Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression
Abstract
1. Introduction
2. Various Preclinical Models
2.1. Human Cancer Cell Xenograft and Allograft Models
2.2. TRAMP Mouse Models
2.3. Other Genetically Engineered/Transgenic Mouse Models Targeting Oncogenes and Tumor Suppressor Genes
2.4. Others
3. Differences in Diets
3.1. Direct Comparison between Two Different Diets Including the High-Fat Diet and Another Diet
3.2. Comparison of the Impact of a High-Fat Diet using Multiple Diets
3.3. Specific Components of Fat
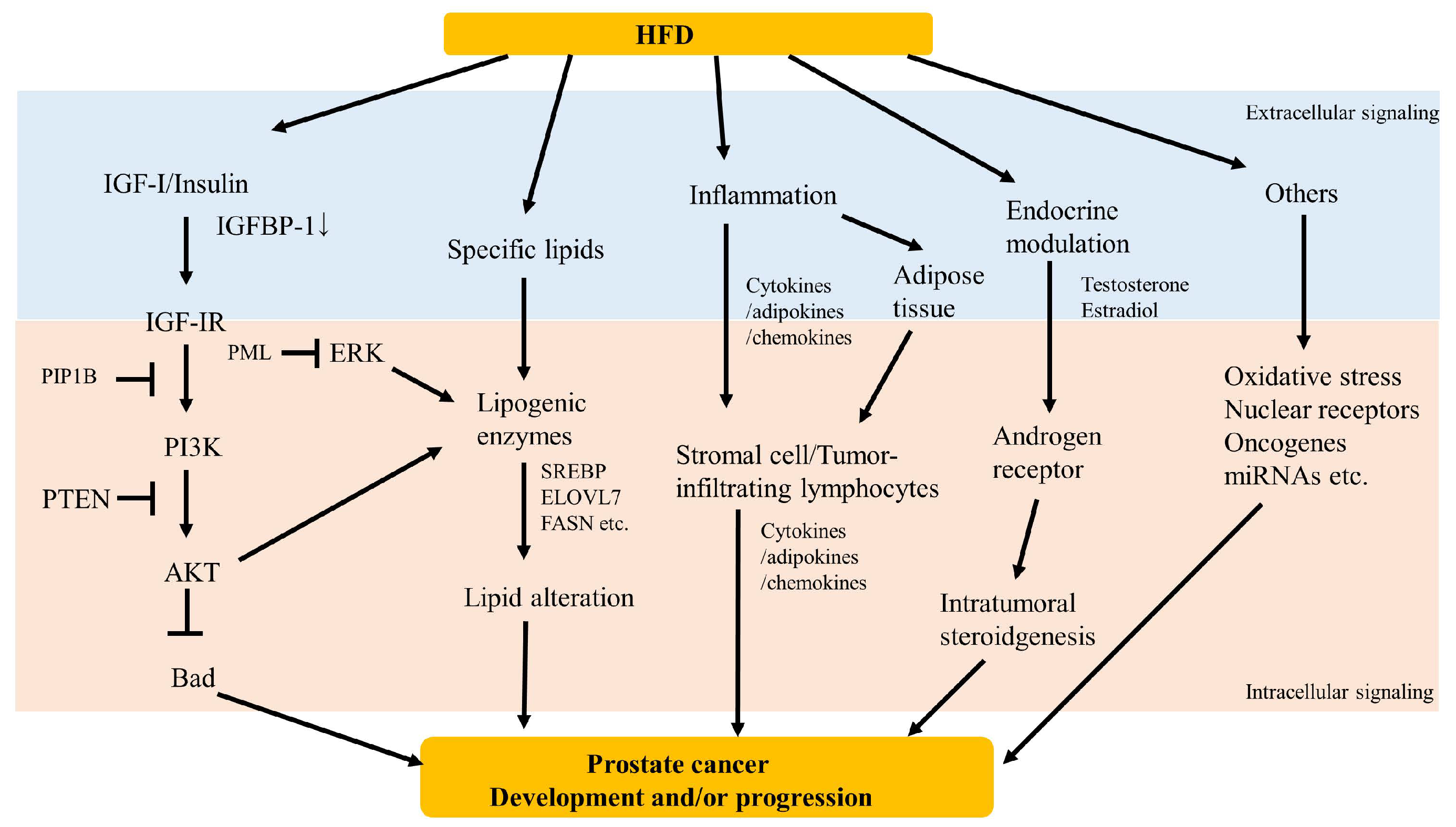
4. Potential Mechanisms
4.1. Growth Factor Signaling
4.2. Lipid Accumulation
4.3. Inflammation
4.4. Endocrine Modulation
4.5. Others
5. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakayama, T.; Shiraishi, T.; Stemmermann, G.N.; Yatani, R. Comparative studies of prostate cancer in Japan versus the United States. A review. Urol. Oncol. 2000, 5, 274–283. [Google Scholar] [CrossRef]
- Breslow, N.; Chan, C.W.; Dhom, G.; Drury, R.A.; Franks, L.M.; Gellei, B.; Lee, Y.S.; Lundberg, S.; Sparke, B.; Sternby, N.H.; et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int. J. Cancer 1977, 20, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Yatani, R.; Henderson, B.E.; Mack, T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef]
- Ornish, D.; Weidner, G.; Fair, W.R.; Marlin, R.; Pettengill, E.B.; Raisin, C.J.; Dunn-Emke, S.; Crutchfield, L.; Jacobs, F.N.; Barnard, R.J.; et al. Intensive lifestyle changes may affect the progression of prostate cancer. J. Urol. 2005, 174, 1065–1069; discussion 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Klotz, L.H. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat. Rev. Urol. 2010, 7, 442–453. [Google Scholar] [CrossRef]
- Lin, P.H.; Aronson, W.; Freedland, S.J. An update of research evidence on nutrition and prostate cancer. Urol. Oncol. 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Corr, J.G.; Thaler, H.T.; Tao, Y.; Fair, W.R.; Heston, W.D. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J. Natl. Cancer Inst. 1995, 87, 1456–1462. [Google Scholar] [CrossRef]
- Connolly, J.M.; Coleman, M.; Rose, D.P. Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutri. Cancer 1997, 29, 114–119. [Google Scholar] [CrossRef]
- Ngo, T.H.; Barnard, R.J.; Tymchuk, C.N.; Cohen, P.; Aronson, W.J. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control. 2002, 13, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Ngo, T.H.; Leung, P.S.; Aronson, W.J.; Golding, L.A. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate 2003, 56, 201–206. [Google Scholar] [CrossRef]
- Ngo, T.H.; Barnard, R.J.; Cohen, P.; Freedland, S.; Tran, C.; de Gregorio, F.; Elshimali, Y.I.; Heber, D.; Aronson, W.J. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin. Cancer Res. 2003, 9, 2734–2743. [Google Scholar]
- Narita, S.; Tsuchiya, N.; Saito, M.; Inoue, T.; Kumazawa, T.; Yuasa, T.; Nakamura, A.; Habuchi, T. Candidate genes involved in enhanced growth of human prostate cancer under high fat feeding identified by microarray analysis. Prostate 2008, 68, 321–335. [Google Scholar] [CrossRef]
