Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
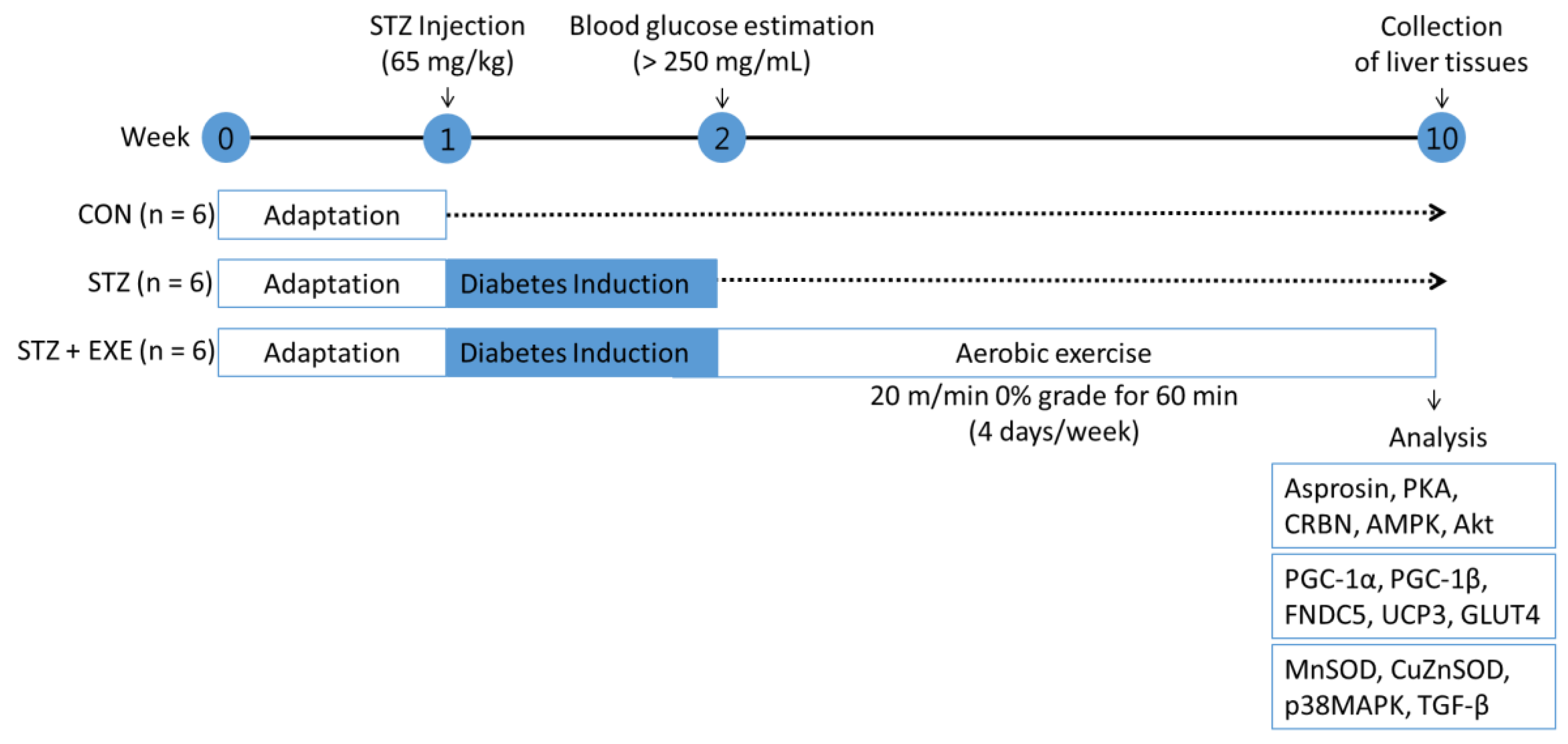
2.2. Diabetes Model
2.3. Aerobic Exercise Training
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
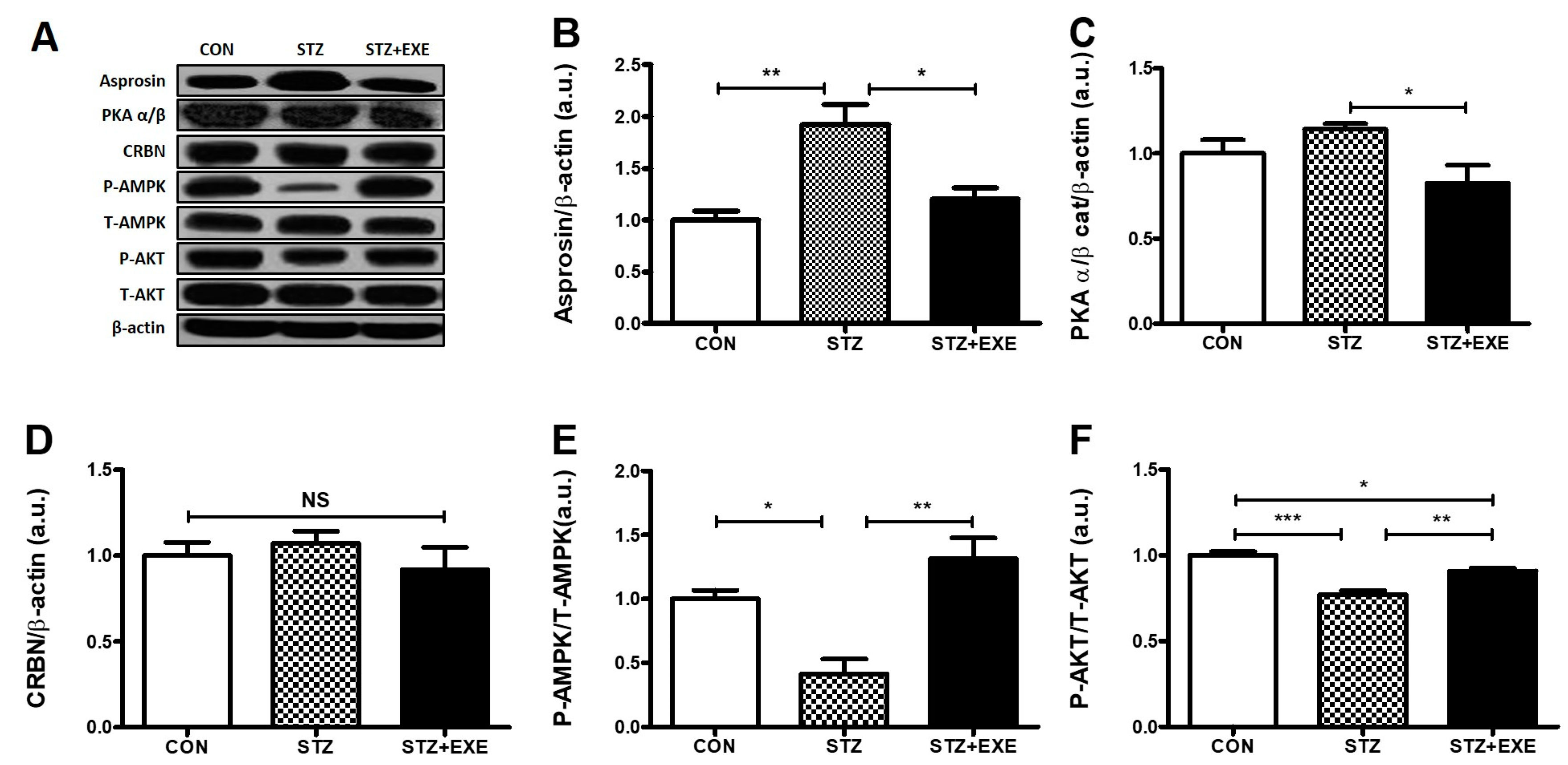
3.1. Aerobic Exercise Training Decreased Asprosin and PKA Levels but Increased AMPK and Akt Levels in the Liver of T1DM Rats
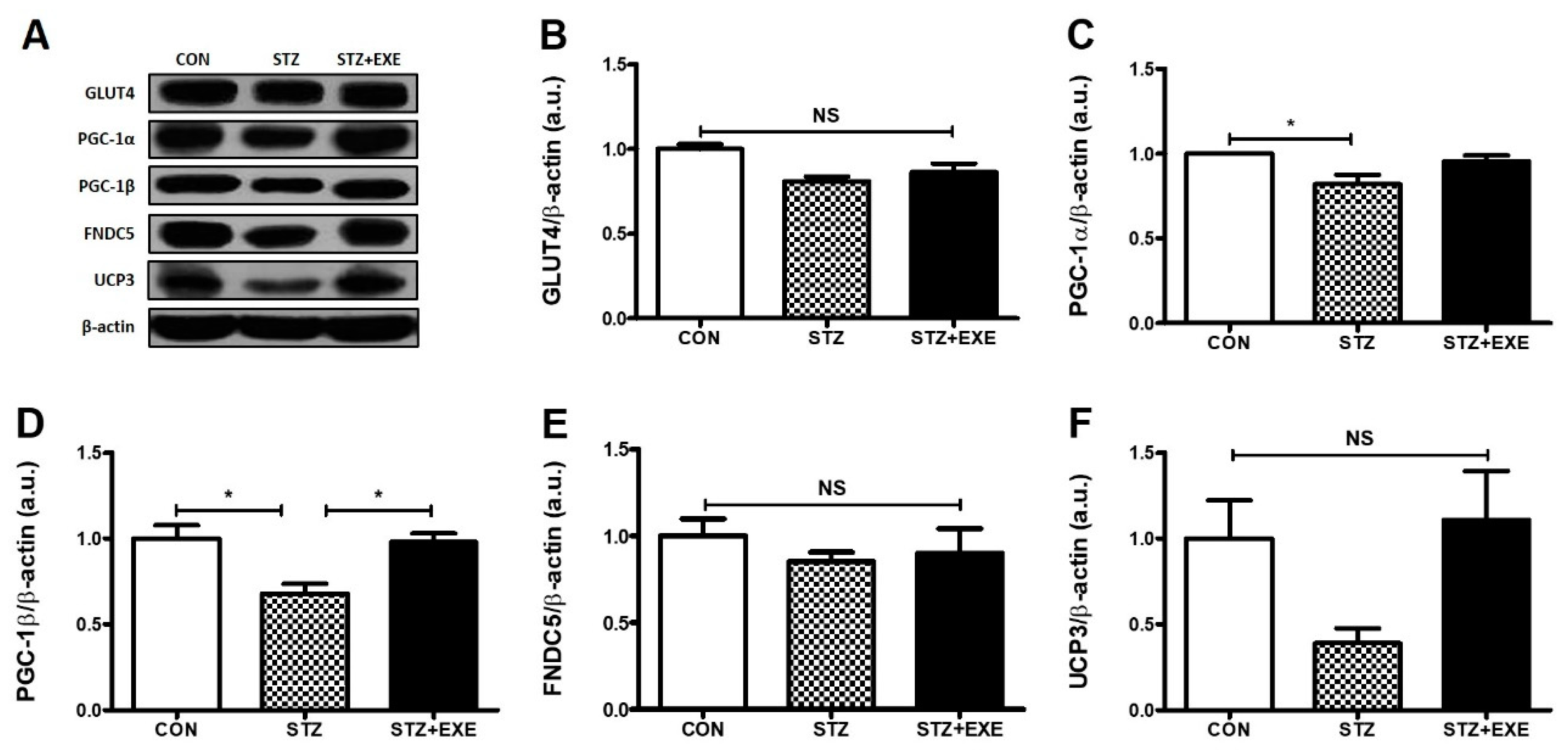
3.2. Aerobic Exercise Training Increased PGC-1β Levels in the Liver of T1DM Rats
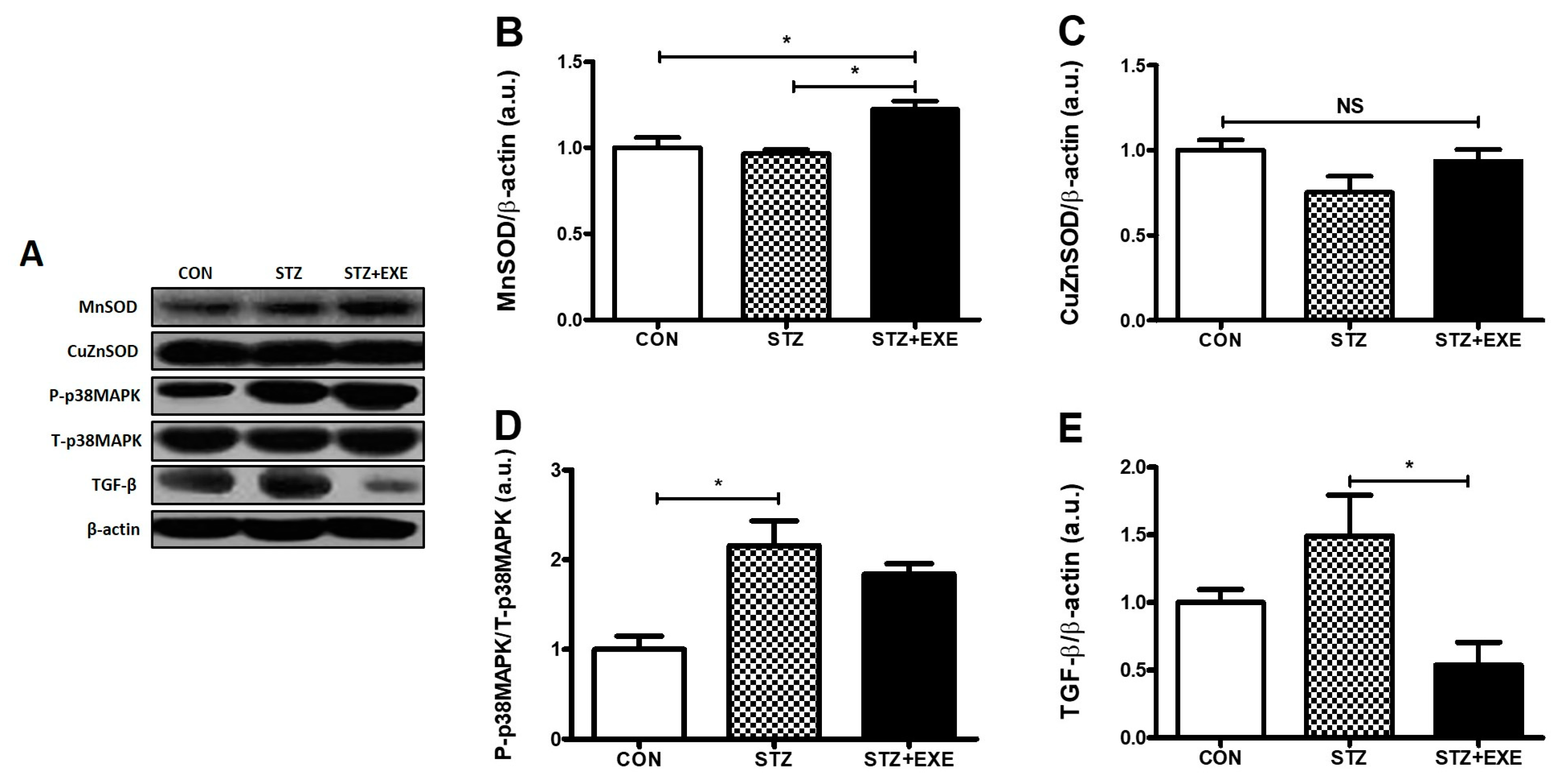
3.3. Aerobic Exercise Training Increased MnSOD Levels and Decreased TGF-β Levels in the Liver of T1DM Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Debnam, E.S.; Smith, M.W.; Sharp, P.A.; Srai, S.K.; Turvey, A.; Keable, S.J. The effects of streptozotocin diabetes on sodium-glucose transporter (SGLT1) expression and function in rat jejunal and ileal villus-attached enterocytes. Pflugers Arch. 1995, 430, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. 2010, 27, 136–142. [Google Scholar] [PubMed]
- Dey, A.; Chandrasekaran, K. Hyperglycemia induced changes in liver: In vivo and in vitro studies. Curr. Diabetes Rev. 2009, 5, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; Del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Zhou, N.; Fu, Y.; Cheng, X. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin. Chim. Acta 2019, 489, 183–188. [Google Scholar] [CrossRef]
