Relationship of Success Rate for Balloon Adhesiolysis with Clinical Outcomes in Chronic Intractable Lumbar Radicular Pain: A Multicenter Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intervention: Percutaneous Epidural Decompression and Adhesiolysis Using an Inflatable Balloon Catheter
2.3. Outcome Assessment and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Ballooning Success Rate Groups for Multiple Target Sites
3.3. The Estimated Proportions of Successful Responders
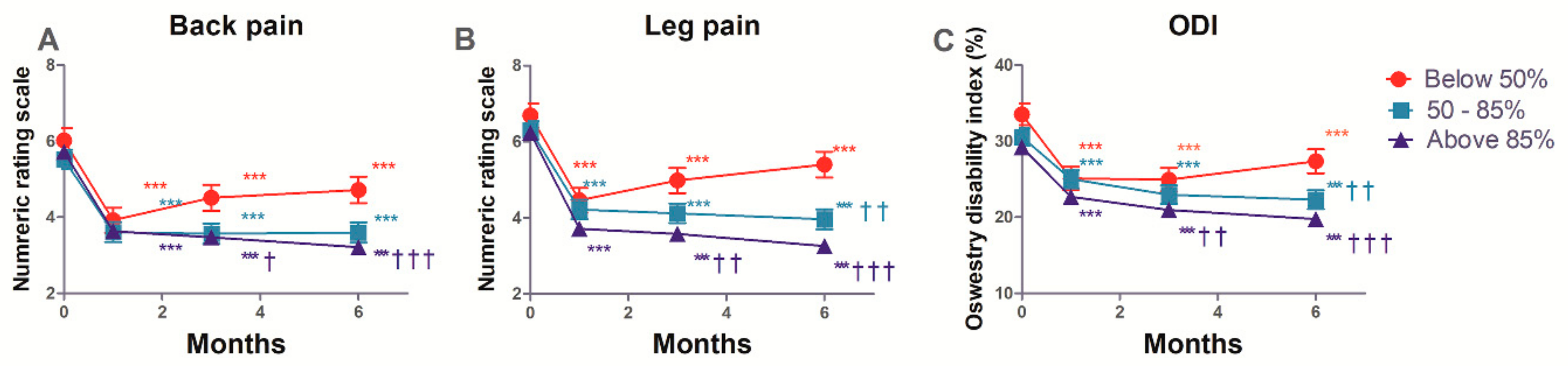
3.4. The Estimated Mean Changes in the Back and Leg Pain, and ODI
3.5. Observed Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Boxem, K.; Cheng, J.; Patijn, J.; Van Kleef, M.; Lataster, A.; Mekhail, N.; Van Zundert, J. 11. Lumbosacral radicular pain. Pain Pract. 2010, 10, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Cole, R.; Kim, D.H.; Li, L.; Suri, P.; Guermazi, A.; Hunter, D.J. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J. 2009, 9, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Sakamaki, T.; Sairyo, K.; Sakai, T.; Tamura, T.; Okada, Y.; Mikami, H. Measurements of ligamentum flavum thickening at lumbar spine using MRI. Arch. Orthop. Trauma. Surg. 2009, 129, 1415. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.G.; Deyo, R.A.; Cherkin, D.C. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine 1995, 20, 11–19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, H.-M.; Kim, H.-S.; Moon, E.-S.; Park, J.-O.; Lee, K.-J.; Moon, S.-H. Life expectancy after lumbar spine surgery: One-to eleven-year follow-up of 1015 patients. Spine 2008, 33, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Verbiest, H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. Clin. Orthop. Relat. Res. 1954, 36, 230–237. [Google Scholar] [CrossRef]
- Tran, D.Q.; Duong, S.; Finlayson, R.J. Lumbar spinal stenosis: A brief review of the nonsurgical management. Can. J. Anaesth. 2010, 57, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Falco, F.; Singh, V.; Benyamin, R.; Racz, G.; Caraway, D.; Calodney, A.; Snook, L.; Smith, H.; Gupta, S. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part I: Introduction and general considerations. Pain Physician. 2013, 16, S1–S48. [Google Scholar] [PubMed]
- Jamison, D.; Hsu, E.; Cohen, S. Epidural adhesiolysis: An evidence-based review. J. Neurosurg. Sci. 2014, 58, 65–76. [Google Scholar]
- Kobayashi, S.; Baba, H.; Uchida, K.; Kokubo, Y.; Kubota, C.; Yamada, S.; Suzuki, Y.; Yoshizawa, H. Effect of mechanical compression on the lumbar nerve root: Localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine 2005, 30, 1699–1705. [Google Scholar] [CrossRef]