- Venkateswaran, V.; Haddad, A.Q.; Fleshner, N.E.; Fan, R.; Sugar, L.M.; Nam, R.; Klotz, L.H.; Pollak, M. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J. Natl. Cancer Inst. 2007, 99, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007, 117, 1866–1875. [Google Scholar] [CrossRef]
- Kobayashi, N.; Barnard, R.J.; Said, J.; Hong-Gonzalez, J.; Corman, D.M.; Ku, M.; Doan, N.B.; Gui, D.; Elashoff, D.; Cohen, P.; et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008, 68, 3066–3073. [Google Scholar] [CrossRef]
- Freedland, S.J.; Mavropoulos, J.; Wang, A.; Darshan, M.; Demark-Wahnefried, W.; Aronson, W.J.; Cohen, P.; Hwang, D.; Peterson, B.; Fields, T.; et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008, 68, 11–19. [Google Scholar] [CrossRef]
- Mavropoulos, J.C.; Buschemeyer, W.C., 3rd; Tewari, A.K.; Rokhfeld, D.; Pollak, M.; Zhao, Y.; Febbo, P.G.; Cohen, P.; Hwang, D.; Devi, G.; et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev. Res. 2009, 2, 557–565. [Google Scholar] [CrossRef]
- Tamura, K.; Makino, A.; Hullin-Matsuda, F.; Kobayashi, T.; Furihata, M.; Chung, S.; Ashida, S.; Miki, T.; Fujioka, T.; Shuin, T.; et al. Novel lipogenic enzyme ELOVL7 is involved in prostate cancer growth through saturated long-chain fatty acid metabolism. Cancer Res. 2009, 69, 8133–8140. [Google Scholar] [CrossRef]
- Kalaany, N.Y.; Sabatini, D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009, 458, 725–731. [Google Scholar] [CrossRef]
- Buschemeyer, W.C., 3rd; Klink, J.C.; Mavropoulos, J.C.; Poulton, S.H.; Demark-Wahnefried, W.; Hursting, S.D.; Cohen, P.; Hwang, D.; Johnson, T.L.; Freedland, S.J. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate 2010, 70, 1037–1043. [Google Scholar] [CrossRef]
- Llaverias, G.; Danilo, C.; Wang, Y.; Witkiewicz, A.K.; Daumer, K.; Lisanti, M.P.; Frank, P.G. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am. J. Pathol. 2010, 177, 3180–3191. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Antonelli, J.A.; Phillips, T.E.; Masko, E.M.; Thomas, J.A.; Poulton, S.H.; Pollak, M.; Freedland, S.J. Effect of isocaloric low fat diet on prostate cancer xenograft progression in a hormone deprivation model. J. Urol. 2010, 183, 1619–1624. [Google Scholar] [CrossRef][Green Version]
- Aronson, W.J.; Barnard, R.J.; Freedland, S.J.; Henning, S.; Elashoff, D.; Jardack, P.M.; Cohen, P.; Heber, D.; Kobayashi, N. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J. Urol. 2010, 183, 345–350. [Google Scholar] [CrossRef]
- Masko, E.M.; Thomas, J.A., 2nd; Antonelli, J.A.; Lloyd, J.C.; Phillips, T.E.; Poulton, S.H.; Dewhirst, M.W.; Pizzo, S.V.; Freedland, S.J. Low-carbohydrate diets and prostate cancer: How low is “low enough”? Cancer Prev. Res. 2010, 3, 1124–1131. [Google Scholar] [CrossRef]
- Akinsete, J.A.; Ion, G.; Witte, T.R.; Hardman, W.E. Consumption of high omega-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 2012, 33, 140–148. [Google Scholar] [CrossRef]
- Mao, G.E.; Harris, D.M.; Moro, A.; Heber, D.; Roy-Burman, P.; Zhang, Z.F.; Rao, J. A joint effect of new Western diet and retinoid X receptor alpha prostate-specific knockout with development of high-grade prostatic intraepithelial neoplasia in mice—A preliminary study. Prostate 2012, 72, 1052–1059. [Google Scholar] [CrossRef]