- Duerrschmid, C.; He, Y.; Wang, C.; Li, C.; Bournat, J.C.; Romere, C.; Saha, P.K.; Lee, M.E.; Phillips, K.J.; Jain, M.; et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017, 23, 1444–1453. [Google Scholar] [PubMed]
- Greenhill, C. Liver: Asprosin—New hormone involved in hepatic glucose release. Nat. Rev. Endocrinol. 2016, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S. Adipose tissue in 2016: Advances in the understanding of adipose tissue biology. Nat. Rev. Endocrinol. 2017, 13, 69–70. [Google Scholar] [CrossRef]
- He, L.; Chang, E.; Peng, J.; An, H.; McMillin, S.M.; Radovick, S.; Stratakis, C.A.; Wondisford, F.E. Activation of the cAMP-PKA pathway antagonizes metformin suppression of hepatic glucose production. J. Biol. Chem. 2016, 291, 10562–10570. [Google Scholar] [CrossRef]
- Unger, R.H.; Aguilar-Parada, E.; Muller, W.A.; Eisentraut, A.M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J. Clin. Investig. 1970, 49, 837–848. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Ko, J.R.; Seo, D.Y.; Park, S.H.; Kwak, H.B.; Kim, M.; Ko, K.S.; Rhee, B.D.; Han, J. Aerobic exercise training decreases cereblon and increases AMPK signaling in the skeletal muscle of STZ-induced diabetic rats. Biochem. Biophys Res. Commun. 