Resting Heart Rate Variability Predicts Vulnerability to Pharmacologically-Induced Ventricular Arrhythmias in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery: Transmitter Implantation
2.3. 24-H Recording of ECG and LOC Signals
2.4. β-Adrenoceptor Pharmacological Stimulation
2.5. ECG Analysis
2.5.1. Heart Rate and Heart Rate Variability
2.5.2. Quantification of Arrhythmic Events
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Owens, S.; Slavens, G.; Riggs, S.; Treiber, F. Effect of physical training on heart-period variability in obese children. J. Pediatr. 1997, 130, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, T.G.; van Doornen, L.J.; de Geus, E.J. Effects of work stress on ambulatory blood pressure, heart rate, and heart rate variability. Hypertension 2000, 35, 880–886. [Google Scholar] [CrossRef]
- Zimmermann-Viehoff, F.; Thayer, J.; Koenig, J.; Herrmann, C.; Weber, C.S.; Deter, H.C. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers—A randomized crossover study. Nutr. Neurosci. 2016, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Barutcu, I.; Esen, A.M.; Kaya, D.; Turkmen, M.; Karakaya, O.; Melek, M.; Esen, O.B.; Basaran, Y. Cigarette smoking and heart rate variability: Dynamic influence of parasympathetic and sympathetic maneuvers. Ann. Noninvasive Electrocardiol. 2005, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Golosheykin, S.; Grant, J.D.; Novak, O.V.; Heath, A.C.; Anokhin, A.P. Genetic influences on heart rate variability. Int. J. Psychophysiol. 2017, 115, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, X.; Su, S.; Li, Z.; Riese, H.; Thayer, J.F.; Treiber, F.; Snieder, H. Genetic influences on heart rate variability at rest and during stress. Psychophysiology 2009, 46, 458–465. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Hohnloser, S.H.; Marcus, F.I.; Mortara, A.; Nohara, R.; Bigger, J.T., Jr.; Camm, A.J.; Schwartz, P.J. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation 2001, 103, 2072–2077. [Google Scholar] [CrossRef]
