Trends of Lipophilic, Antioxidant and Hematological Parameters Associated with Conventional and Electronic Smoking Habits in Middle-Age Romanians
Abstract
1. Introduction
2. Study Design and Participants Analysis
2.1. Study Design
2.2. Ethical Statement
2.3. Blood Samples Analysis
2.4. Statistical Analysis
3. Results
3.1. Characterisation of Study Volunteers
3.2. Characterisation of Blood Components
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Analiza de situatie. Ziua Nationala fara tutun 2017. Available online: http://insp.gov.ro/sites/cnepss/wp-content/uploads/2017/10/ANALIZA-DE-SITUA%C5%A2IE-TUTUN.pdf (accessed on 3 March 2019).
- Share of individuals who currently smoke cigarettes, cigars, cigarillos or a pipe in selected European countries in 2017. Available online: https://www.statista.com/statistics/433390/individuals-who-currently-smoke-cigarettes-in-european-countries/ (accessed on 3 March 2019).
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Szczeklik, A.; Gryglewski, R.J.; Domagala, B.; Dworski, R.; Basista, M. Dietary supplementation with vitamin E in hyperlipoproteinemias: Effects on plasma lipid peroxides, antioxidant activity, prostacyclin generation and platelet aggregability. Thromb Haemost. 1985, 54, 425–430. [Google Scholar] [CrossRef]
- Liu, M.; Wallmon, A.; Olsson-Mortlock, C.; Wallin, R.; Saldeen, T. Mixed tocopherols inhibit platelet aggregation in humans: Potential mechanisms. Am. J. Clin. Nutr. 2003, 77, 700–706. [Google Scholar] [CrossRef]
- Qasim, H.; Karim, Z.A.; Rivera, J.O.; Khasawneh, F.T.; Alshbool, F.Z. Impact of electronic cigarettes on the cardiovascular system. J. Am. Heart Assoc. 2017, 6, e006353. [Google Scholar] [CrossRef] [PubMed]
- Voigt, K. Smoking norms and the regulation of e-cigarettes. Am. J. Public Health 2015, 105, 1967–1972. [Google Scholar] [CrossRef]
- Burton, A. Does the smoke ever really clear? Thirdhand smoke exposure raises new concerns. Environ. Health Perspect. 2011, 119, A70–A74. [Google Scholar] [CrossRef]
- Protano, C.; Vitali, M. The new danger of thirdhand smoke: Why passive smoking does not stop at secondhand smoke. Environ. Health Perspect. 2011, 119, a422. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.E.; Quintana, P.J.E.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Bals, R. Basic science of electronic cigarettes: Assessment in cell culture and in vivo models. Respir Res. 2016, 17, 127. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Azimi, S.; Mehdipour, M.; Amini, S.; Elmi, Z.; Namazi, Z. Effect of cigarette smoke on salivary total antioxidant capacity. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 9, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, R.; Pandurang, P.; Kamath, S.U.; Goyal, R.; Ballal, S.; Shanbhogue, A.Y.; Kamath, U.; Bhat, G.S.; Bhat, K.M. Association of cigarette smoking with superoxide dismutase enzyme levels in subjects with chronic periodontitis. J. Periodontol. 2009, 80, 657–662. [Google Scholar] [CrossRef]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2018, 50, e12926. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, B.; Pauwels, F.; Vaneechoutte, M.; Van Beeumen, J.J. Exogenous glutathione completes the defense against oxidative stress in haemophilus influenza. J. Bacteriol. 2003, 185, 1572–1581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kariya, C.; Chu, H.W.; Huang, J.; Leitner, H.; Martin, R.J.; Day, B.J. Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress. Infect. Immun. 2008, 76, 4455–4462. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Amini, H.; Heydarpour, P.; Amini Chermahini, F.; Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2018, 18, 32126–32133. [Google Scholar] [CrossRef]
- Preston, A.M. Cigarette smoking-nutritional implications. Prog. Food Nutr. Sci. 1991, 15, 183–217. [Google Scholar] [PubMed]
- Flouris, A.D.; Poulianiti, K.P.; Chorti, M.S.; Jamurtas, A.Z.; Kouretas, D.; Owolabi, E.O.; Tzatzarakis, M.N.; Tsatsakis, A.M.; Koutedakis, Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012, 50, 3600–3603. [Google Scholar] [CrossRef] [PubMed]
- Gossett, L.K.; Johnson, H.M.; Piper, M.E.; Fiore, M.C.; Baker, T.B.; Stein, J.H. Smoking intensity and lipoprotein abnormalities in active smokers. J. Clin. Lipidol. 2009, 3, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Luzardo, O.P.; González-Antuña, A.; Zumbado, M.; Rogozea, L.; Floroian, L.; Alexandrescu, D.; Moga, M.; Gaman, L.; Radoi, M.; et al. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ. Res. 2018, 166, 269–275. [Google Scholar] [CrossRef]
- Olimid, A.P.; Rogozea, L.M.; Olimid, D.A. Ethical approach to the genetic, biometric and health data protection and processing in the new EU General Data Protection Regulation. Romanian journal of morphology and embryology. Revue Roum. Morphol. Embryol. 2018, 59, 631–636. [Google Scholar]
- Popescu, I.G.; Sechel, G.; Leaşu, F.G.; Ţânţu, M.M.; Cotoi, B.V.; Rogozea, L.M. Correlations on the protection of personal data and intellectual property rights in medical research. Rom. J. Morphol. Embryol. 2018, 59, 1001–1005. [Google Scholar]
- Laborator Synevo. Referintele specifice tehnologiei de lucru utilizate. 2010. Available online: https://www.synevo.ro/hemograma/ (accessed on 18 December 2018).
- Laborator Synevo. Referinte specifice tehnologiei de lucru utilizate 2015. Available online: https://pim-eservices.roche.com/eLD_SF/ro/en/Documents/GetDocument?documentId=96a814bd-13b5-e711-b392-00215a9b3428 (accessed on 18 December 2018).
- Cleeman, J.I. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA 2001, 285, y2486–y2497. [Google Scholar]
- Cobas Integra System. Available online: http://www.roche-diagnostics.ch/content/dam/corporate/roche-dia_ch/documents/broschueren/professional_diagnostics/serumarbeitsplatz/swa_systeme/cobas-integra-400-plus-analyzer/03273873001_EN_EA_COBAS-INTEGRA-400-plus.pdf (accessed on 18 December 2018).
- Turak, O.; Ozcan, F.; Tok, D.; Işleyen, A.; Sökmen, E.; Taşoğlu, I.; Aydoğdu, S.; Sen, N.; McFann, K.; Johnson, R.J.; et al. Serum uric acid, inflammation, and nondipping circadian pattern in essential hypertension. J. Clin. Hypertens. 2013, 15, 7–13. [Google Scholar] [CrossRef]
- Vitamin A/E HPLC Kit. Available online: http://www.immundiagnostik.com/fileadmin/pdf/Vitamin%20AE_KC1600.pdf (accessed on 18 December 2018).