- Bonorden, M.J.; Grossmann, M.E.; Ewing, S.A.; Rogozina, O.P.; Ray, A.; Nkhata, K.J.; Liao, D.J.; Grande, J.P.; Cleary, M.P. Growth and Progression of TRAMP Prostate Tumors in Relationship to Diet and Obesity. Prostate Cancer 2012, 2012, 543970. [Google Scholar] [CrossRef]
- Konijeti, R.; Koyama, S.; Gray, A.; Barnard, R.J.; Said, J.W.; Castor, B.; Elashoff, D.; Wan, J.; Beltran, P.J.; Calzone, F.J.; et al. Effect of a low-fat diet combined with IGF-1 receptor blockade on 22Rv1 prostate cancer xenografts. Mol. Cancer Ther. 2012, 11, 1539–1546. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Numakura, K.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; Tsuchiya, N.; Habuchi, T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate 2012, 72, 1779–1788. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Suburu, J.; Gu, Z.; Cai, J.; Axanova, L.S.; Cramer, S.D.; Thomas, M.J.; Perry, D.L.; Edwards, I.J.; et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis. 2012, 33, 404–412. [Google Scholar] [CrossRef]
- Vandersluis, A.D.; Venier, N.A.; Colquhoun, A.J.; Sugar, L.; Pollak, M.; Kiss, A.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Exercise does not counteract the effects of a “westernized” diet on prostate cancer xenografts. Prostate 2013, 73, 1223–1232. [Google Scholar] [CrossRef]
- Pommier, A.J.; Dufour, J.; Alves, G.; Viennois, E.; De Boussac, H.; Trousson, A.; Volle, D.H.; Caira, F.; Val, P.; Arnaud, P.; et al. Liver x receptors protect from development of prostatic intra-epithelial neoplasia in mice. PLoS Genet. 2013, 9, e1003483. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Inoue, T.; Tsuchiya, N.; Satoh, S.; Nanjo, H.; Sasaki, T.; Habuchi, T. Diet-induced macrophage inhibitory cytokine 1 promotes prostate cancer progression. Endocr. Relat. Cancer 2014, 21, 39–50. [Google Scholar] [CrossRef]
- Moiola, C.P.; De Luca, P.; Zalazar, F.; Cotignola, J.; Rodriguez-Segui, S.A.; Gardner, K.; Meiss, R.; Vallecorsa, P.; Pignataro, O.; Mazza, O.; et al. Prostate tumor growth is impaired by CtBP1 depletion in high-fat diet-fed mice. Clin. Cancer Res. 2014, 20, 4086–4095. [Google Scholar] [CrossRef]
- Chang, S.N.; Han, J.; Abdelkader, T.S.; Kim, T.H.; Lee, J.M.; Song, J.; Kim, K.S.; Park, J.H. High animal fat intake enhances prostate cancer progression and reduces glutathione peroxidase 3 expression in early stages of TRAMP mice. Prostate 2014, 74, 1266–1277. [Google Scholar] [CrossRef]
- Liu, J.; Ramakrishnan, S.K.; Khuder, S.S.; Kaw, M.K.; Muturi, H.T.; Lester, S.G.; Lee, S.J.; Fedorova, L.V.; Kim, A.J.; Mohamed, I.E.; et al. High-calorie diet exacerbates prostate neoplasia in mice with haploinsufficiency of Pten tumor suppressor gene. Mol. Metab. 2015, 4, 186–198. [Google Scholar] [CrossRef]