- Schwartz, P.J. The autonomic nervous system and sudden death. Eur. Heart J. 1998, 19, F72–F80. [Google Scholar] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kluttig, A.; Kuss, O.; Greiser, K.H. Ignoring lack of association of heart rate variability with cardiovascular disease and risk factors: Response to the manuscript “The relationship of autonomic imbalance, heart rate variability cardiovascular disease risk factors” by Julian F. Thayer, Shelby S. Yamamoto, Jos F. Brosschot. Int. J. Cardiol. 2010, 145, 375–376. [Google Scholar] [CrossRef]
- Frenneaux, M.P. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart 2004, 90, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Stein, P.K. Clinical application of heart rate variability after acute myocardial infarction. Front. Physiol. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.; Makijarvi, M.; Fetsch, T.; Martinez-Rubio, A.; Bocker, D.; Block, M.; Borggrefe, M.; Breithardt, G. Reduced beat-to-beat changes of heart rate: An important risk factor after acute myocardial infarction. Cardiology 1996, 87, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, G.R.; Brodie, D.A. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin. Electrophysiol. 2006, 29, 892–904. [Google Scholar] [CrossRef]
- De Bruyne, M.C.; Kors, J.A.; Hoes, A.W.; Klootwijk, P.; Dekker, J.M.; Hofman, A.; van Bemmel, J.H.; Grobbee, D.E. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: The Rotterdam study. Am. J. Epidemiol. 1999, 150, 1282–1288. [Google Scholar] [CrossRef]
- Dekker, J.M.; Schouten, E.G.; Klootwijk, P.; Pool, J.; Swenne, C.A.; Kromhout, D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen study. Am. J. Epidemiol. 1997, 145, 899–908. [Google Scholar] [CrossRef]
