngol. Ital. 2007, 27, 134–138. [Google Scholar] [PubMed]
- Al-Mazrou, K.A.; Al-Khattaf, A.S. Adherent biofilms in adenotonsillar diseases in children. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 20–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Winther, B.; Gross, B.C.; Hendley, J.O.; Early, S.V. Location of bacterial biofilm in the mucus overlying the adenoid by light microscopy. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 1239–1245. [Google Scholar] [CrossRef]
- Kosikowska, U.; Korona-Głowniak, I.; Niedzielski, A.; Malm, A. Nasopharyngeal and Adenoid Colonization by Haemophilus influenzae and Haemophilus parainfluenzae in Children Undergoing Adenoidectomy and the Ability of Bacterial Isolates to Biofilm Production. Medicine 2015, 94, e799. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.A.; Lin, C.D.; Hsu, H.Y.; Peng, M.T.; Kuo, Y.Y.; Tien, N.; Li, J.P.; Wang, C.K.; Wu, H.S.; Tsai, M.H.; et al. Association of β-Lactam-sensitive Haemophilus influenza Type B with adenoid biofilm formation in patients with adenoidectomy syrgery. Surg. Infect. 2015, 16, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Homøe, P.; Bjarnsholt, T.; Wessman, M.; Sørensen, H.C.; Johansen, H.K. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur. Arch. Otorhinolaryngol. 2009, 266, 1533–1538. [Google Scholar] [CrossRef]
- Zuliani, G.; Carlisle, M.; Duberstein, A.; Haupert, M.; Syamal, M.; Berk, R.; Du, W.; Coticchia, J. Biofilm density in the pediatric nasopharynx: Recurrent acute otitis media versus obstructive sleep apnea. Ann. Otol. Rhinol. Laryngol. 2009, 118, 519–524. [Google Scholar] [CrossRef]
- Hoa, M.; Syamal, M.; Schaeffer, M.A.; Sachdeva, L.; Berk, R.; Coticchia, J. Biofilms and chronic otitis media: An initial exploration into the role of biofilms in the pathogenesis of chronic otitis media. Am. J. Otolaryngol. 2010, 31, 241–245. [Google Scholar] [CrossRef]
- Saylam, G.; Tatar, E.C.; Tatar, I.; Ozdek, A.; Korkmaz, H. Association of adenoid surface biofilm formation and chronic otitis media with effusion. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 550–555. [Google Scholar] [CrossRef]
- Nistico, L.; Kreft, R.; Gieseke, A.; Coticchia, J.M.; Burrows, A.; Khampang, P.; Liu, Y.; Kerschner, J.E.; Post, J.C.; Lonergan, S.; et al. Adenoid reservoir for pathogenic biofilm bacteria. J. Clin. Microbiol. 2011, 49, 1411–1420. [Google Scholar] [CrossRef]
- Daniel, M.; Imtiaz-Umer, S.; Fergie, N.; Birchall, J.P.; Bayston, R. Bacterial involvement in otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1416–1422. [Google Scholar] [CrossRef]
- Saafan, M.E.; Ibrahim, W.S.; Tomoum, M.O. Role of adenoid biofilm in chronic otitis media with effusion in children. Eur. Arch. Otorhinolaryngol. 2013, 270, 2417–2425. [Google Scholar] [CrossRef]