- Agilent 1290 Infinity II LC. Available online: http://www.ingenieria-analitica.com/downloads/dl/file/id/2365/product/321/1290_infinity_ii_lc_system_manual_and_quick_reference.pdf (accessed on 18 December 2018).
- FREND™ Vitamin D. Available online: http://www.nanoentek.com/upload/product/39/FREND%20Vitamin%20D_Package%20insert(V.0.1).pdf (accessed on 18 December 2018).
- Di Lorenzo, C.; Badea, M.; Colombo, F.; Orgiu, F.; Frigerio, G.; Pastor, R.F.; Restani, P. Antioxidant activity of wine assessed by different in vitro methods. In Proceedings of the 40th World Congress of Vine and Wine, Sofia, Bulgaria, 29 May–2 June 2017. [Google Scholar]
- Finak, G.; Langweiler, M.; Jaimes, M.; Malek, M.; Taghiyar, J.; Korin, Y.; Raddassi, K.; Devine, L.; Obermoser, G.; Pekalski, M.L.; et al. Standardizing flow cytometry Immunophenotyping analysis from the Human ImmunoPhenotyping Consortium. Sci. Rep. 2016, 6, 20686. [Google Scholar] [CrossRef]
- Rajwa, B.; Wallace, P.K.; Griffiths, E.A.; Dundar, M. Automated assessment of disease progression in acute myeloid leukemia by probabilistic analysis of flow cytometry data. IEEE 2017, 64, 1089–1098. [Google Scholar] [CrossRef]
- Gormley, N.J.; Turley, D.M.; Dickey, J.S.; Farrell, A.T.; Reaman, G.H.; Stafford, E.; Carrington, L.; Marti, G.E. Regulatory perspective on minimal residual disease flow cytometry testing in multiple myeloma, Cytometry. Part B Clin. Cytometry 2016, 90, 73–80. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: A report of the Surgeon General. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24455788 (accessed on 18 December 2018).
- Gregory Lande R, Army Substance Abuse Program. 2018. Available online: https://emedicine.medscape.com/article/287555-overview#a4 (accessed on 18 December 2018).
- Smokeless Tobacco Use in the United States. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/use_us/index.htm (accessed on 18 December 2018).
- Use of E-Cigarettes Rising Among Middle and High School Students. Available online: https://www.cancer.org/latest-news/use-of-e-cigarettes-rising-among-middle-and-high-school-students.html (accessed on 18 December 2018).
- Alexander, J.P.; Williams, P.; Lee, Y.O. Youth who use e-cigarettes regularly: A qualitative study of behavior, attitudes, and familial norms. Prev. Med. Rep. 2019, 13, 93–97. [Google Scholar] [CrossRef]
- Simmons, V.N.; Quinn, G.P.; Harrell, P.T.; Meltzer, L.R.; Correa, J.B.; Unrod, M.; Brandon, T.H. E-cigarette use in adults: A qualitative study of users’ perceptions and future use intentions. Addict. Res. Theory. 2016, 24, 313–321. [Google Scholar] [CrossRef]
- Dawkins, L.; Cox, S.; Goniewicz, M.; McRobbie, H.; Kimber, C.; Doig, M.; Kosmider, L. ‘Real world’ compensatory behaviour with low nicotine concentration e-liquid: Subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction 2018, 113, 1874–1882. [Google Scholar] [CrossRef]
- Avino, P.; Scungio, M.; Stabile, L.; Cortellessa, G.; Buonanno, G.; Manigrasso, M. Second-hand aerosol from tobacco and electronic cigarettes: Evaluation of the smoker emission rates and doses and lung cancer risk of passive smokers and vapers. Sci. Total Environ. 2018, 9, 137–147. [Google Scholar] [CrossRef]
- WHO Report on the Global Tobacco Epidemic. Available online: https://www.who.int/tobacco/surveillance/policy/country_profile/rou.pdf (accessed on 18 December 2018).
- Muttarak, R.; Gallus, S.; Franchi, M.; Faggiano, F.; Pacifici, R.; Colombo, P.; La Vecchia, C. Why do smokers start? Eur. J. Cancer Prev. 2013, 22, 181–186. [Google Scholar] [CrossRef] [PubMed]
- İçmeli, Ö.S.; Türker, H.; Gündoğuş, B.; Çiftci, M.; Aka Aktürk, Ü. Behaviours and opinions of adolescent students on smoking. Tuberk Toraks. 2016, 64, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tobacco Control Committee of the American Thoracic Society; Folan, P.; Fardellone, C.; Spatarella, A. Withdrawal and Relapse from Tobacco Use. Available online: https://www.thoracic.org/patients/patient-resources/resources/withdrawal-and-relapse.pdf (accessed on 18 December 2018).
- Poulianiti, K.; Karatzaferi, C.; Flouris, AD.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. Antioxidant responses following active and passive smoking of tobacco and electronic cigarettes. Toxicol. Mech. Methods 2016, 26, 455–461. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21452462 (accessed on 18 December 2018).
- Gepner, A.D.; Piper, M.E.; Johnson, H.M.; Fiore, M.C.; Baker, T.B.; Stein, J.H. Effects of smoking and smoking cessation on lipids and lipoproteins: Outcomes from a randomized clinical trial. Am. Heart J. 2011, 161, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.Y.; Palomaki, G.E.; Johnson, A.M.; Haddow, J.E. Cigarette smoking-associated changes in blood lipid and lipoprotein levels in the 8- to 19-year-old age group: A meta-analysis. Pediatrics 1990, 85, 155–158. [Google Scholar] [PubMed]
- Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310413/ (accessed on 18 December 2018).
- Craig, W.Y.; Palomaki, G.E.; Haddow, J.E. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ 1989, 298, 784–788. [Google Scholar] [CrossRef] [PubMed]
- DeJarnett, N.; Conklin, D.J.; Riggs, D.W.; Myers, J.A.; O’Toole, T.E.; Hamzeh, I.; Wagner, S.; Chugh, A.; Ramos, K.S.; Srivastava, S.; et al. Acrolein exposure is associated with increased cardiovascular disease risk. J. Am. Heart Assoc. 2014, 3, e000934. [Google Scholar] [CrossRef] [PubMed]
- How Smoking Affects Your Cholesterol and Heart. Available online: https://www.verywellhealth.com/how-does-smoking-affect-your-cholesterol-and-heart-698284 (accessed on 18 December 2018).
