Osteoarthritis Changes Hip Geometry and Biomechanics Regardless of Bone Mineral Density—A Quantitative Computed Tomography Study
Abstract
1. Introduction
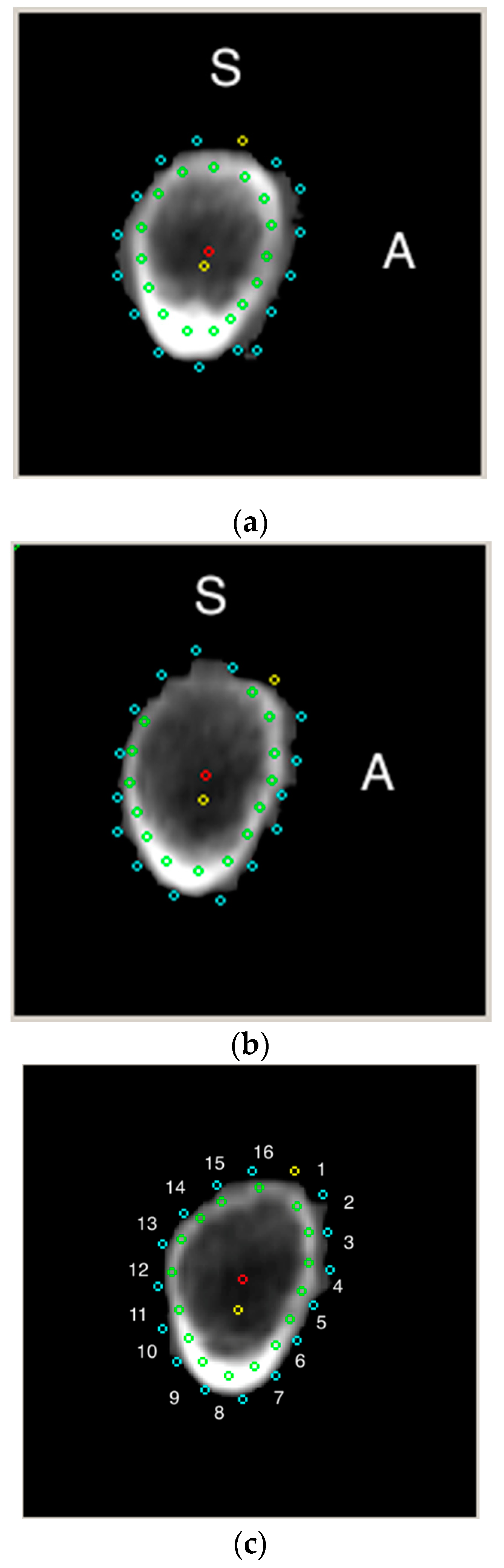
2. Materials and Methods
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Morphological and Densitometric Findings
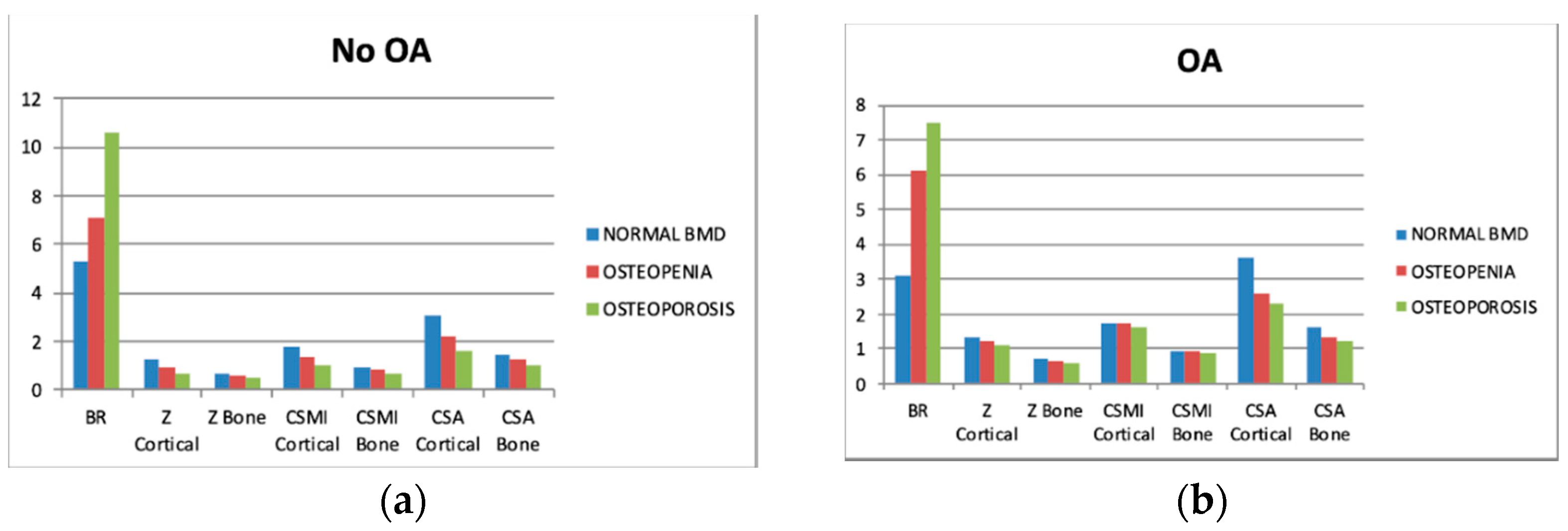
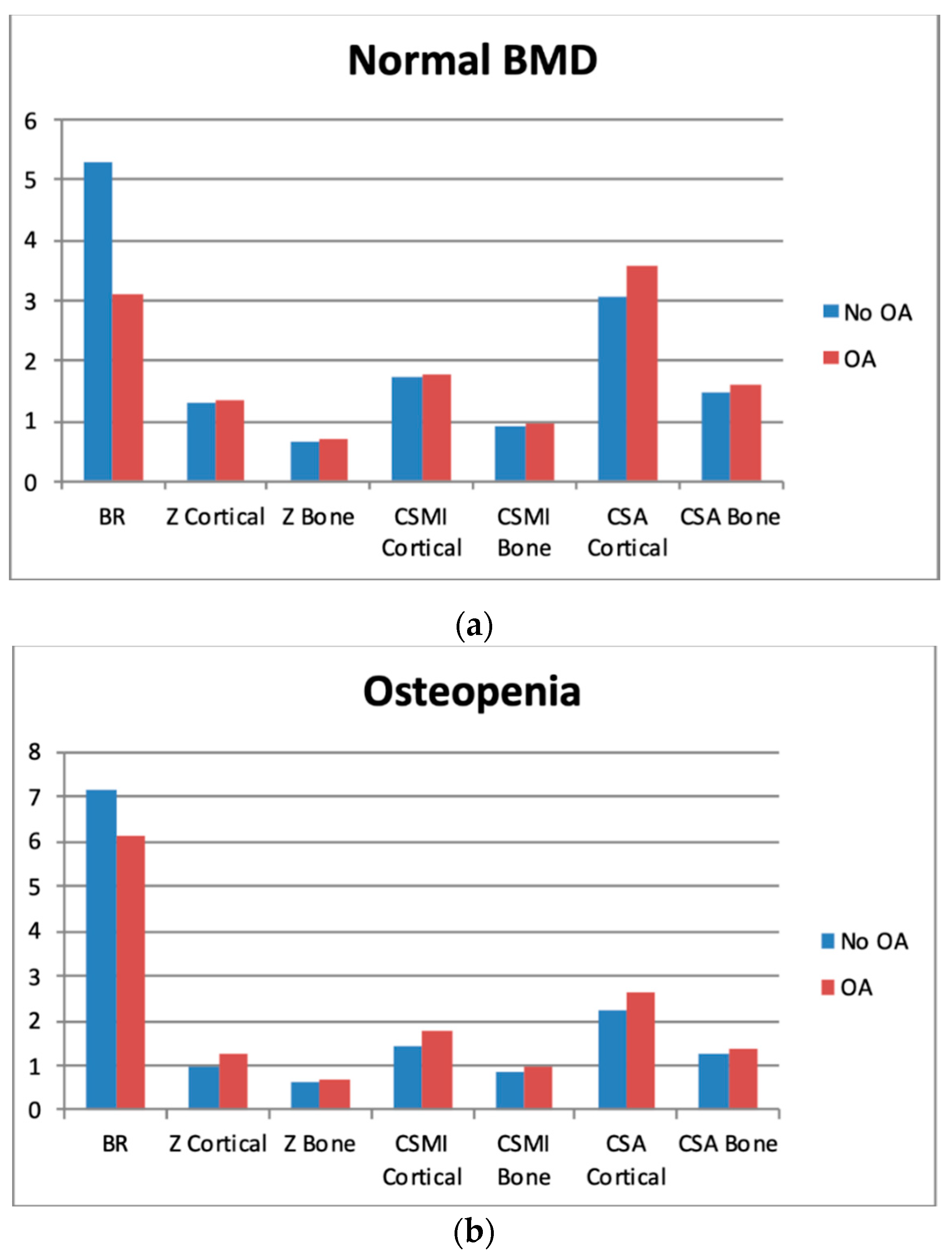
3.3. Biomechanical Findings
3.4. Sectors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Postler, A.; Ramos, A.L.; Goronzy, J.; Gunther, K.P.; Lange, T.; Schmitt, J.; Zink, A.; Hoffmann, F. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: An analysis based on health insurance claims data. Clin. Interv. Aging 2018, 13, 2339–2349. [Google Scholar] [CrossRef]
- Endres, H.; Schneider, O.; Scharf, H.P.; Kaufmann-Kolle, P.; Knapstein, S.; Hermann, C.; Lembeck, B.; Flechtenmacher, J. Hip Osteoarthritis—Epidemiology and Current Medical Care Situation—Health Care Data of 2.4 Million AOK Baden-Wurttemberg Insurees Aged 40 Years or Older. Z. Orthop. Unfall. 2018, 156, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip Osteoarthritis: A Primer. Perm. J. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Kiadaliri, A.A.; Lohmander, L.S.; Moradi-Lakeh, M.; Petersson, I.F.; Englund, M. High and rising burden of hip and knee osteoarthritis in the Nordic region, 1990–2015. Acta Orthop. 2018, 89, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.K.; Sharma, B. Prevalence of osteoporosis in otherwise healthy Indian males aged 50 years and above. Arch. Osteoporos. 2013, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Schray, D.; Neuerburg, C.; Stein, J.; Gosch, M.; Schieker, M.; Bocker, W.; Kammerlander, C. Value of a coordinated management of osteoporosis via Fracture Liaison Service for the treatment of orthogeriatric patients. Eur. J. Trauma Emerg. Surg. 2016, 42, 559–564. [Google Scholar] [CrossRef]
- Cipriani, C.; Pepe, J.; Bertoldo, F.; Bianchi, G.; Cantatore, F.P.; Corrado, A.; Di Stefano, M.; Frediani, B.; Gatti, D.; Giustina, A.; et al. The epidemiology of osteoporosis in Italian postmenopausal women according to the National Bone Health Alliance (NBHA) diagnostic criteria: A multicenter cohort study. J. Endocrinol. Investig. 2018, 41, 431–438. [Google Scholar] [CrossRef]