2018, 501, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Biedenbach, J.; Huber, Y.; Straub, B.K.; Galle, P.R.; Simon, P.; Schattenberg, J.M. Voluntary exercise in mice fed an obesogenic diet alters the hepatic immune phenotype and improves metabolic parameters an animal model of life style intervention in NAFLD. Sci. Rep. 2019, 9, 4007. [Google Scholar] [CrossRef]
- Palmer, W.K. Introduction to the symposium: Cyclic AMP regulation of fuel metabolism during exercise. Med. Sci. Sports Exerc. 1988, 20, 523–524. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Acute anaerobic exercise affects the secretion of asprosin, irisin, and other cytokines—A comparison between sexes. Front. Physiol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Huang, C.; Wang, C.; Zheng, J.; Zhang, P.; Xu, Y.; Chen, H.; Shen, W. Ginsenoside Rg3 improves cardiac mitochondrial population quality: Mimetic exercise training. Biochem. Biophys. Res. Commun. 2013, 441, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Kwak, H.B.; Park, H.; Seo, K.W.; Noh, Y.H.; Song, K.M.; Ryu, J.K.; Ko, K.S.; Rhee, B.D.; et al. Exercise training causes a partial improvement through increasing testosterone and eNOS for erectile function in middle-aged rats. Exp. Gerontol. 