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Fu, X.; McDonald, T.O.; Green, P.S.; Uchida, K.; O’Brien, K.D.; Oram, J.F.; Heinecke, J.W. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J. Biol Chem. 2005, 280, 36386–36396. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Holme, R.L.; Chen, Y.; Thomas, M.J.; Sorci-Thomas, M.G.; Silverstein, R.L.; Pritchard, K.A., Jr.; Sahoo, D. Acrolein impairs the cholesterol transport functions of high density lipoproteins. PLoS ONE 2015, 10, e0123138. [Google Scholar] [CrossRef]
- Raveh, O.; Pinchuk, I.; Schnitzer, E.; Fainaru, M.; Schaffer, Z.; Lichtenberg, D. Kinetic analysis of copper-induced peroxidation of HDL, autoaccelerated and tocopherol-mediated peroxidation. Free Radic. Biol. Med. 2000, 29, 131–146. [Google Scholar] [CrossRef]
- Shao, B.; Heinecke, J.W. Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway. J. Proteom. 2011, 74, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, G.S.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans-an overview. Environ. Sci Pollut. Res. Int. 2013, 20, 4356–4369. [Google Scholar] [CrossRef]
- He, B.M.; Zhao, H.P.; Peng, Z.Y. Effects of cigarette smoking on HDL quantity and function: Implications for atherosclerosis. J. Cell. Biochem. 2013, 114, 2431–2436. [Google Scholar] [CrossRef]
- Forey, B.A.; Fry, J.S.; Peter, N.L.; Thornton, A.J.; Coombs, K.J. The effect of quitting smoking on HDL-cholesterol—A review based on within-subject changes. Biomark Res. 2013, 1, 26. [Google Scholar] [CrossRef]
- Chelland Campbell, S.; Moffatt, R.J.; Stamford, B.A. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis 2008, 201, 225–235. [Google Scholar] [CrossRef]
- Hellsten, Y.; Tullson, P.C.; Richter, E.A.; Bangsbo, J. Oxidation of urate in human skeletal muscle during exercise. Free Radic. Biol. Med. 1997, 22, 169–174. [Google Scholar] [CrossRef]
- Mathru, M.; Dries, D.J.; Barnes, L.; Tonino, P.; Sukhani, R.; Rooney, M.W. Tourniquet-induced exsanguination in patients requiring lower limb surgery. An ischemia–reperfusion model of oxidant and antioxidant metabolism. Anesthesiology 1996, 84, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Waring, W.S.; Convery, A.; Mishra, V.; Shenkin, A.; Webb, D.J.; Maxwell, S.R. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin. Sci. 2003, 105, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Haj Mouhamed, D.; Ezzaher, A.; Neffati, F.; Douki, W.; Gaha, L.; Fadhel Najjar, M. Effect of cigarette smoking on plasma uric acid concentrations. Environ. Health Prev. Med. 2011, 16, 307–312. [Google Scholar] [CrossRef]
- Waring, W.S.; McKnight, J.A.; Webb, D.J.; Maxwell, S.R.J. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 2006, 55, 3127–3132. [Google Scholar] [CrossRef]
- Kirschbaum, B. Renal regulation of plasma total antioxidant capacity. Med. Hypotheses 2001, 56, 625–629. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta. 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Boldt, J. Use of albumin: An update. Br. J. Anaesth. 2010, 104, 276–284. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensiv. Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Turell, L.; Carballal, S.; Botti, H.; Radi, R.; Alvarez, B. Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz. J. Med. Biol. Res. 2009, 42, 305–311. [Google Scholar] [CrossRef]