- Cho, H.J.; Kwon, G.T.; Park, H.; Song, H.; Lee, K.W.; Kim, J.I.; Park, J.H. A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: Possible contribution of adipose tissue-derived cytokines. Nutrients 2015, 7, 2539–2561. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.B.; Bai, P.D.; Zhu, W.H.; Liu, S.H.; Hou, J.Y.; Xiong, Z.Q.; Ding, Q.; Jiang, H.W. Proinflammatory cytokines in prostate cancer development and progression promoted by high-fat diet. BioMed Res. Int. 2015, 2015, 249741. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, H.W.; Ding, Q. Insulin-Like growth factor 1 related pathways and high-fat diet promotion of transgenic adenocarcinoma mouse prostate (TRAMP) cancer progression. Actas Urol. Esp. 2015, 39, 161–168. [Google Scholar] [CrossRef]
- Lo, J.C.; Clark, A.K.; Ascui, N.; Frydenberg, M.; Risbridger, G.P.; Taylor, R.A.; Watt, M.J. Obesity does not promote tumorigenesis of localized patient-derived prostate cancer xenografts. Oncotarget. 2016, 7, 47650–47662. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Schokrpur, S.; Wu, L.; Doan, N.; Said, J.; Grogan, T.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effect of Dietary Omega-3 Fatty Acids on Tumor-Associated Macrophages and Prostate Cancer Progression. Prostate 2016, 76, 1293–1302. [Google Scholar] [CrossRef]
- Huang, M.; Koizumi, A.; Narita, S.; Inoue, T.; Tsuchiya, N.; Nakanishi, H.; Numakura, K.; Tsuruta, H.; Saito, M.; Satoh, S.; et al. Diet-induced alteration of fatty acid synthase in prostate cancer progression. Oncogenesis 2016, 5, e195. [Google Scholar] [CrossRef]
- Labbe, D.P.; Uetani, N.; Vinette, V.; Lessard, L.; Aubry, I.; Migon, E.; Sirois, J.; Haigh, J.J.; Begin, L.R.; Trotman, L.C.; et al. PTP1B Deficiency Enables the Ability of a High-Fat Diet to Drive the Invasive Character of PTEN-Deficient Prostate Cancers. Cancer Res. 2016, 76, 3130–3135. [Google Scholar] [CrossRef]
- Kwon, O.J.; Zhang, B.; Zhang, L.; Xin, L. High fat diet promotes prostatic basal-to-luminal differentiation and accelerates initiation of prostate epithelial hyperplasia originated from basal cells. Stem Cell Res. 2016, 16, 682–691. [Google Scholar] [CrossRef]
- Zhang, T.; Tseng, C.; Zhang, Y.; Sirin, O.; Corn, P.G.; Li-Ning-Tapia, E.M.; Troncoso, P.; Davis, J.; Pettaway, C.; Ward, J.; et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016, 7, 11674. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.Y.; Wen, H.C.; Huang, C.Y.; Hung, M.S.; Lu, H.C.; Chen, W.L.; Chang, C.H. Caveolin-1 Secreted from Adipose Tissues and Adipocytes Functions as an Adipogenesis Enhancer. Obesity 2017, 25, 1932–1940. [Google Scholar] [CrossRef]
- Kim, S.; Yang, X.; Li, Q.; Wu, M.; Costyn, L.; Beharry, Z.; Bartlett, M.G.; Cai, H. Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice. J. Biol. Chem. 2017, 292, 18422–18433. [Google Scholar] [CrossRef]
- Nara, T.; Narita, S.; Mingguo, H.; Yoshioka, T.; Koizumi, A.; Numakura, K.; Tsuruta, H.; Maeno, A.; Saito, M.; Inoue, T.; et al. Altered miRNA expression in high-fat diet-induced prostate cancer progression. Carcinogenesis 2016, 37, 1129–1137. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Inoue, T.; Koizumi, A.; Saito, M.; Tsuruta, H.; Numakura, K.; Satoh, S.; Nanjo, H.; Sasaki, T.; et al. Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget 2017, 8, 111780–111794. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef]