- Winter, J.; Tipton, M.J.; Shattock, M.J. Autonomic conflict exacerbates long QT associated ventricular arrhythmias. J. Mol. Cell Cardiol. 2018, 116, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Koplan, B.A.; Stevenson, W.G. Ventricular tachycardia and sudden cardiac death. Mayo Clin. Proc. 2009, 84, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; Cobb, L.A.; Edwards, J.E.; Kuller, L.; Moss, A.J.; Bigger, J.T., Jr.; Fleiss, J.L.; Rolnitzky, L.; Serokman, R. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am. J. Cardiol. 1988, 61, 8–15. [Google Scholar] [CrossRef]
- Ng, G.A. Neuro-cardiac interaction in malignant ventricular arrhythmia and sudden cardiac death. Auton. Neurosci. 2016, 199, 66–79. [Google Scholar] [CrossRef]
- Hillebrand, S.; Gast, K.B.; de Mutsert, R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace 2013, 15, 742–749. [Google Scholar] [CrossRef]
- Carnevali, L.; Sgoifo, A. Vagal modulation of resting heart rate in rats: The role of stress, psychosocial factors, and physical exercise. Front. Physiol. 2014, 5, 118. [Google Scholar] [CrossRef]
- Sgoifo, A.; Carnevali, L.; Grippo, A.J. The socially stressed heart. Insights from studies in rodents. Neurosci. Biobehav. Rev. 2014, 39, 51–60. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.; van Roon, A.; Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Bentho, O.; Park, M.Y.; Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 2011, 96, 1255–1261. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Goedhart, A.D.; Willemsen, G.; Houtveen, J.H.; Boomsma, D.I.; De Geus, E.J. Comparing low frequency heart rate variability and preejection period: Two sides of a different coin. Psychophysiology 2008, 45, 1086–1090. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Carnevali, L.; Mastorci, F.; Graiani, G.; Razzoli, M.; Trombini, M.; Pico-Alfonso, M.A.; Arban, R.; Grippo, A.J.; Quaini, F.; Sgoifo, A. Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol. Behav. 2012, 106, 142–150. [Google Scholar] [CrossRef]