- Aldini, G.; Vistoli, G.; Regazzoni, L.; Gamberoni, L.; Facino, R.M.; Yamaguchi, S.; Uchida, K.; Carini, M. Albumin is the main nucleophilic target of human plasma: A protective role against pro-atherogenic electrophilic reactive carbonyl species? Chem. Res. Toxicol. 2008, 2, 824–835. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986. [Google Scholar] [CrossRef]
- Karademirci, M.; Kutlu, R.; Kilinc, I. Relationship between smoking and total antioxidant status, total oxidant status, oxidative stress index, vit C, vit E. Clin. Respir. J. 2018, 12, 2006–2012. [Google Scholar] [CrossRef]
- Bruno, R.S.; Traber, M.G. Cigarette smoke alters human vitamin E requirements. J. Nutr. 2005, 135, 671–674. [Google Scholar] [CrossRef]
- Yokus, B.; Mete, N.; Cakir, U.D.; Toprak, G. Effects of active and passive smoking on antioxidant enzymes and antioxidant micronutrients. Biotechnol. Biotechnol. Equip. 2005, 117–123. [Google Scholar] [CrossRef]
- van Antwerpen, V.L.; Theron, A.J.; Richards, G.A.; Steenkamp, K.J.; van der Merwe, C.A. Vitamin E, pulmonary functions, and phagocyte-mediated oxidative stress in smokers and nonsmokers. Free Radic. Biol. Med. 1995, 31, 935–941. [Google Scholar] [CrossRef]
- Reilly, M.; Delany, N.; Lawson, J.A. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 1996, 94, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wurzel, H.; Yeh, C.C.; Gairola, C.; Chow, C.K. Oxidative damage and antioxidant status in the lungs and bronchoalveolar lavage fluid of rats exposed chronically to cigarette smoke. J. Biochem. Toxicol. 1995, 10, 11–17. [Google Scholar]
- Traber, M.G.; Ramakrishnan, R.; Kayden, H.J. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1994, 91, 10005–10008. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, Y.M.; Hall, W.L.; Proteggente, A.R.; Lodge, J.K. Cigarette smokers have decreased lymphocyte and platelet alpha-tocopherol levels and increased excretion of the gamma-tocopherol metabolite gamma-carboxyethyl-hydroxychroman (gamma-CEHC). Free Radic. Res. 2004, 38, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Traber, M.G. Vitamin E biokinetics, oxidative stress and cigarette smoking. Pathophysiology 2006, 13, 143–149. [Google Scholar] [CrossRef]
- Schwartz, J.; Weiss, S.T. Cigarette smoking and peripheral blood leukocyte differentials. Ann. Epidemiol. 1994, 4, 236–242. [Google Scholar] [CrossRef]
- Petitti, D.B.; Kipp, H. The leukocyte count: Associations with intensity of smoking and persistence of effect after quitting. Am. J. Epidemiol. 1986, 123, 89–95. [Google Scholar] [CrossRef]
- Freedman, D.S.; Flanders, W.D.; Barboriak, J.J.; Malarcher, A.M.; Gates, A.L. Cigarette smoking and leukocyte subpopulations in men. Ann. Epidemiol. 1996, 6, 299–306. [Google Scholar] [CrossRef]
- Lao, X.Q.; Jiang, C.Q.; Zhang, W.S. Smoking, smoking cessation and inflammatory markers in older Chinese men: The Guangzhou biobank cohort study. Atherosclerosis 2009, 203, 304–310. [Google Scholar] [CrossRef]