- Thornton, R.B.; Wiertsema, S.P.; Kirkham, L.A.; Rigby, P.J.; Vijayasekaran, S.; Coates, H.L.; Richmond, P.C. Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media—A potential treatment target. PLoS ONE 2013, 8, e53837. [Google Scholar] [CrossRef] [PubMed]
- Szalmás, A.; Papp, Z.; Csomor, P.; Kónya, J.; Sziklai, I.; Szekanecz, Z.; Karosi, T. Microbiological profile of adenoid hypertrophy correlates to clinical diagnosis in children. Biomed. Res. Int. 2013, 2013, 629607. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, H.; De Paepe, A.S.; Lambert, E.; Van Belleghem, J.D.; Cools, P.; Van Simaey, L.; Deschaght, P.; Vaneechoutte, M.; Dhooge, I. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur. Arch. Otorhinolaryngol. 2016, 273, 3553–3560. [Google Scholar] [CrossRef]
- Tawfik, S.A.; Ibrahim, A.A.; Talaat, I.M.; El-Alkamy, S.S.; Youssef, A. Role of bacterial biofilm in development of middle ear effusion. Eur. Arch. Otorhinolaryngol. 2016, 273, 4003–4009. [Google Scholar] [CrossRef]
- de la Torre González, C.; Huante-Guido, M.; Velázquez Guadarrama, N.; Preciado, D.; Patiño López, G. Changes in biofilm in chronic cholesteatomatous otitis media in children following the application of sodium 2-mercaptoethanesulfonate (MESNA). Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hoa, M.; Tomovic, S.; Nistico, L.; Hall-Stoodley, L.; Stoodley, P.; Sachdeva, L.; Berk, R.; Coticchia, J.M. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Calò, L.; Giuliani, M.; Sergi, B.; Lucidi, D.; Meucci, D.; Bassotti, E.; Sanguinetti, M.; Paludetti, G. Biofilm’s Role in Chronic Cholesteatomatous Otitis Media: A Pilot Study. Otolaryngol. Head Neck Surg. 2016, 154, 914–916. [Google Scholar] [CrossRef]
- Wessman, M.; Bjarnsholt, T.; Eickhardt-Sørensen, S.R.; Johansen, H.K.; Homøe, P. Mucosal biofilm detection in chronic otitis media: A study of middle ear biopsies from Greenlandic patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 1079–1085. [Google Scholar] [CrossRef]
- Bakaletz, L.O. Bacterial biofilms in the upper airway—Evidence for role in pathology and implications for treatment of otitis media. Paediatr. Respir. Rev. 2012, 13, 154–159. [Google Scholar] [CrossRef]
- Rayner, M.G.; Zhang, Y.; Gorry, M.C.; Chen, Y.; Post, J.C.; Ehrlich, G.D. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 1998, 28, 296–299. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Keyoumu, Y.; Long, L.; Zhang, H. Detection of bacterial biofilms in different types of chronic otitis media. Eur. Arch. Otorhinolaryngol. 2014, 271, 2877–2883. [Google Scholar] [CrossRef]
- Saunders, J.; Murray, M.; Alleman, A. Biofilms in chronic suppurative otitis media and cholesteatoma: Scanning electron microscopy findings. Am. J. Otolaryngol. 2011, 32, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Hotomi, M.; Shimada, J.; Billal, D.S.; Fujihara, K.; Yamanaka, N. Formation of biofilm by Haemophilus influenzae isolated from pediatric intractable otitis media. Auris Nasus Larynx 2009, 36, 525–531. [Google Scholar] [CrossRef]