2018, 108, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Damm, E.; Buech, T.R.; Gudermann, T.; Breit, A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol. Endocrinol. 2012, 26, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Cheng, H.; Lv, H.; Cheng, G.; Ci, X. Nrf2-mediated liver protection by esculentoside a against acetaminophen toxicity through the AMPK/Akt/GSK3beta pathway. Free Radic. Biol. Med. 2016, 101, 401–412. [Google Scholar] [CrossRef]
- Vozenin-Brotons, M.C.; Sivan, V.; Gault, N.; Renard, C.; Geffrotin, C.; Delanian, S.; Lefaix, J.L.; Martin, M. Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts. Free Radic. Biol. Med. 2001, 30, 30–42. [Google Scholar] [CrossRef]
- Sundaram, B.; Singhal, K.; Sandhir, R. Ameliorating effect of chromium administration on hepatic glucose metabolism in streptozotocin-induced experimental diabetes. Biofactors 2012, 38, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cipponeri, E.; Vitturi, N.; Mariano, V.; Boscari, F.; Galasso, S.; Crepaldi, C.; Fadini, G.P.; Vigili de Kreutzenberg, S.; Marescotti, M.C.; Iori, E.; et al. Vitamin D status and non-alcoholic fatty liver disease in patients with type 1 diabetes. J. Endocrinol. Invest. 2019. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ashford, M.L. AMPK: Regulating energy balance at the cellular and whole body levels. Physiology 2014, 29, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, H.; Xiong, X.; Qiu, Y.; Liao, Y.; Chen, Y.; Zheng, Y.; Zheng, H. Plasma asprosin concentrations areincreased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediat. Inflamm. 2018, 9471583. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef]
- Ferretti, A.C.; Tonucci, F.M.; Hidalgo, F.; Almada, E.; Larocca, M.C.; Favre, C. AMPK and PKA interaction in the regulation of survival of liver cancer cells subjected to glucose starvation. Oncotarget 2016, 7, 17815–17828. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Zhang, C.; Zhang, Z.; Tan, Y.; Feng, W.; Skibba, M.; Xin, Y.; Cai, L. The protective effect of FGF21 on diabetes-induced male germ cell apoptosis is associated with up-regulated testicular AKT and AMPK/Sirt1/PGC-1alpha signaling. Endocrinology 2015, 156, 1156–1170. [Google Scholar] [CrossRef]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007, 17, 1646–1656. [Google Scholar] [CrossRef]
- Yadav, H.; Devalaraja, S.; Chung, S.T.; Rane, S.G. TGF-beta1/Smad3 Pathway Targets PP2A-AMPK-FoxO1 signaling to regulate hepatic gluconeogenesis. J. Biol. Chem. 2017, 292, 3420–3432. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, Y.M.; Yan, H.; Yang, W.B.; Shen, Z.; Sudath, A.D.; Guo, S. Regulation of hepatic glucose production by transforming growth factor beta 1 via protein kinase A. Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Duca, F.A.; Cote, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.R.; Seo, D.Y.; Kim, T.N.; Park, S.H.; Kwak, H.-B.; Ko, K.S.; Rhee, B.D.; Han, J. Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats. J. Clin. Med. 2019, 8, 666. https://doi.org/10.3390/jcm8050666
Ko JR, Seo DY, Kim TN, Park SH, Kwak H-B, Ko KS, Rhee BD, Han J. Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats. Journal of Clinical Medicine. 2019; 8(5):666. https://doi.org/10.3390/jcm8050666
Chicago/Turabian StyleKo, Jeong Rim, Dae Yun Seo, Tae Nyun Kim, Se Hwan Park, Hyo-Bum Kwak, Kyung Soo Ko, Byoung Doo Rhee, and Jin Han. 2019. "Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats" Journal of Clinical Medicine 8, no. 5: 666. https://doi.org/10.3390/jcm8050666
APA StyleKo, J. R., Seo, D. Y., Kim, T. N., Park, S. H., Kwak, H.-B., Ko, K. S., Rhee, B. D., & Han, J. (2019). Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats. Journal of Clinical Medicine, 8(5), 666. https://doi.org/10.3390/jcm8050666