- Corre, F.; Lellouch, J.; Schwartz, D. Smoking and leucocyte counts. Lancet 1971, 2, 632–634. [Google Scholar] [CrossRef]
- Nielsen, H. A quantitative and qualitative study of blood monocytes in smokers. Eur. J. Respir. Dis. 1985, 66, 3277332. [Google Scholar]
- Bridges, R.B.; Wyatt, R.J.; Rehm, S.R. Effect of smoking on peripheral blood leucocytes and serum antiproteases. Eur. J. Respiv. Dis. 1985, 66, 24–33. [Google Scholar]
- Hallgren, R.; Bjermer, L.; Lundgren, R.; Venge, P. The eosinophil component of the alveolitis in idiopathic pulmonary lung fibrosis. Am. Rev. Respir. Dis. 1989, 139, 373–377. [Google Scholar] [CrossRef]
- Hubcher, T. Role of the eosinophil in the allergic reactions: II. Release of prostaglandins from human eosinophilic leucocytes. J. Immunol. 1975, 114, 1389–1393. [Google Scholar]
- Kirby, J.G.; Hargreave, F.E.; Gleich, G.J.; O’Byrne, P.M. Bronchoalveolar cell profiles of asthmatic and non- asthmatic subjects. Am. Rev. Respir. Dis. 1987, 136, 379–383. [Google Scholar] [CrossRef]
- Kay, A.B. Leucocytes in asthma. Immol. Investig. 1988, 17, 6799805. [Google Scholar] [CrossRef]
- Hallgren, R.; Samuelson, T.; Venge, P.; Modig, J. Eosinophi1 activation in the lung is related to lung damage in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1987, 135, 639–642. [Google Scholar]
- Burrows, B.; Hasan, F.M.; Barbee, R.A.; Halonen, M.; Lebowitz, M.D. Epidemiologic observations on eosinophilia and its relation to respiratory disorders. Am. Rev. Respir. Dis. 1980, 125, 708719. [Google Scholar]
- Taylor, R.G.; Gross, E.; Joyce, H.; Holland, F.; Pride, N.B. Smoking, allergy, and the differential white blood cell count. Thorax 1985, 40, 17–22. [Google Scholar] [CrossRef]
- Kaufmann, F.K.; Neukirch, F.; Korobaeff, M.; Marne, M.-J.; Claud, J.-R.; Lellouch, J. Eosinophils, smoking, and lung function. Am. Rev. Respir. Dis. 1986, 134, 1172–1175. [Google Scholar]
- Juel Jensen, E.; Pedersen, B.; Narvestadt, E.; Dahl, R. Blood eosinophil and monocyte counts are related to smoking and lung function. Respir. Med. 1998, 92, 63–69. [Google Scholar] [CrossRef]
- Hussein, S.E.O. Effect of cigarettes smoking on the serum levels of calcium and phosphate in Sudanese males in Khartoum. Int. J. Res. Pharm. Biosci. 2015, 2, 4–9. [Google Scholar]
- Aitchison, R.; Russell, N. Smoking—a major cause of polycythaemia. J. R. Soc. Med. 1988, 81, 89–91. [Google Scholar] [CrossRef]
- Malenica, M.; Prnjavorac, B.; Bego, T.; Du, T.; Skrbo, S.; Gusic, A.; Hadzic, A.; Causevic, A. Effect of Cigarette Smoking Haematological Parameters Population. Med. Arch. 2017, 71, 132–136. [Google Scholar] [CrossRef]
- Taylor, G.M.J.; Dalili, M.N.; Semwal, M.; Civljak, M.; Sheikh, A.; Car, J. Internet-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2017, CD007078. [Google Scholar] [CrossRef]
- Hsu, G.; Sun, J.Y.; Zhu, S.H. Evolution of electronic cigarette brands from 2013–2014 to 2016–2017: Analysis of brand websites. J. Med. Internet Res. 2018, 20, e80. [Google Scholar] [CrossRef]
- Noar, S.; Francis, D.; Bridges, C.; Sontag, J.; Ribisl, K.; Brewer, N. The impact of strengthening cigarette pack warnings: Systematic review of longitudinal observational studies. Soc. Sci. Med. 2016, 164, 118–129. [Google Scholar] [CrossRef]