- Mizrahi, A.; Cohen, R.; Varon, E.; Bonacorsi, S.; Bechet, S.; Poyart, C.; Levy, C.; Raymond, J. Non typable-Haemophilus influenzae biofilm formation and acute otitis media. BMC Infect. Dis. 2014, 19, 400. [Google Scholar] [CrossRef] [PubMed]
- Osgood, R.; Salamone, F.; Diaz, A.; Casey, J.R.; Bajorski, P.; Pichichero, M.E. Effect of pH and oxygen on biofilm formation in acute otitis media associated NTHi clinical isolates. Laryngoscope 2015, 125, 2204–2208. [Google Scholar] [CrossRef] [PubMed]

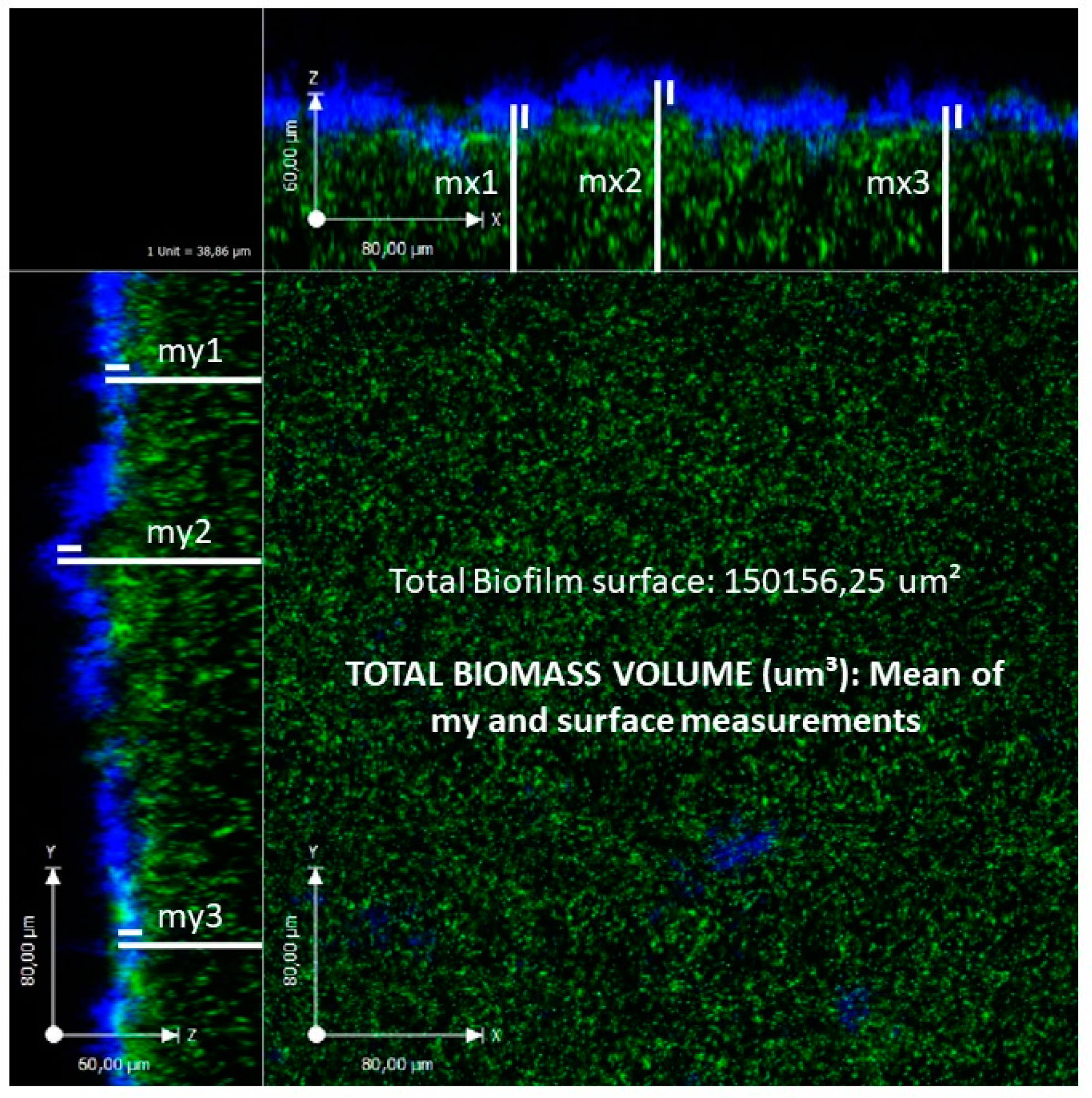
| Authors; Year | No. of Patients; Samples | Mean Age ± SD, years | Analytical Technique | Prevalence of Bacterial Biofilm (%) | Prevalence of Isolated BPB |
|---|---|---|---|---|---|
| Galli et al. [15], 2007 | 15; 15 | - | SEM | 100 | H. influenzae = 67% S. pyogenese = 7% Alpha-hemolytic streptococcus i = 13% |
| Al-Mazrou et al. [16], 2008 | 76; 76 (adenoids and tonsils) | 5.7 ± 3.3 | SEM | 85 | Staphylococcus spp. and Streptococcus spp. |
| Winther et al. [17], 2009 | -; 9 | - | PAS of Carnoy fluid and FISH | 91 | - |
| Torretta et al. [9], 2011 | 42; 84 | 7.0 ± 2.7 | Spectrophotometry | BPB in 74 NPS and 69 B | S. aureus = 55% (NPS); 79% (B) M. catarrhalis = 19% (NPS); 21% (B) H. influenzae = 10% (NPS); 14% (B) S. pneumoniae = 35% (NPS); 10% (B) P. aeruginosa = 3% (NPS); 10% (B) S. pyogenes = 10% (NPS); 7% (B) H. parainfluenzae = 23% (NPS); 0% (B) |
| Torretta et al. [12], 2012 | 113; - | Median: 40 (range: 10–132), months | Spectrophotometry | BPO in 41 patients with recurrent middle ear infections and 14 controls | M. catarrhalis = 6 (patients); 34 (controls) S. pneumoniae = 17 (patients); 33 (controls) S. pyogenes = 6 (patients); 33 (controls) H. influenzae= 71 (patients); 0 (controls) |
| Torretta et al. [11], 2013 | 45; 135 | Median: 7 (range: 4–13) | Spectrophotometry | BPB in 72 (ET); 53 (NPD) | S. aureus = 45% (ET); 50% (NPD) S. pneumoniae = 18% (ET); 12% (NPD) M. catarrhalis = 20% (ET); 12% (NPD) S. pyogenes = 9% (ET); 9% (NPD) H. influenzae = 3% (ET); 4% (NPD) Coagulase negative staphylococci = 3% (ET); 4% (NPD) H. parainfluenzae = 1% (ET); 4% (NPD) P. fluorescens = 1% (ET); 4% (NPD) |
| Kosikowska et al. [18], 2015 | 164; 328 | Range: 2–5 | Spectrophotometry | BP Haemophilus species in 97% of patients. | 67% of H. influenzae samples were biofilm producers 56% of H. parainfluenzae samples were biofilm producers 86% of other H. spp samples were biofilm producers |
| Tsou et al. [19], 2015 | 32; - | Range: 4–13 | Scanning electron microscopy | BP β-lactam-resistant Haemophilus influenzae type b more frequently detected in children with chronic adenoiditis than in those with adenoidal hypertrophy without infections. | |
| Authors; Year | No. of Patients; Samples | Mean Age ± SD, Years | Disease | Analytical Technique | Prevalence of Bacterial Biofilm (%) | Prevalence of Isolated BPB |
|---|---|---|---|---|---|---|