- Khoo, B.C.; Brown, J.K.; Prince, R.L. Reconsideration of the Effects of Age on Proximal Femur Structure: Implications for Joint Replacement and Hip Fracture. PLoS ONE 2016, 11, e0164949. [Google Scholar] [CrossRef][Green Version]
- Foss, M.V.; Byers, P.D. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann. Rheum. Dis. 1972, 31, 259–264. [Google Scholar] [CrossRef]
- Stewart, A.; Black, A.J. Bone mineral density in osteoarthritis. Curr. Opin. Rheumatol. 2000, 12, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Bultink, I.E.; Lems, W.F. Osteoarthritis and osteoporosis: What is the overlap? Curr. Rheumatol. Rep. 2013, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Borggrefe, J.; de Buhr, T.; Shrestha, S.; Marshall, L.M.; Orwoll, E.; Peters, K.; Black, D.M.; Gluer, C.C.; Osteoporotic Fractures in Men Study Research, G. Association of 3D Geometric Measures Derived From Quantitative Computed Tomography With Hip Fracture Risk in Older Men. J. Bone Miner. Res. 2016, 31, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.F.; Guglielmi, G.; van Kuijk, C.; De Serio, A.; Cammisa, M.; Genant, H.K. Measurement of bone mineral density at the spine and proximal femur by volumetric quantitative computed tomography and dual-energy X-ray absorptiometry in elderly women with and without vertebral fractures. Bone 2002, 30, 247–250. [Google Scholar] [CrossRef]
- Lang, T.F.; Keyak, J.H.; Heitz, M.W.; Augat, P.; Lu, Y.; Mathur, A.; Genant, H.K. Volumetric quantitative computed tomography of the proximal femur: Precision and relation to bone strength. Bone 1997, 21, 101–108. [Google Scholar] [CrossRef]
- Glinkowski, W.; Ciszek, B. Anatomy of the Proximal Femur-geometry and architecture. Morphologic investigation and literature review. Ortop. Traumatol. Rehabil. 2002, 4, 200–208. [Google Scholar] [PubMed]
- Hayes, W.C.; Piazza, S.J.; Zysset, P.K. Biomechanics of fracture risk prediction of the hip and spine by quantitative computed tomography. Radiol. Clin. N. Am. 1991, 29, 1–18. [Google Scholar]
- Kroger, H.; Lunt, M.; Reeve, J.; Dequeker, J.; Adams, J.E.; Birkenhager, J.C.; Diaz Curiel, M.; Felsenberg, D.; Hyldstrup, L.; Kotzki, P.; et al. Bone density reduction in various measurement sites in men and women with osteoporotic fractures of spine and hip: The European quantitation of osteoporosis study. Calcif. Tissue Int. 1999, 64, 191–199. [Google Scholar] [CrossRef]