- Sgoifo, A.; Stilli, D.; Medici, D.; Gallo, P.; Aimi, B.; Musso, E. Electrode positioning for reliable telemetry ECG recordings during social stress in unrestrained rats. Physiol. Behav. 1996, 60, 1397–1401. [Google Scholar] [CrossRef]
- Carnevali, L.; Vacondio, F.; Rossi, S.; Macchi, E.; Spadoni, G.; Bedini, A.; Neumann, I.D.; Rivara, S.; Mor, M.; Sgoifo, A. Cardioprotective effects of fatty acid amide hydrolase inhibitor URB694, in a rodent model of trait anxiety. Sci. Rep. 2015, 5, 18218. [Google Scholar] [CrossRef]
- Curtis, M.J.; Hancox, J.C.; Farkas, A.; Wainwright, C.L.; Stables, C.L.; Saint, D.A.; Clements-Jewery, H.; Lambiase, P.D.; Billman, G.E.; Janse, M.J.; et al. The lambeth conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013, 139, 213–248. [Google Scholar] [CrossRef]
- Pitzalis, M.V.; Mastropasqua, F.; Massari, F.; Forleo, C.; Di Maggio, M.; Passantino, A.; Colombo, R.; Di Biase, M.; Rizzon, P. Short- and long-term reproducibility of time and frequency domain heart rate variability measurements in normal subjects. Cardiovasc. Res. 1996, 32, 226–233. [Google Scholar] [CrossRef]
- Schmidt, L.A.; Santesso, D.L.; Miskovic, V.; Mathewson, K.J.; McCabe, R.E.; Antony, M.M.; Moscovitch, D.A. Test-retest reliability of regional electroencephalogram (EEG) and cardiovascular measures in social anxiety disorder (SAD). Int. J. Psychophysiol. 2012, 84, 65–73. [Google Scholar] [CrossRef]
- Almeida-Santos, M.A.; Barreto-Filho, J.A.; Oliveira, J.L.; Reis, F.P.; da Cunha Oliveira, C.C.; Sousa, A.C. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 2016, 63, 1–8. [Google Scholar] [CrossRef]
- Junior, E.C.; Oliveira, F.M. Attenuation of vagal modulation with aging: Univariate and bivariate analysis of HRV. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3178–3181. [Google Scholar] [CrossRef]