- Report of the Scientific Committee on Tobacco and Health. Available online: http://www.archive.official-documents.co.uk/document/doh/tobacco/report.htm (accessed on 18 December 2018).
- Harvey, J.; Chadi, N. Canadian Paediatric Society, Adolescent Health Committee, Preventing smoking in children and adolescents: Recommendations for practice and policy. Paediatr. Child. Health 2016, 21, 209–214. [Google Scholar] [CrossRef]
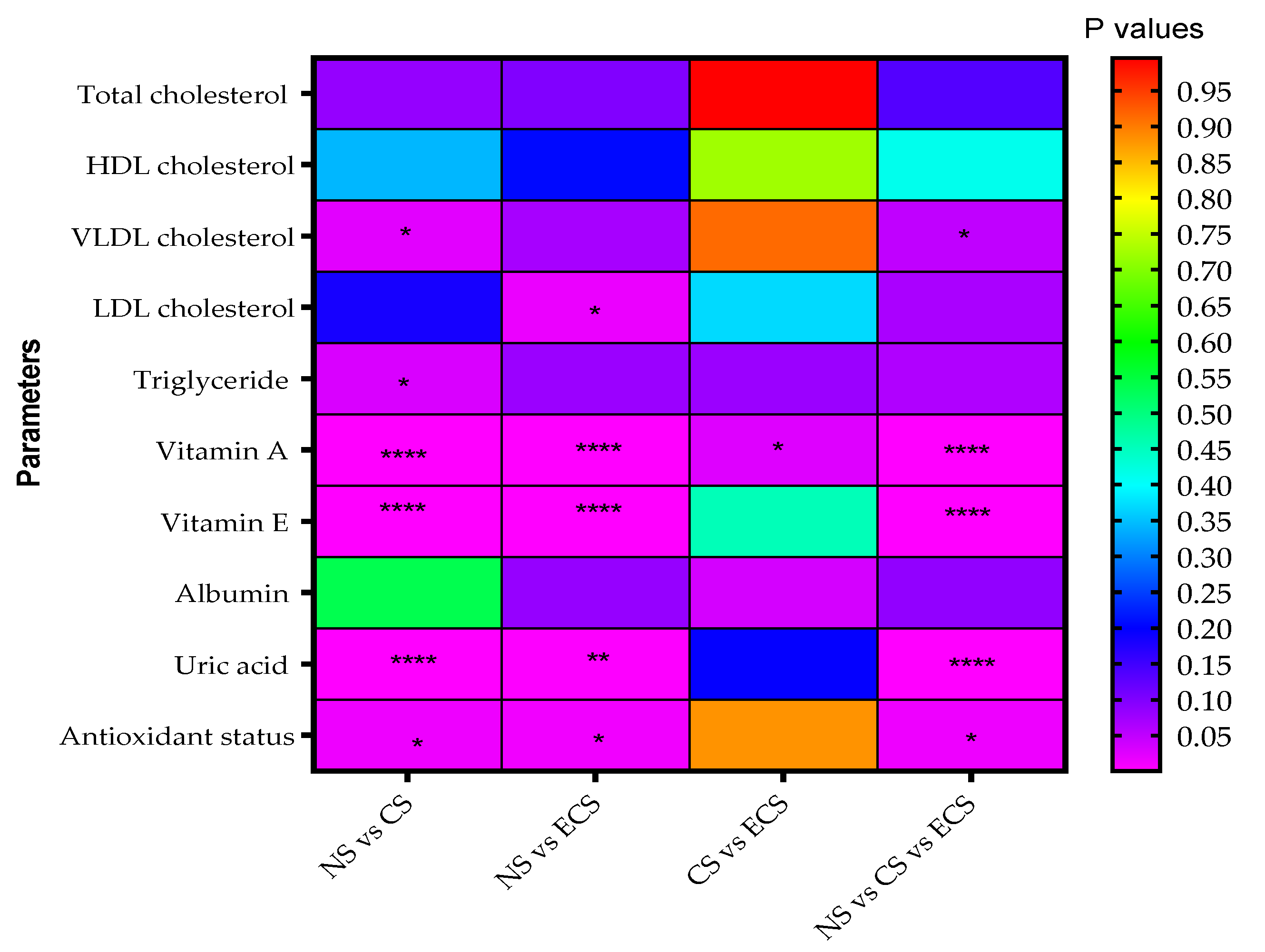
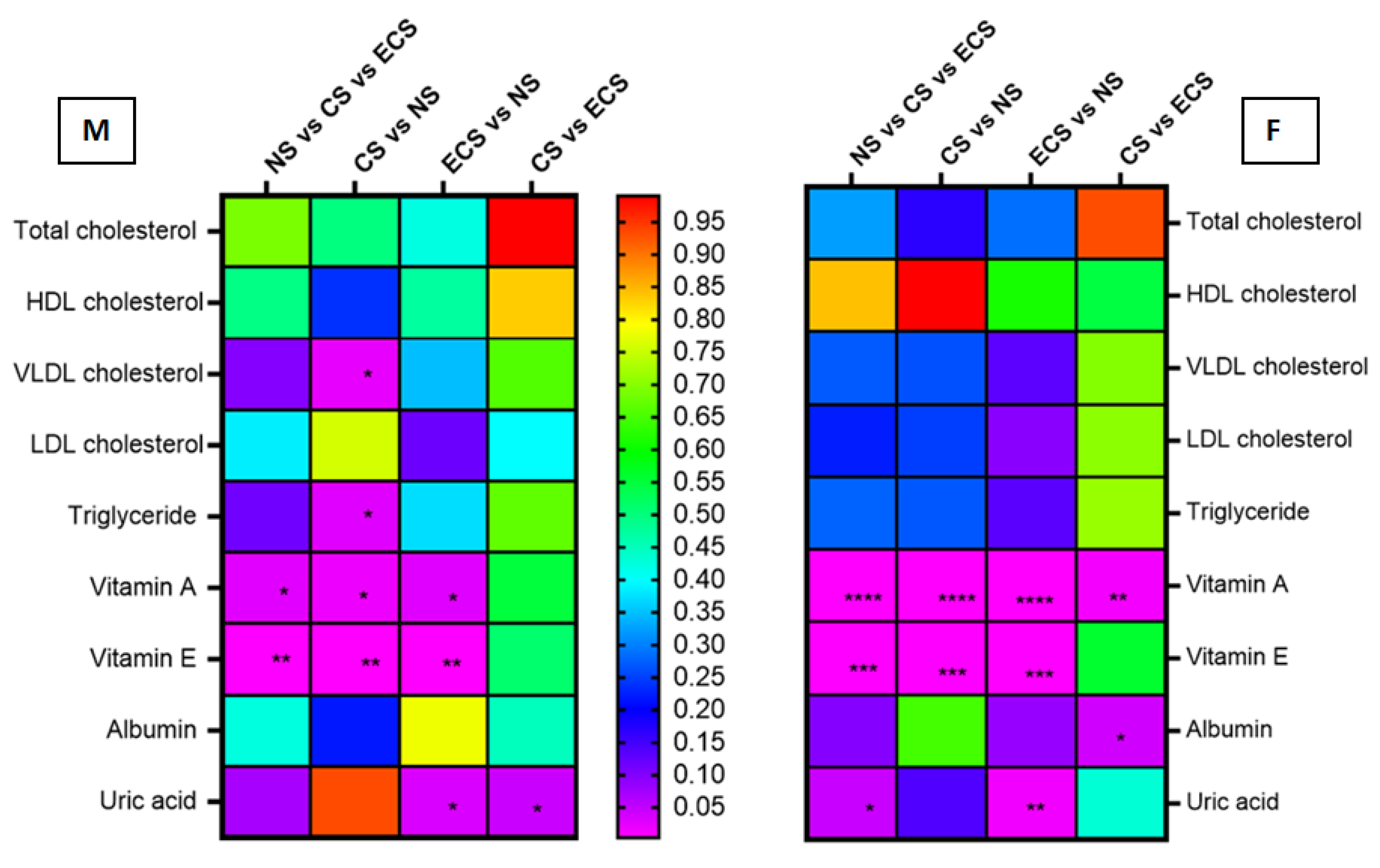
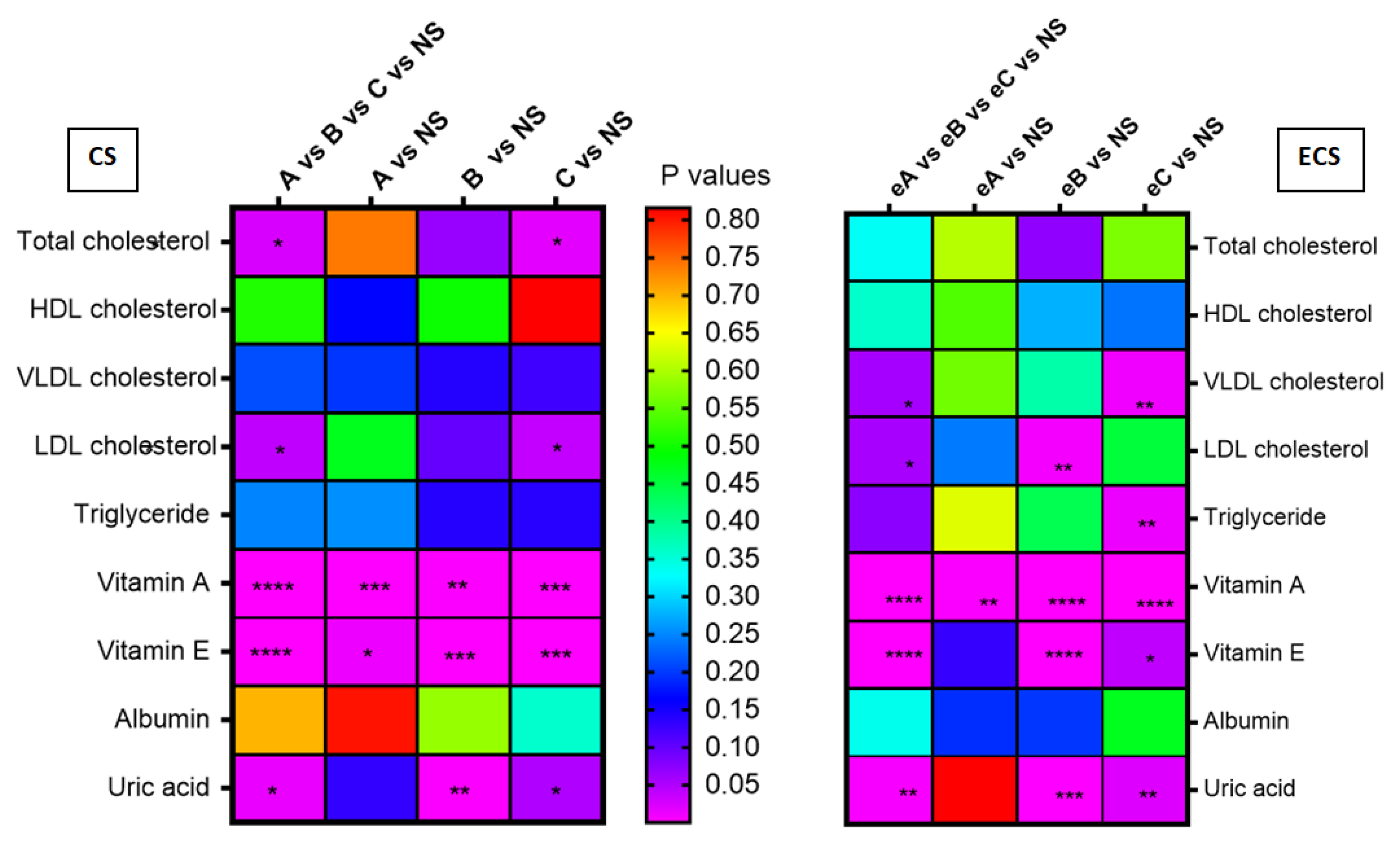
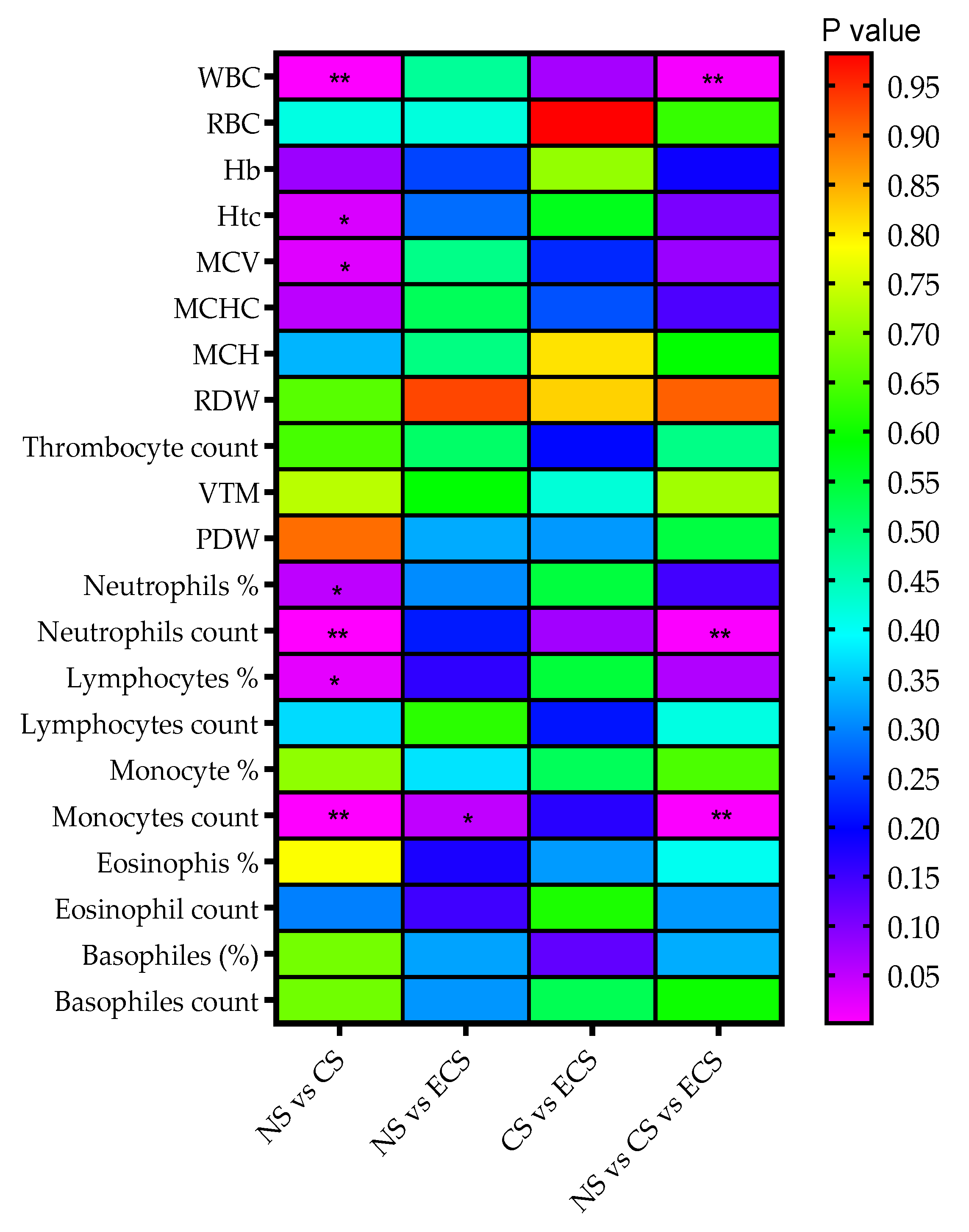
| Characteristics | Non-Smokers (n = 58) | Cigarette Smokers (n = 58) | E-Cigarette Users (n = 34) | P Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | 0.307 | ||||||
| Male | 10 | 17.2 | 17 | 29.3 | 8 | 23.5 | |
| Female | 48 | 82.8 | 41 | 70.7 | 26 | 76.5 | |
| Habitat | 0.012 (a) | ||||||
| Urban | 39 | 67.2 | 45 | 77.6 | 32 | 94.1 | |
| Rural | 19 | 32.8 | 13 | 22.4 | 2 | 5.9 | |
| BMI (kg/m2) (b) | 0.078 | ||||||
| <18.5 | 8 | 14.0 | 3 | 5.3 | 1 | 2.9 | |
| 18.5–24.99 | 39 | 68.4 | 35 | 61.4 | 19 | 55.9 | |
| 25–29.99 | 8 | 14.0 | 14 | 24.6 | 8 | 23.5 | |
| >30 | 2 | 3.5 | 5 | 8.8 | 6 | 17.6 | |
| Age (years) (mean ± STDEV) | 24.5 ± 6.7 | 28.4 ± 10.8 | 35.2 ± 9.4 | <0.001 (c) | |||
| Age start smoking (mean ± STDEV) (years) | - | 17.3 ± 3.9 | 18.4 ± 6.1 | 0.002 (d) | |||
| Time smoking cigarettes (mean ± STDEV) (years) | - | 10.5 ± 5.9 | 14.3 ± 8.0 * | ||||
| Cigarettes/day (mean ± STDEV) | - | 11.0 ± 5.9 | 13.0 ± 5.7 * | ||||
| Time using e-cigarettes (mean ± STDEV) (months) | 16.2 ± 15.5 | n.a. | |||||
| Item | Suggested Answer | Cigarette Smokers (n = 58) | E-cigarette Users (n = 34) | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Reasons of start smoking | Social entourage | 45 | 77.59% | 26 | 76.47% |
| Partner pressure | 1 | 1.72% | 0 | 0.00% | |
| Children pressure | 0 | 0.00% | 0 | 0.00% | |
| Work pressure | 10 | 17.24% | 3 | 8.82% | |
| Religious | 0 | 0.00% | 0 | 0.00% | |
| Esthetic | 1 | 1.72% | 0 | 0.00% | |
| Existent disease | 0 | 0.00% | 0 | 0.00% | |
| Other reasons | 12 | 20.69% | 6 | 17.65% | |
| Previous attempts to quit smoking | No | 8 | 13.79% | 9 | 26.47% |
| Just a decreasing of frequency | 17 | 29.31% | 13 | 38.24% | |
| Yes | 33 | 56.90% | 12 | 35.29% | |
| Re-starting smoking/withdrawal symptoms | Trembling hands | 2 | 6.06% | 1 | 8.33% |
| Tongue tremors | 0 | 0.00% | 0 | 0.00% | |
| Eye tremors | 0 | 0.00% | 0 | 0.00% | |
| Nausea and vomiting | 0 | 0.00% | 0 | 0.00% | |
| Weakness | 2 | 6.06% | 2 | 16.67% | |
| Tachycardia | 1 | 3.03% | 0 | 0.00% | |
| Sweating | 0 | 0.00% | 0 | 0.00% | |
| Entourage | 16 | 45.45% | 12 | 100.00% | |
| "Just a cigarette" | 20 | 60.61% | 6 | 50.00% | |
| Other reasons | 8 | 24.24% | 7 | 58.33% | |
| Reported adverse effects | No | 28 | 48.28% | 28 | 82.35% |
| No answer | 4 | 6.90% | 2 | 5.88% | |
| Yes | 26 | 44.83% | 4 | 11.76% | |
| Examples of adverse effects | Constipation | 0 | 0.00% | 0 | 0.00% |