- Lochmuller, E.M.; Burklein, D.; Kuhn, V.; Glaser, C.; Muller, R.; Gluer, C.C.; Eckstein, F. Mechanical strength of the thoracolumbar spine in the elderly: Prediction from in situ dual-energy X-ray absorptiometry, quantitative computed tomography (QCT), upper and lower limb peripheral QCT, and quantitative ultrasound. Bone 2002, 31, 77–84. [Google Scholar] [CrossRef]
- Khoo, B.C.; Brown, K.; Cann, C.; Zhu, K.; Henzell, S.; Low, V.; Gustafsson, S.; Price, R.I.; Prince, R.L. Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporos. Int. 2009, 20, 1539–1545. [Google Scholar] [CrossRef]
- Adams, J.E. Quantitative computed tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef]
- Prevrhal, S.; Fuerst, T.; Fan, B.; Njeh, C.; Hans, D.; Uffmann, M.; Srivastav, S.; Genant, H.K. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporos. Int. 2001, 12, 28–34. [Google Scholar] [CrossRef]
- Engelke, K.; Adams, J.E.; Armbrecht, G.; Augat, P.; Bogado, C.E.; Bouxsein, M.L.; Felsenberg, D.; Ito, M.; Prevrhal, S.; Hans, D.B.; et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 123–162. [Google Scholar] [CrossRef]
- Johannesdottir, F.; Turmezei, T.; Poole, K.E. Cortical bone assessed with clinical computed tomography at the proximal femur. J. Bone Miner. Res. 2014, 29, 771–783. [Google Scholar] [CrossRef]
- Engelke, K.; Lang, T.; Khosla, S.; Qin, L.; Zysset, P.; Leslie, W.D.; Shepherd, J.A.; Shousboe, J.T. Clinical Use of Quantitative Computed Tomography-Based Advanced Techniques in the Management of Osteoporosis in Adults: The 2015 ISCD Official Positions—Part III. J. Clin. Densitom. 2015, 18, 393–407. [Google Scholar] [CrossRef]
- Marques, E.A.; Gudnason, V.; Sigurdsson, G.; Lang, T.; Johannesdottir, F.; Siggeirsdottir, K.; Launer, L.; Eiriksdottir, G.; Harris, T.B. Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik study. Osteoporos. Int. 2016, 27, 1765–1776. [Google Scholar] [CrossRef]
- Sfeir, J.G.; Drake, M.T.; Atkinson, E.J.; Achenbach, S.J.; Camp, J.J.; Tweed, A.J.; McCready, L.K.; Yu, L.; Adkins, M.C.; Amin, S.; et al. Evaluation of cross-sectional and longitudinal changes in volumetric bone mineral density in postmenopausal women using single- versus dual-energy quantitative computed tomography. Bone 2018, 112, 145–152. [Google Scholar] [CrossRef]
- Fazzalari, N.L.; Forwood, M.R.; Smith, K.; Manthey, B.A.; Herreen, P. Assessment of cancellous bone quality in severe osteoarthrosis: Bone mineral density, mechanics, and microdamage. Bone 1998, 22, 381–388. [Google Scholar] [CrossRef]