- Felber Dietrich, D.; Schindler, C.; Schwartz, J.; Barthelemy, J.C.; Tschopp, J.M.; Roche, F.; von Eckardstein, A.; Brandli, O.; Leuenberger, P.; Gold, D.R.; et al. Heart rate variability in an ageing population and its association with lifestyle and cardiovascular risk factors: Results of the SAPALDIA study. Europace 2006, 8, 521–529. [Google Scholar] [CrossRef]
- Japundzic-Zigon, N.; Sarenac, O.; Lozic, M.; Vasic, M.; Tasic, T.; Bajic, D.; Kanjuh, V.; Murphy, D. Sudden death: Neurogenic causes, prediction and prevention. Eur. J. Prev. Cardiol. 2018, 25, 29–39. [Google Scholar] [CrossRef]
- Rossi, S.; Fortunati, I.; Carnevali, L.; Baruffi, S.; Mastorci, F.; Trombini, M.; Sgoifo, A.; Corradi, D.; Callegari, S.; Miragoli, M.; et al. The effect of aging on the specialized conducting system: A telemetry ECG study in rats over a 6 month period. PLoS ONE 2014, 9, e112697. [Google Scholar] [CrossRef][Green Version]
- Tan, J.P.H.; Beilharz, J.E.; Vollmer-Conna, U.; Cvejic, E. Heart rate variability as a marker of healthy ageing. Int. J. Cardiol. 2019, 275, 101–103. [Google Scholar] [CrossRef]
- Kuss, O.; Schumann, B.; Kluttig, A.; Greiser, K.H.; Haerting, J. Time domain parameters can be estimated with less statistical error than frequency domain parameters in the analysis of heart rate variability. J. Electrocardiol. 2008, 41, 287–291. [Google Scholar] [CrossRef]
- Kennedy, H.L.; Whitlock, J.A.; Sprague, M.K.; Kennedy, L.J.; Buckingham, T.A.; Goldberg, R.J. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N. Engl. J. Med. 1985, 312, 193–197. [Google Scholar] [CrossRef]
- Grimm, W.; Menz, V.; Hoffmann, J.; Maisch, B. Reversal of tachycardia induced cardiomyopathy following ablation of repetitive monomorphic right ventricular outflow tract tachycardia. Pacing Clin. Electrophysiol. 2001, 24, 166–171. [Google Scholar] [CrossRef]