| Diarrhea | 2 | 7.69% | 0 | 0.00% | |
| Dizziness | 14 | 53.85% | 3 | 75.00% | |
| Unclear view | 0 | 0.00% | 1 | 25.00% | |
| Upset stomach | 4 | 15.38% | 0 | 0.00% | |
| Insomnia | 0 | 0.00% | 0 | 0.00% | |
| Sleepiness | 1 | 3.85% | 0 | 0.00% | |
| Nausea | 6 | 23.08% | 2 | 50.00% | |
| Others | 12 | 46.15% | 1 | 25.00% | |
| Parameter | Non-Smokers (n = 58) | Cigarette Smokers (n = 58) | E-Cigarette Users (n = 34) | |||
|---|---|---|---|---|---|---|
| Mean ± STDEV | Median (p25th–p75th) | Mean ± STDEV | Median (p25th–p75th) | Mean ± STDEV | Median (p25th–p75th) | |
| Total cholesterol (mg/dL) | 164.29 ± 33.68 | 160.00 (138.75–185) | 179.64 ± 47.84 | 167.50 (146.75–207.75) | 173.24 ± 27.19 | 174.00 (156.50–189.75) |
| HDL cholesterol (mg/dL) | 58.72 ± 12.28 | 56.95 (50.55–66.55) | 58.22 ± 18.01 | 56.00 (47.18–66.78) | 55.29 ± 13.66 | 54.35 (45.65–65.98) |
| VLDL cholesterol (mg/dL) | 15.59 ± 6.84 | 13 (11.00–18.00) | 23.19 ± 25.33 | 17.50 (11.75–24.25) | 20.15 ±12.40 | 16.50 (12.00–25.25) |
| LDL cholesterol (mg/dL) | 100.16 ± 32.10 | 95 (74.00–117.50) | 111.00 ± 41.88 | 102.50 (85.75–132.75) | 112.03 ± 26.67 | 113 (94.50–134.50) (a) |
| Triglycerides (mg/dL) | 78.22 ± 34.07 | 66.50 (56.00 –89.25) | 115.78 ± 126.63 | 87.00 (57.50–122.00) | 100.91 ± 61.95 | 83.50 (60.50–125.25) |
| Vitamin A (mg/L) | 0.39 ± 0.10 | 0.37 (0.32–0.46) | 0.52 ± 0.14 | 0.49 (0.42–0.62) | 0.59 ± 0.14 | 0.56 (0.49–0.67) (a) |
| Vitamin E (mg/L) | 10.35 ± 3.12 | 9.93 (8.27 –11.91) | 13.98 ± 6.25 | 12.91 (10.69–15.5) | 14.07 ± 4.20 | 13.29 (10.65–16.88) (a) |
| Albumin (g/dL) | 4.86 ± 0.25 | 4.85 (4.65–5.00) | 4.84 ± 0.27 | 4.82 (4.67–4.97) | 4.95 ± 0.33 | 4.94 (4.79–5.12) (a) |
| Uric acid (mg/dL) | 4.36 ± 0.97 | 4.30 (3.70–5.00) | 4.85 ± 1.28 | 4.7 (3.7–5.7) | 5.24 ± 1.43 | 5.2 (4.15–6.2) (a) |
| Antioxidant status (Edel units/s) | −1.15 ± 0.32 | −1.112 (−1.81–−0.72) | −1.29 ± 0.31 | −1.252 (−1.838–−0.76) | −1.28 ± 0.24 | −1.27 (−1.72–−0.88) |
| Parameter | Non-Smokers (n = 58) | Cigarette Smokers (n = 58) | E-Cigarette Users (n = 34) | |||
|---|---|---|---|---|---|---|
| Mean ± STDEV | Median (p25th–p75th) | Mean ± STDEV | Median (p25th–p75th) | Mean ± STDEV | Median (p25th–p75th) | |
| WBC count (103μL) | 6.47 ± 1.52 | 6.37 (5.41–7.39) | 7.42 ± 1.87 | 7.39 (6.23–8.75) | 6.74 ± 1.63 | 6.6 (5.39–8.13) |
| RBC count (103/μL) | 4.74 ± 0.39 | 4.69 (4.43–4.91) | 4.82 ± 0.49 | 4.73 (4.42–5.13) | 4.8 ± 0.36 | 4.71 (4.56–5.04) |
| Hb (g/dL) | 13.69 ± 1.47 | 13.70 (12.85–14.33) | 14.25 ± 1.47 | 14 (13.2–15.3) | 13.94 ± 1.71 | 13.95 (13.1–14.93) |
| Htc (%) | 40.97 ± 3.41 | 40.85 (39.00–42.73) | 42.53 ± 3.72 | 42.20 (39.83–44.53) | 41.71 ± 4.14 | 41.75 (39.28–44.55) |
| MCV (fL) | 86.47 ± 3.83 | 86.50 (84.98–89.40) | 88.37 ± 4.35 | 88.6 (85.4–90.45) | 86.8 ± 5.7 | 87.00 (85.35–89.63) |
| MCHC (pg/cell) | 28.88 ± 2.11 | 29.10 (28.08–30.10) | 29.58 ± 1.75 | 29.7 (28.7–30.43) | 29 ± 2.67 | 29.05 (28.40–30.25) |
| MCH (g/dL) | 33.38 ± 1.48 | 33.20 (32.60–34.30) | 33.46 ± 1.00 | 33.35 (32.8–34.15) | 33.34 ± 1.27 | 33.30 (32.80–34.13) |
| RDW (%) | 13.07 ± 1.43 | 12.70 (12.38–13.20) | 12.81 ± 0.79 | 12.70 (12.20–13.40) | 13.00 ± 1.33 | 12.70 (12.38–13.05) |