- Massillo, C.; Dalton, G.N.; Porretti, J.; Scalise, G.D.; Farre, P.L.; Piccioni, F.; Secchiari, F.; Pascuali, N.; Clyne, C.; Gardner, K.; et al. CTBP1/CYP19A1/estradiol axis together with adipose tissue impacts over prostate cancer growth associated to metabolic syndrome. Int. J. Cancer 2019, 144, 1115–1127. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef]
- Hu, M.B.; Xu, H.; Zhu, W.H.; Bai, P.D.; Hu, J.M.; Yang, T.; Jiang, H.W.; Ding, Q. High-fat diet-induced adipokine and cytokine alterations promote the progression of prostate cancer in vivo and in vitro. Oncol. Lett. 2018, 15, 1607–1615. [Google Scholar] [CrossRef]
- Ngo, T.H.; Barnard, R.J.; Anton, T.; Tran, C.; Elashoff, D.; Heber, D.; Freedland, S.J.; Aronson, W.J. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004, 64, 1252–1254. [Google Scholar] [CrossRef]
- Budhu, S.; Wolchok, J.; Merghoub, T. The importance of animal models in tumor immunity and immunotherapy. Curr. Opin. Genet. Dev. 2014, 24, 46–51. [Google Scholar] [CrossRef]
- Gingrich, J.R.; Barrios, R.J.; Morton, R.A.; Boyce, B.F.; DeMayo, F.J.; Finegold, M.J.; Angelopoulou, R.; Rosen, J.M.; Greenberg, N.M. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996, 56, 4096–4102. [Google Scholar]
- Irshad, S.; Abate-Shen, C. Modeling prostate cancer in mice: something old, something new, something premalignant, something metastatic. Cancer Metastasis Rev. 2013, 32, 109–122. [Google Scholar] [CrossRef]
- Hu, M.B.; Hu, J.M.; Jiang, L.R.; Yang, T.; Zhu, W.H.; Hu, Y.; Wu, X.B.; Jiang, H.W.; Ding, Q. Differential expressions of integrin-linked kinase, beta-parvin and cofilin 1 in high-fat diet induced prostate cancer progression in a transgenic mouse model. Oncol. Lett. 2018, 16, 4945–4952. [Google Scholar] [CrossRef]
- Ellwood-Yen, K.; Graeber, T.G.; Wongvipat, J.; Iruela-Arispe, M.L.; Zhang, J.; Matusik, R.; Thomas, G.V.; Sawyers, C.L. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003, 4, 223–238. [Google Scholar] [CrossRef]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2. [Google Scholar] [CrossRef]
- Shappell, S.B.; Thomas, G.V.; Roberts, R.L.; Herbert, R.; Ittmann, M.M.; Rubin, M.A.; Humphrey, P.A.; Sundberg, J.P.; Rozengurt, N.; Barrios, R.; et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004, 64, 2270–2305. [Google Scholar] [CrossRef]
- Buettner, R.; Scholmerich, J.; Bollheimer, L.C. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Khodadadi, S.; Sobhani, N.; Mirshekar, S.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M.; Dehsoukhteh, S.S. Tumor Cells Growth and Survival Time with the Ketogenic Diet in Animal Models: A Systematic Review. Int. J. Pre. Med. 2017, 8, 35. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Mourtzakis, M. The role of dietary fat throughout the prostate cancer trajectory. Nutrients 2014, 6, 6095–6109. [Google Scholar] [CrossRef]
- Heber, D.; Kritchevsky, D. Dietary Fats, Lipids, Hormones, and Tumorigenesis. Available online: https://www.springer.com/la/book/9780306453175 (accessed on 30 April 2019).