| Homoe et al. [20], 2009 | 10; 13 | Range: 2–15 | CSOM OME | Peptide nucleic acid-FISH of MEM and MEF | 83% 0% | S. aureus = 67% S. maltophilia = 17% |
| Zuliani et al. [21], 2009 | 68; 68 | Range: 3 months–15 years | RAOM OSA | SEM of adenoidal mucosa | 93% of adenoidal mucosa covered by biofilm in children with RAOM; 1% of adenoidal mucosa covered by biofilm in children with OSA | |
| Hoa et al. [22], 2010 | 30; 30 | Range: 9 months–10 years | RAOM OME OSA | SEM of adenoidal mucosa | 98% of adenoidal mucosa covered by biofilm in children with RAOM; 28% of adenoidal mucosa covered by biofilm in children with OME; and <1% of adenoidal mucosa covered by biofilm in children with OSA | |
| Saylam et al. [23], 2011 | 17; 17 | 7.5 ± 2.6 | OME | SEM of adenoidal mucosa | 100% | - |
| Nistico et al. [24], 2011 | 35; - | 4.1 (range: 1–10) | COM OSA | CLSM and FISH of adenoidal mucosa | H. influenzae, S. pneumoniae, S. aureus polymicrobic biofilm in most samples | |
| Daniel et al. [25], 2012 | 42; 62 | Median: 4.5 (range: 1–75) | OME | CLSM of MEM | 49% | Coagulase-negative staphylococci = 3 S. aureus = 2 S. pneumoniae = 3 Bacillus spp. = 2 M. catarrhalis = 2 Pseudomonas spp. = 5 Other = 14 |
| Saafan et al. [26], 2013 | 100; - | 5.7 (range: 3–14) | OME | SEM and multiplex PCR of adenoidal mucosa and MEM | 74% (adenoidal mucosa) | - |
| Thornton et al. [27], 2013 | 24; 38 | Median: 17.9 (range: 9.7–36.0) months | RAOM | FISH on MEM | 70% | S. pneumoniae = 73 H. influenzae = 65 M. catarrhalis = 27 S. aureus = 27 P. aeruginosa = 4 |
| Szalmas et al. [28], 2013 | 59; - | 5.1 (range: 3–11) | RAOM OME OSA | Hematoxylin-eosin and Gram staining of adenoidal mucosa | 80% 0% 6% | - |
| Van Hoecke et al. [29], 2016 | 21; 34 | 3.3 (range: 1.1–6.6) | OME | FISH and CLSM of MEE | 62% H. influenza biofilm | |
| Tawfik et al. [30], 2016 | 40; - | Range: 1–16 | OME | SEM of adenoidal mucosa | 100% | - |
| De la Torre et al. [31], 2018 | 10; 20 | 10 (range: 6–17) | Cholesteatoma | CLSM of cholesteatoma | 100% | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torretta, S.; Drago, L.; Marchisio, P.; Ibba, T.; Pignataro, L. Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. J. Clin. Med. 2019, 8, 671. https://doi.org/10.3390/jcm8050671
Torretta S, Drago L, Marchisio P, Ibba T, Pignataro L. Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. Journal of Clinical Medicine. 2019; 8(5):671. https://doi.org/10.3390/jcm8050671
Chicago/Turabian StyleTorretta, Sara, Lorenzo Drago, Paola Marchisio, Tullio Ibba, and Lorenzo Pignataro. 2019. "Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease" Journal of Clinical Medicine 8, no. 5: 671. https://doi.org/10.3390/jcm8050671
APA StyleTorretta, S., Drago, L., Marchisio, P., Ibba, T., & Pignataro, L. (2019). Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. Journal of Clinical Medicine, 8(5), 671. https://doi.org/10.3390/jcm8050671