| Thrombocyte count (103/μL) | 270.97 ± 75.40 | 255.00 (217.50–308.75) | 258.93 ± 48.27 | 254.00 (224.00–293.25) | 269.35 ± 47.45 | 267.50 (234.00–297.25) |
| VTM (fL) | 10.60 ± 0.99 | 10.40 (9.80–11.20) | 10.50 ± 0.85 | 10.35 (9.80–11.23) | 10.61 ± 0.79 | 10.50 (10.18–11.30) |
| PDW (fL) | 12.65 ± 2.23 | 12.00 (11.18–13.80) | 12.49 ± 1.75 | 12.15 (11.20–13.33) | 12.76 ± 1.66 | 12.55 (11.75–13.85) |
| Neutrophils (%) | 54.19 ± 8.57 | 53.65 (48.98–58.53) | 57.30 ± 8.14 | 55.85 (52.00–64.35) | 56.16 ± 8.31 | 54.85 (50.55–61.03) |
| Neutrophils count (103/μL) | 3.55 ± 1.23 | 3.45 (2.60–4.04) | 4.33 ± 1.50 | 4.21 (3.39–5.14) | 3.82 ± 1.20 | 3.69 (2.93–4.44) |
| Lymphocytes (%) | 34.56 ± 7.93 | 35.05 (29.43–39.33) | 31.42 ± 6.85 | 31.95 (25.75–36.83) | 32.46 ± 7.64 | 32.9 (27.23–36.88) |
| Lymphocytes count (103/μL) | 2.20 ± 0.61 | 2.12 (1.71–2.64) | 2.28 ± 0.58 | 2.36 (1.84–2.60) | 2.16 ± 0.68 | 1.96 (1.70–2.57) |
| Monocytes (%) | 8.10 ± 1.85 | 7.90 (6.60–9.33) | 8.16 ± 1.56 | 7.95 (6.88–9.33) | 8.35 ± 1.56 | 8.30 (7.28–9.40 |
| Monocytes count (103/μL) | 0.52 ± 0.18 | 0.49 (0.41–0.57) | 0.59 ± 0.14 | 0.59 (0.48–0.68) | 0.55 ± 0.13 | 0.56 (0.47–0.61) |
| Eosinophil (%) | 2.57 ± 3.43 | 1.85 (1.00–2.83) | 2.56 ± 2.63 | 1.90 (1.28–2.65) | 2.41 ± 1.23 | 2.05 (1.58–3.10) |
| Eosinophil count (103/μL) | 0.17 ± 0.22 | 0.12 (0.06–0.18) | 0.18 ± 0.19 | 0.14 (0.08–0.21) | 0.16 ± 0.09 | 0.15 (0.11–0.24) |
| Basophiles (%) | 0.58 ± 0.27 | 0.50 (0.40–0.70) | 0.55 ± 0.26 | 0.50 (0.40–0.70) | 0.62 ± 0.25 | 0.60 (0.40–0.83) |
| Basophiles (103/μL) | 0.04 ± 0.01 | 0.04 (0.03–0.05) | 0.04 ± 0.02 | 0.04 (0.03–0.04) | 0.04 ± 0.02 | 0.04 (0.03–0.06) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, M.; Gaman, L.; Delia, C.; Ilea, A.; Leașu, F.; Henríquez-Hernández, L.A.; Luzardo, O.P.; Rădoi, M.; Rogozea, L. Trends of Lipophilic, Antioxidant and Hematological Parameters Associated with Conventional and Electronic Smoking Habits in Middle-Age Romanians. J. Clin. Med. 2019, 8, 665. https://doi.org/10.3390/jcm8050665
Badea M, Gaman L, Delia C, Ilea A, Leașu F, Henríquez-Hernández LA, Luzardo OP, Rădoi M, Rogozea L. Trends of Lipophilic, Antioxidant and Hematological Parameters Associated with Conventional and Electronic Smoking Habits in Middle-Age Romanians. Journal of Clinical Medicine. 2019; 8(5):665. https://doi.org/10.3390/jcm8050665
Chicago/Turabian StyleBadea, Mihaela, Laura Gaman, Corina Delia, Anca Ilea, Florin Leașu, Luis Alberto Henríquez-Hernández, Octavio P. Luzardo, Mariana Rădoi, and Liliana Rogozea. 2019. "Trends of Lipophilic, Antioxidant and Hematological Parameters Associated with Conventional and Electronic Smoking Habits in Middle-Age Romanians" Journal of Clinical Medicine 8, no. 5: 665. https://doi.org/10.3390/jcm8050665
APA StyleBadea, M., Gaman, L., Delia, C., Ilea, A., Leașu, F., Henríquez-Hernández, L. A., Luzardo, O. P., Rădoi, M., & Rogozea, L. (2019). Trends of Lipophilic, Antioxidant and Hematological Parameters Associated with Conventional and Electronic Smoking Habits in Middle-Age Romanians. Journal of Clinical Medicine, 8(5), 665. https://doi.org/10.3390/jcm8050665