- Manchikanti, L.; Bakhit, C.E. Percutaneous lysis of epidural adhesions. Pain Physician. 2000, 3, 46–64. [Google Scholar] [PubMed]
- Manchikanti, L.; Singh, V.; Bakhit, C.; Fellows, B. Interventional techniques in the management of chronic pain: Part 1.0. Pain Physician. 2000, 3, 7–42. [Google Scholar]
- Lee, J.H.; Lee, S.-H. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician. 2012, 15, 213–221. [Google Scholar] [PubMed]
- Birkenmaier, C.; Baumert, S.; Schroeder, C.; Jansson, V.; Wegener, B. A biomechanical evaluation of the epidural neurolysis procedure. Pain Physician. 2012, 15, E89–E97. [Google Scholar]
- Hsu, E.; Atanelov, L.; Plunkett, A.R.; Chai, N.; Chen, Y.; Cohen, S.P. Epidural lysis of adhesions for failed back surgery and spinal stenosis: Factors associated with treatment outcome. Anesth. Analg. 2014, 118, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.H. Clinical effectiveness of percutaneous adhesiolysis and predictive factors of treatment efficacy in patients with lumbosacral spinal stenosis. Pain Med. 2013, 14, 1497–1504. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Cash, K.A.; Pampati, V. Assessment of effectiveness of percutaneous adhesiolysis and caudal epidural injections in managing post lumbar surgery syndrome: 2-year follow-up of a randomized, controlled trial. J. Pain Res. 2012, 5, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Choi, W.-J.; Suh, J.H.; Jeon, S.-R.; Hwang, C.J.; Koh, W.-U.; Lee, C.; Leem, J.G.; Lee, S.C.; Shin, J.-W. Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: A randomized, controlled, double-blind trial. Pain Physician. 2013, 16, 213–224. [Google Scholar]
- Shin, J.W. A new approach to neuroplasty. Korean J. Pain 2013, 26, 323–326. [Google Scholar] [CrossRef]
- Choi, S.S.; Joo, E.Y.; Hwang, B.S.; Lee, J.H.; Lee, G.; Suh, J.H.; Leem, J.G.; Shin, J.W. A novel balloon-inflatable catheter for percutaneous epidural adhesiolysis and decompression. Korean J. Pain 2014, 27, 178–185. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, J.-H.; Kim, D.; Kim, H.K.; Lee, S.; Song, K.J.; Park, J.K.; Shim, J.H. Effectiveness and factors associated with epidural decompression and adhesiolysis using a balloon-inflatable catheter in chronic lumbar spinal stenosis: 1-year follow-up. Pain Med. 2015, 17, 476–487. [Google Scholar] [CrossRef]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Gallizzi, M.; Gagnon, C.; Harden, R.N.; Stanos, S.; Khan, A. Medication Quantification Scale Version III: Internal validation of detriment weights using a chronic pain population. Pain Pract. 2008, 8, 1–4. [Google Scholar] [CrossRef]