- Niwano, S.; Wakisaka, Y.; Niwano, H.; Fukaya, H.; Kurokawa, S.; Kiryu, M.; Hatakeyama, Y.; Izumi, T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009, 95, 1230–1237. [Google Scholar] [CrossRef]
- Carnevali, L.; Trombini, M.; Porta, A.; Montano, N.; de Boer, S.F.; Sgoifo, A. Vagal withdrawal and susceptibility to cardiac arrhythmias in rats with high trait aggressiveness. PLoS ONE 2013, 8, e68316. [Google Scholar] [CrossRef][Green Version]
- Mantravadi, R.; Gabris, B.; Liu, T.; Choi, B.R.; de Groat, W.C.; Ng, G.A.; Salama, G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ. Res. 2007, 100, e72–e80. [Google Scholar] [CrossRef]
- Ng, G.A.; Mantravadi, R.; Walker, W.H.; Ortin, W.G.; Choi, B.R.; de Groat, W.; Salama, G. Sympathetic nerve stimulation produces spatial heterogeneities of action potential restitution. Heart Rhythm 2009, 6, 696–706. [Google Scholar] [CrossRef]
- Vaseghi, M.; Gima, J.; Kanaan, C.; Ajijola, O.A.; Marmureanu, A.; Mahajan, A.; Shivkumar, K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: Intermediate and long-term follow-up. Heart Rhythm 2014, 11, 360–366. [Google Scholar] [CrossRef]
- Carnevali, L.; Trombini, M.; Rossi, S.; Graiani, G.; Manghi, M.; Koolhaas, J.M.; Quaini, F.; Macchi, E.; Nalivaiko, E.; Sgoifo, A. Structural and electrical myocardial remodeling in a rodent model of depression. Psychosom. Med. 2013, 75, 42–51. [Google Scholar] [CrossRef]
- Kalla, M.; Herring, N.; Paterson, D.J. Cardiac sympatho-vagal balance and ventricular arrhythmia. Auton. Neurosci. 2016, 199, 29–37. [Google Scholar] [CrossRef]
- Coote, J.H. Myths and realities of the cardiac vagus. J. Physiol. 2013, 591, 4073–4085. [Google Scholar] [CrossRef]
- Lewis, M.E.; Al-Khalidi, A.H.; Bonser, R.S.; Clutton-Brock, T.; Morton, D.; Paterson, D.; Townend, J.N.; Coote, J.H. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J. Physiol. 2001, 534, 547–552. [Google Scholar] [CrossRef]
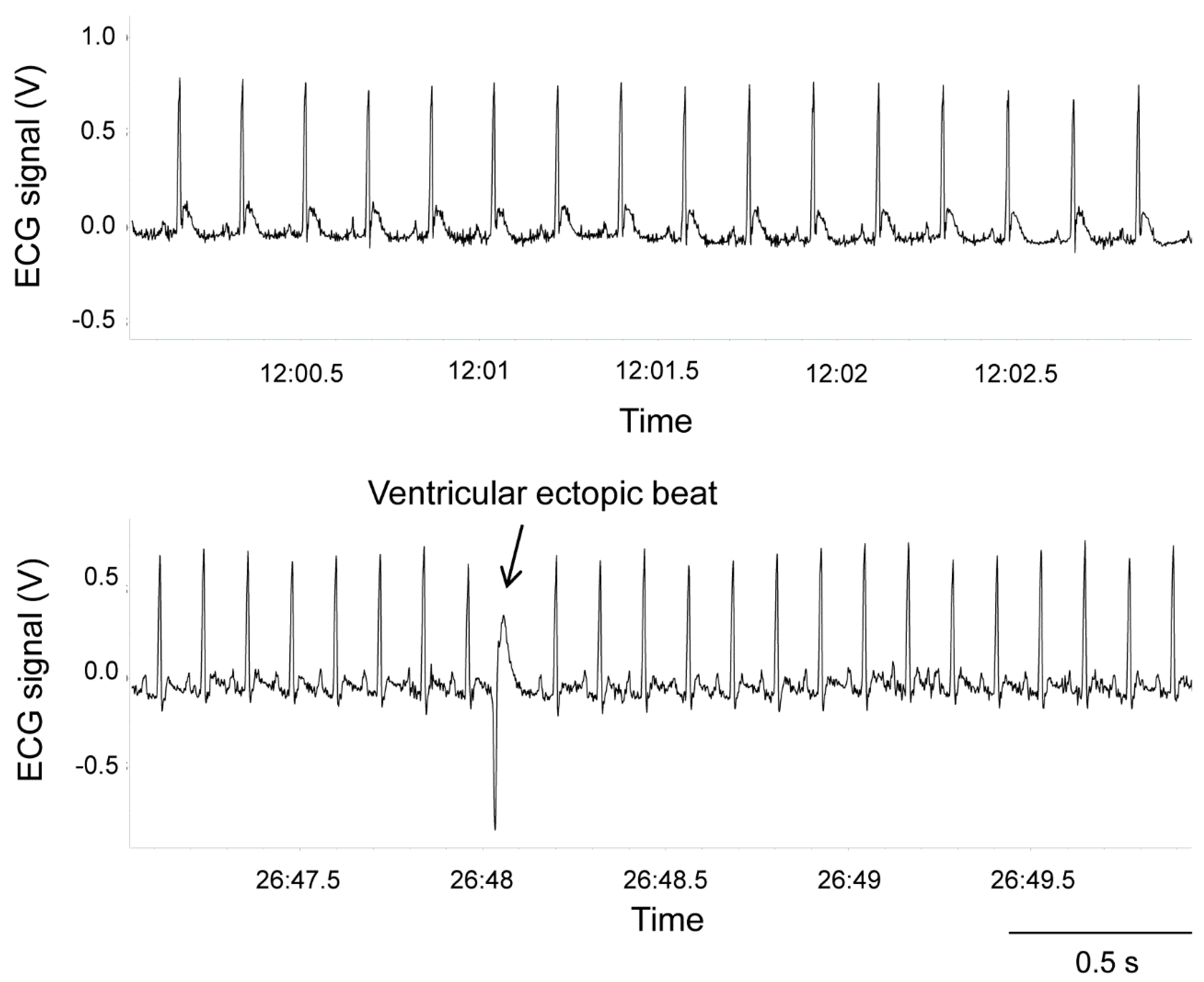
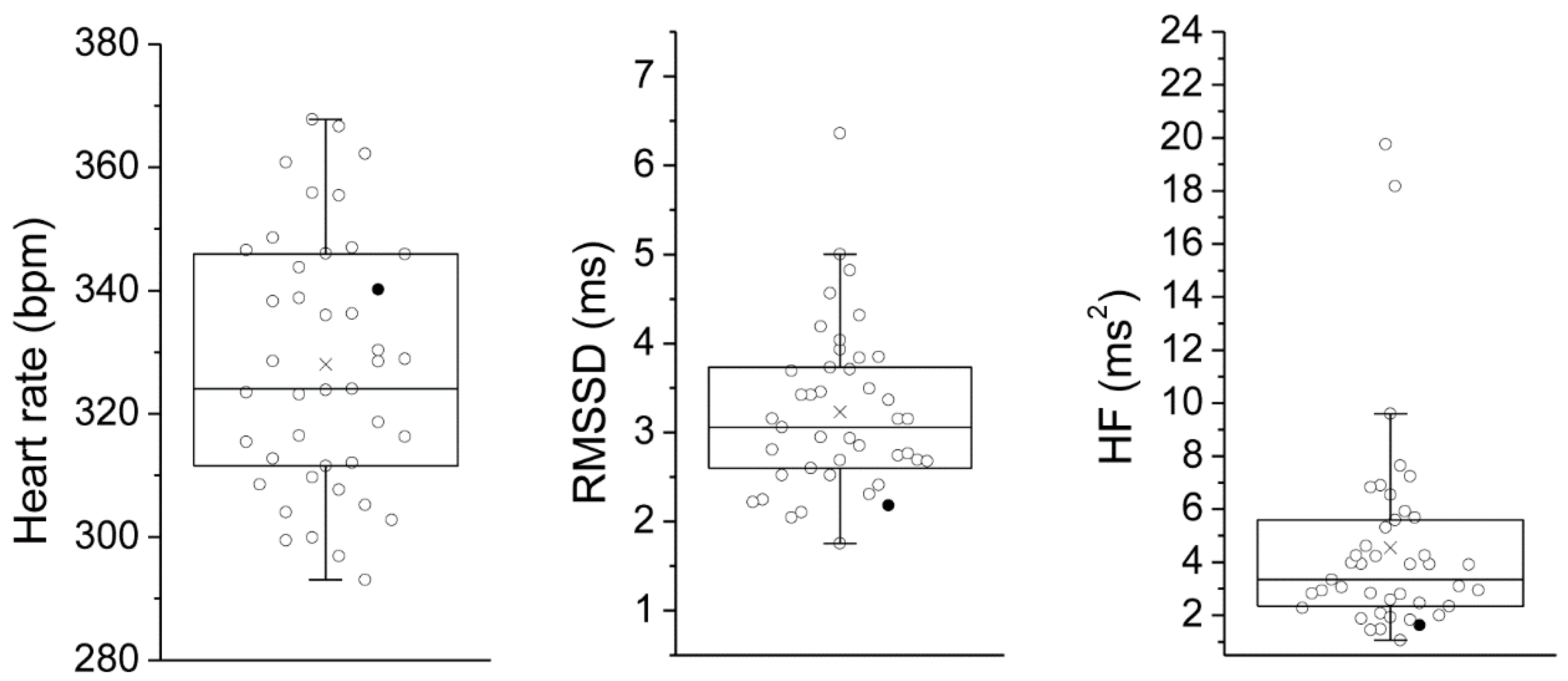