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutrition Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Muller, B.; Brockmoller, S.; Seppanen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Zhou, D.; Li, Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci. Rep. 2014, 4, 5959. [Google Scholar] [CrossRef]
- Othman, R. Dietary lipids and cancer. Libyan J. Med. 2007, 2, 180–184. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Berquin, I.M.; Edwards, I.J.; Kridel, S.J.; Chen, Y.Q. Polyunsaturated fatty acid metabolism in prostate cancer. Cancer Metastasis Rev. 2011, 30, 295–309. [Google Scholar] [CrossRef]
- Spencer, L.; Mann, C.; Metcalfe, M.; Webb, M.; Pollard, C.; Spencer, D.; Berry, D.; Steward, W.; Dennison, A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer 2009, 45, 2077–2086. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Scholmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef]
- Allott, E.H.; Masko, E.M.; Freedland, A.R.; Macias, E.; Pelton, K.; Solomon, K.R.; Mostaghel, E.A.; Thomas, G.V.; Pizzo, S.V.; Freeman, M.R.; et al. Serum cholesterol levels and tumor growth in a PTEN-null transgenic mouse model of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 196–203. [Google Scholar] [CrossRef]
- Babcook, M.A.; Joshi, A.; Montellano, J.A.; Shankar, E.; Gupta, S. Statin Use in Prostate Cancer: An Update. Nutr. Metab. Insights 2016, 9, 43–50. [Google Scholar] [CrossRef]
- Murtola, T.J.; Tammela, T.L.; Maattanen, L.; Huhtala, H.; Platz, E.A.; Ala-Opas, M.; Stenman, U.H.; Auvinen, A. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int. J. Cancer 2010, 127, 1650–1659. [Google Scholar] [CrossRef]
- Roberts, D.L.; Dive, C.; Renehan, A.G. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu. Rev. Med. 2010, 61, 301–316. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Giovannucci, E.; Mucci, L.; Qiu, W.; Nguyen, P.L.; Gaziano, J.M.; Pollak, M.; Stampfer, M.J. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet. Oncol. 2008, 9, 1039–1047. [Google Scholar] [CrossRef]
- Poloz, Y.; Stambolic, V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015, 6, e2037. [Google Scholar] [CrossRef]
- Lin, J.; Yang, R.; Tarr, P.T.; Wu, P.H.; Handschin, C.; Li, S.; Yang, W.; Pei, L.; Uldry, M.; Tontonoz, P.; et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 2005, 120, 261–273. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Gronberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Deyoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007, 56, 16–23. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Tsuchiya, N.; Ma, Z.; Numakura, K.; Obara, T.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; et al. Overexpression of Fn14 promotes androgen-independent prostate cancer progression through MMP-9 and correlates with poor treatment outcome. Carcinogenesis. 2011, 32, 1589–1596. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J. The effects of diet on circulating sex hormone levels in men. Nutr. Res. Rev. 2000, 13, 159–184. [Google Scholar] [CrossRef]
| Authors | Years | Animal Models | Tumors | Diet Summary | End Point | Summary of the Results |
|---|---|---|---|---|---|---|