- Van Zundert, J.; Patijn, J.; Kessels, A.; Lamé, I.; van Suijlekom, H.; van Kleef, M. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: A double blind sham controlled randomized clinical trial. Pain 2007, 127, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Park, J.Y.; Cho, S.-S.; Cho, H.-S.; Lee, J.-Y.; Kim, Y.J.; Choi, S.-S. Comparative effectiveness of percutaneous epidural adhesiolysis for different sacrum types in patients with chronic pain due to lumbar disc herniation: A propensity score matching analysis. Medicine 2016, 95, e4647. [Google Scholar] [CrossRef]
- Sprague, S.; Matta, J.M.; Bhandari, M.; Investigators, A.T.H.A.C. Multicenter collaboration in observational research: Improving generalizability and efficiency. JBJS 2009, 91, 80–86. [Google Scholar] [CrossRef]
- Bhandari, M.; Schemitsch, E.H. Beyond the basics: The organization and coordination of multicenter trials. Tech. Ortho. 2004, 19, 83–87. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.-H. Clinical effectiveness of percutaneous adhesiolysis versus transforaminal epidural steroid injection in patients with postlumbar surgery syndrome. Reg. Anesth. Pain Med. 2014, 39, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Katz, N.P.; Kerns, R.D.; Stucki, G.; Allen, R.R.; Bellamy, N. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geurts, J.W.; van Wijk, R.M.; Wynne, H.J.; Hammink, E.; Buskens, E.; Lousberg, R.; Knape, J.T.; Groen, G.J. Radiofrequency lesioning of dorsal root ganglia for chronic lumbosacral radicular pain: A randomised, double-lind, controlled trial. Lancet 2003, 361, 21–26. [Google Scholar] [CrossRef]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond repeated measures ANOVA: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 2012, 37, 99. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young Jr, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; Henrica, C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Fielding, S.; Fayers, P.; Ramsay, C.R. Analysing randomised controlled trials with missing data: Choice of approach affects conclusions. Contemp. Clin. Trials 2012, 33, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Dulhunty, J.M.; Boots, R.J.; Paratz, J.D.; Lipman, J. Determining authorship in multicenter trials: A systematic review. Acta Anaesthesiol Scand. 2011, 55, 1037–1043. [Google Scholar] [CrossRef]