- Kaneko, M.; Ohnishi, I.; Matsumoto, T.; Ohashi, S.; Bessho, M.; Hayashi, N.; Tanaka, S. Prediction of proximal femur strength by a quantitative computed tomography-based finite element method—Creation of predicted strength data of the proximal femur according to age range in a normal population. Mod. Rheumatol. 2016, 26, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Glinkowski, W.; Wojnarowski, J. Finite element modeling of strength of proximal femoral end during osteoporosis.(pol.). Post. Osteoartrol. 1995, 7, 61–66. [Google Scholar]
- Cann, C.E.; Adams, J.E.; Brown, J.K.; Brett, A.D. CTXA hip—An extension of classical DXA measurements using quantitative CT. PLoS ONE 2014, 9, e91904. [Google Scholar] [CrossRef]
- Glinkowski, W.; Ciszek, B. The topographic and comparative study of the calcar femorale. Folia Morphol. 1989, 48, 183–191. [Google Scholar]
- Yates, L.B.; Karasik, D.; Beck, T.J.; Cupples, L.A.; Kiel, D.P. Hip structural geometry in old and old-old age: Similarities and differences between men and women. Bone 2007, 41, 722–732. [Google Scholar] [CrossRef][Green Version]
- Johannesdottir, F.; Aspelund, T.; Reeve, J.; Poole, K.E.; Sigurdsson, S.; Harris, T.B.; Gudnason, V.G.; Sigurdsson, G. Similarities and differences between sexes in regional loss of cortical and trabecular bone in the mid-femoral neck: The AGES-Reykjavik longitudinal study. J. Bone Miner. Res. 2013, 28, 2165–2176. [Google Scholar] [CrossRef]
- Johannesdottir, F.; Poole, K.E.; Reeve, J.; Siggeirsdottir, K.; Aspelund, T.; Mogensen, B.; Jonsson, B.Y.; Sigurdsson, S.; Harris, T.B.; Gudnason, V.G.; et al. Distribution of cortical bone in the femoral neck and hip fracture: A prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone 2011, 48, 1268–1276. [Google Scholar] [CrossRef]
- Smith, J.A.; Vento, J.A.; Spencer, R.P.; Tendler, B.E. Aortic calcification contributing to bone densitometry measurement. J. Clin. Densitom. 1999, 2, 181–183. [Google Scholar] [CrossRef]
- Liu, G.; Peacock, M.; Eilam, O.; Dorulla, G.; Braunstein, E.; Johnston, C.C. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos. Int. 1997, 7, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Floriani, I.; Torri, V.; Li, J.; van Kuijk, C.; Genant, H.K.; Lang, T.F. Effect of spinal degenerative changes on volumetric bone mineral density of the central skeleton as measured by quantitative computed tomography. Acta Radiol. 