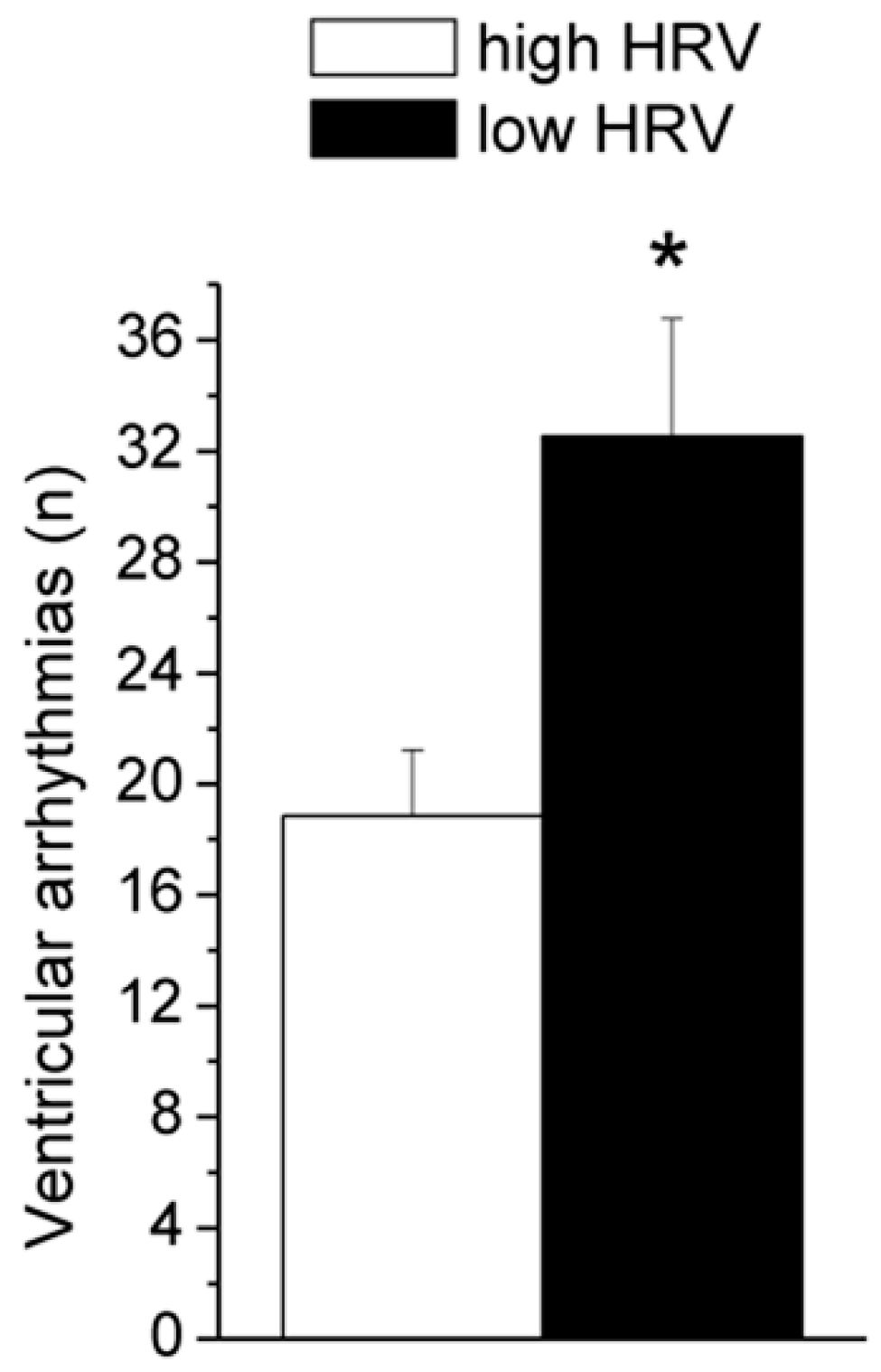

| Mean (SEM) | HR | lnRMSSD | lnHF | SVA | LOC | |
|---|---|---|---|---|---|---|
| HR | 328 (3) | - | ||||
| lnRMSSD | 1.14 (0.04) | −0.38 * | - | |||
| lnHF | 1.30 (0.10) | −0.41 * | 0.98 * | - | ||
| SVA | 0.49 (0.11) | 0.19 | 0.09 | 0.05 | - | |
| LOC | 4.52 (0.12) | 0.23 | −0.01 | −0.05 | 0.09 | - |
| Pre-Isoproterenol | Post-Isoproterenol | |
|---|---|---|
| Heart rate (beats/minute) | 358 ± 7 | 485 ± 3 * |
| lnRMSSD | 0.86 ± 0.05 | 0.64 ± 0.05 * |
| Ventricular arrhythmias (n) | 0.3 ± 0.1 | 25.9 ± 2.7 * |
| Model 1 | B | SE | β | 95%CI |
| Constant | 27.48 | 5.03 | ||
| SVA | −0.88 | 2.39 | −0.06 | −5.71; 3.96 |
| r2 = 0.00 | ||||
| Model 2 | B | SE | β | 95%CI |
| Constant | 51.44 | 12.57 | ||
| SVA | −1.25 | 2.30 | −0.83 | −5.91; 3.42 |
| lnRMSSD | −20.36 | 9.86 | −0.32 * | −40.31; −0.40 |
| r2 = 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnevali, L.; Statello, R.; Sgoifo, A. Resting Heart Rate Variability Predicts Vulnerability to Pharmacologically-Induced Ventricular Arrhythmias in Male Rats. J. Clin. Med. 2019, 8, 655. https://doi.org/10.3390/jcm8050655
Carnevali L, Statello R, Sgoifo A. Resting Heart Rate Variability Predicts Vulnerability to Pharmacologically-Induced Ventricular Arrhythmias in Male Rats. Journal of Clinical Medicine. 2019; 8(5):655. https://doi.org/10.3390/jcm8050655
Chicago/Turabian StyleCarnevali, Luca, Rosario Statello, and Andrea Sgoifo. 2019. "Resting Heart Rate Variability Predicts Vulnerability to Pharmacologically-Induced Ventricular Arrhythmias in Male Rats" Journal of Clinical Medicine 8, no. 5: 655. https://doi.org/10.3390/jcm8050655
APA StyleCarnevali, L., Statello, R., & Sgoifo, A. (2019). Resting Heart Rate Variability Predicts Vulnerability to Pharmacologically-Induced Ventricular Arrhythmias in Male Rats. Journal of Clinical Medicine, 8(5), 655. https://doi.org/10.3390/jcm8050655