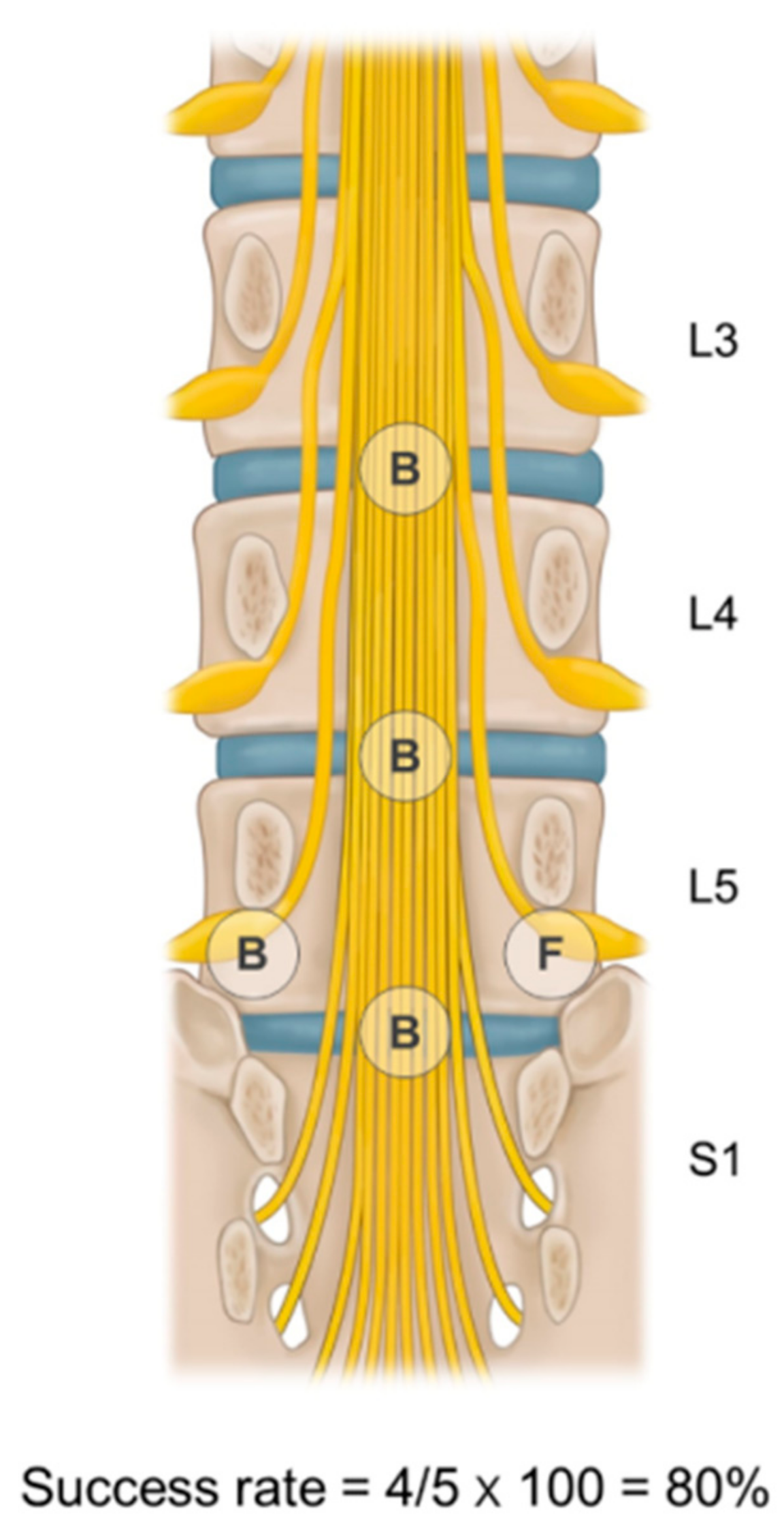

| Parameter | Below 50% (n = 48) | 50–85% (n = 79) | 85–100% (n = 148) | p-Value |
|---|---|---|---|---|
| Age, years | 68.9 (12.2) | 61.9 (12.8) | 61.6 (13.1) | 0.003 |
| Gender, n (%) | ||||
| Male/female | 22/26 (45.8/54.2) | 48/31 (60.8/39.2) | 79/69 (53.4/46.6) | 0.251 |
| Body mass index, kg/m2 | 23.2 (4.1) | 24.8 (2.9) | 24.2 (2.9) | 0.019 |
| Areas of pain, n (%) | 0.943 | |||
| Back/leg/both | 7/4/37 (14.6/8.3/77.1) | 11/8/60 (13.9/10.1/76.0) | 17/17/114 (11.5/11.5/77.0) | |
| Duration of pain (months) | 12.0 (4.0–24.0) | 12.0 (7.0–36.0) | 12.0 (6.0–24.0) | 0.771 |
| Concurrent disease, n (%) | ||||
| Diabetes | 10 (20.8) | 8 (10.1) | 27 (18.2) | 0.189 |
| Hypertension | 30 (62.5) | 38 (48.1) | 74 (50.0) | 0.244 |
| Cardiovascular disease | 14 (29.2) | 35 (44.3) | 43 (29.1) | 0.053 |
| Spinal stenosis grading, n (%) | ||||
| Central canal (A/B/C/D) | 9/11/9/1 (18.8/22.9/18.8/2.1) | 16/15/23/0 (20.3/19.0/29.1/0.0) | 35/20/25/3 (23.6/13.5/16.9/2.0) | 0.355 |
| Foraminal (mild/moderate/severe) | 11/17/20 (22.9/35.4/41.7) | 28/22/24 (35.4/27.8/30.4) | 51/33/34 (34.5/22.3/23.0) | 0.190 |
| Spondylolisthesis, n (%) | 6 (12.5) | 5 (6.3) | 13 (8.8) | 0.480 |
| MQS, points | 11.8 (8.0–15.0) | 11.0 (8.0–12.2) | 8.0 (7.6–11.2) | 0.100 |
| Pain intensity (NRS) | ||||
| Back | 7.0 (4.0–8.0) | 6.0 (4.0–7.0) | 6.0 (4.0–8.0) | 0.414 |
| Leg | 7.0 (5.5–8.0) | 6.0 (5.0–8.0) | 7.0 (5.0–8.0) | 0.515 |
| ODI (%) | 34.0 (24.5–39.5) | 30.0 (23.0–38.0) | 28.0 (22.0–35.0) | 0.071 |
| BDI, points | 7.0 (5.0–10.0) | 6.0 (5.0–9.0) | 6.0 (4.0–11.0) | 0.595 |
| Parameter | Below 50% (n = 48) | 50–85% (n = 79) | 85–100% (n = 148) | p-Value |
|---|---|---|---|---|
| Target level, n (%) | 0.284 | |||
| 1 level (L3-4/L4-5/L5-S1) 2 levels (L3-4-5/L4-5-S1) 3 levels (L2-3-4-5/L3-4-5-S1) 4 levels (L2-3-4-5-S1) | 0/24/3 (0.0/50.0/6.3) 5/15 (10.4/31.3) 0/1 (0.0/2.1) 0 (0.0) | 2/31/10 (2.5/39.2/12.7) 9/24 (11.4/30.4) 0/3 (0.0/3.8) 0 (0.0) | 2/56/9 (1.4/37.8/6.1) 21/40 (14.2/27.0) 5/12 (3.4/8.1) 3 (2.0) | |
| Target site, n (%) | 0.302 | |||
| Left/right/both/central/Lt, central/Rt, central/both, central | 8/6/14/1/4/5/ 10 (16.7/12.5/29.2/2.1/8.3/10.4/20.8) | 13/6/24/2/4/7/23 (16.5/7.6/30.4/2.5/5.1/8.9/29.1) | 19/13/40/17/17/12/30 (12.8/8.8/27.0/11.5/11.5/8.1/20.3) | |
| Number of target sites, n (%) | 0.368 | |||
| 2–3/4–5/above 6 | 18/22/8 (37.5/45.8/16.7) | 21/35/23 (26.6/44.3/29.1) | 54/57/37 (36.5/38.5/25.0) |