2005, 46, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sugano, N.; Ohzono, K.; Nishii, T.; Haraguchi, K.; Sakai, T.; Ochi, T. Computed-tomography-based computer preoperative planning for total hip arthroplasty. Comput. Aided Surg. 1998, 3, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, A.; Radmer, S.; Wagner, M.; Roessler, T.; Hamm, B.; Sparmann, M. Computed tomography for preoperative planning in total hip arthroplasty: What radiologists need to know. Skelet. Radiol. 2014, 43, 1041–1051. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Maeda, T.; Kajino, Y.; Fujita, K.; Hasegawa, K.; Yamamoto, T.; Tsuchiya, H. Value of computed tomography-based three-dimensional surgical preoperative planning software in total hip arthroplasty with developmental dysplasia of the hip. J. Orthop. Sci. 2015, 20, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Javaid, M.K.; Judge, A.; Murray, D.; Carr, A.; Cooper, C.; Arden, N.K. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: Population based retrospective cohort study. BMJ 2011, 343, d7222. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO); The Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Hangartner, T.N.; Gilsanz, V. Evaluation of cortical bone by computed tomography. J. Bone Miner. Res. 1996, 11, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narloch, J.; Glinkowski, W.M. Osteoarthritis Changes Hip Geometry and Biomechanics Regardless of Bone Mineral Density—A Quantitative Computed Tomography Study. J. Clin. Med. 2019, 8, 669. https://doi.org/10.3390/jcm8050669
Narloch J, Glinkowski WM. Osteoarthritis Changes Hip Geometry and Biomechanics Regardless of Bone Mineral Density—A Quantitative Computed Tomography Study. Journal of Clinical Medicine. 2019; 8(5):669. https://doi.org/10.3390/jcm8050669
Chicago/Turabian StyleNarloch, Jerzy, and Wojciech M. Glinkowski. 2019. "Osteoarthritis Changes Hip Geometry and Biomechanics Regardless of Bone Mineral Density—A Quantitative Computed Tomography Study" Journal of Clinical Medicine 8, no. 5: 669. https://doi.org/10.3390/jcm8050669
APA StyleNarloch, J., & Glinkowski, W. M. (2019). Osteoarthritis Changes Hip Geometry and Biomechanics Regardless of Bone Mineral Density—A Quantitative Computed Tomography Study. Journal of Clinical Medicine, 8(5), 669. https://doi.org/10.3390/jcm8050669