| Follow-Up (Months) | Below 50% (n = 48), Estimated Proportion (95% CI) | 50–85% (n = 79), Estimated Proportion (95% CI) | 85–100% (n = 148), Estimated Proportion (95% CI) | p- Value | |
|---|---|---|---|---|---|
| Successful | 1 | 0.688 (0.556–0.819) | 0.633 (0.527–0.739) | 0.662 (0.586–0.738) | 0.812 |
| Responder | 3 | 0.542 (0.401–0.683) | 0.582 (0.474–0.691) | 0.628 (0.551–0.706) | 0.528 |
| 6 | 0.292 (0.163–0.420) | 0.468 (0.358–0.578) | 0.507 (0.426–0.587) | 0.038 |
| Variables * | Time (Months) | Below 50% (n = 48) Values (95% CI) | 50–85% (n = 79) Values (95% CI) | 85–100% (n = 148) Values (95% CI) | p-Value † |
|---|---|---|---|---|---|
| GPES | 1 | 4.80 (4.43–5.17) | 4.83 (4.55–5.11) | 4.86 (4.64–5.09) | 0.955 |
| 3 | 4.86 (4.05–5.67) | 4.23 (3.47–4.99) | 4.48 (3.98–4.99) | 0.532 | |
| 6 | 4.09 (3.68–4.49) | 4.31 (4.01–4.61) | 4.88 (4.64–5.12) | 0.001 |
| Complication | Number (%) |
|---|---|
| Dura matter puncture | 9 (3.3) |
| Subdural injection | 5 (1.8) |
| Vascular injection | 4 (1.5) |
| Disc injection | 6 (2.2) |
| Hypotension | 4 (1.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Ji, G.Y.; Lee, S.W.; Park, J.K.; Ha, D.; Park, Y.; Cho, S.-S.; Moon, S.H.; Shin, J.-W.; Kim, D.J.; et al. Relationship of Success Rate for Balloon Adhesiolysis with Clinical Outcomes in Chronic Intractable Lumbar Radicular Pain: A Multicenter Prospective Study. J. Clin. Med. 2019, 8, 606. https://doi.org/10.3390/jcm8050606
Park J-Y, Ji GY, Lee SW, Park JK, Ha D, Park Y, Cho S-S, Moon SH, Shin J-W, Kim DJ, et al. Relationship of Success Rate for Balloon Adhesiolysis with Clinical Outcomes in Chronic Intractable Lumbar Radicular Pain: A Multicenter Prospective Study. Journal of Clinical Medicine. 2019; 8(5):606. https://doi.org/10.3390/jcm8050606
Chicago/Turabian StylePark, Jun-Young, Gyu Yeul Ji, Sang Won Lee, Jin Kyu Park, Dongwon Ha, Youngmok Park, Seong-Sik Cho, Sang Ho Moon, Jin-Woo Shin, Dong Joon Kim, and et al. 2019. "Relationship of Success Rate for Balloon Adhesiolysis with Clinical Outcomes in Chronic Intractable Lumbar Radicular Pain: A Multicenter Prospective Study" Journal of Clinical Medicine 8, no. 5: 606. https://doi.org/10.3390/jcm8050606
APA StylePark, J.-Y., Ji, G. Y., Lee, S. W., Park, J. K., Ha, D., Park, Y., Cho, S.-S., Moon, S. H., Shin, J.-W., Kim, D. J., Shin, D. A., & Choi, S.-S. (2019). Relationship of Success Rate for Balloon Adhesiolysis with Clinical Outcomes in Chronic Intractable Lumbar Radicular Pain: A Multicenter Prospective Study. Journal of Clinical Medicine, 8(5), 606. https://doi.org/10.3390/jcm8050606