| Wang [11] | 1995 | Nude mice | LNCaP | 40.5%, 30.8%, 21.2%, 11.6%, or 2.3l% fat | Tumor growth rates, tumor weights, ratios of final tumor weights to animal weights, PSA | Groups that continued to receive a 40.5l% fat diet were substantially greater tumor growth rates, final tumor weights, and ratios of final tumor weights to animal weights than those whose diets were changed to 2.3 kcal%, 11.6 kcal%, or 21.2 kcal% fat. |
| Connoly [12] | 1997 | Nude mice | a) DU145 subcutaneous xenograft, b) DU145 into prostate | a) 18:2 ω-6-rich vs. 18:3 ω-3-rich vs. 20:5 and 22:6 ω-3-rich, b) ω-6-rich vs. a LF | Tumor growth | a) 18:2 ω-6-rich vs. 18:3 ω-3-rich mice were similar; a 30% reduction in tumor growth was observed in the 20:5 and 22:6 ω-3-rich groups. b) The mean tumor weight in the ω-6-rich group was twice that in the low-fat group. |
| Ngo [13] | 2002 | LNCaP cultured with human serum | Before and after residential diet and exercise | Cell growth, apoptosis, necrosis | Serum-stimulated LNCaP cell growth was reduced by 30% in post-11-day serum and by 44% in long-term serum relative to baseline. LNCaP cells incubated with post-diet and exercise serum showed higher apoptosis/necrosis, compared to baseline. | |
| Barnard [14] | 2003 | LNCaP cultured with human serum | Volunteer serum (control, LF and exercise, exercise alone) | Cell growth | Both the LF/exercise and exercise alone groups had reduced LNCaP cell growth compared to control. | |
| Ngo [15] | 2003 | CB17 SCID | a) LAPC-4 xenograft, b) LAPC-4 culture with 10% mouse serum | HFD (42%) vs. LFD (12%) | a) tumor growth, PSA, b) cell growth | LFD mice had significantly slower tumor growth rates and lower serum PSA levels compared to HFD mice. LAPC-4 cells cultured in vitro with media containing serum from LFD mice demonstrated slower growth than LAPC-4 cells cultured in media containing HFD mice serum. |
| Ngo [16] | 2004 | CB17 SCID | LAPC-4 xeograft | HFD (42%) vs LFD (12%) | Tumor growth, survival | Tumor latency and mouse survival were significantly longer in the LFD castration versus HFD castration group. |
| Venkateswaran [17] | 2007 | Swiss nu/nu | LNCaP xenograft | HC + HFD vs. LC + HFD | Tumor growth | Mice on the HC–HFD diet experienced increased tumor growth. |
| Berquin [18] | 2007 | Prostate-specific Pten deletion mouse | High ω-6 vs. ω-3 diet | Prostate weight, rate of invasive carcinoma | Prostate weight was significantly lower in mice fed high ω-3; half of the mice fed ω-3 develop invasive carcinoma, whereas 80% of the mice fed high ω-6 diet had invasive carcinoma. | |
| Kobayashi [19] | 2008 | Prostate specific High-Myc transgenic mouse | a)LNCaP, b)MycCap with mice serum | HFD (42%) vs LFD (12%) | Rate of mPIN and cancer incidence | The number of mice that developed invasive adenocarcinoma at 7 months was 27 % less in the LFD group (12/28) compared to the HFD group (23/33, p = 0.04). Epithelial cells in PIN lesions in the LFD group had a significantly lower proliferative index compared to epithelial cells in the HFD group (21.7% vs. 28.9%, p < 0.05). |
| Freedland [20] | 2008 | SCID | LAPC-4 xenograft | NCKD (84% fat) vs. LFD (12% fat) vs. WD (40% fat) | Tumor growth, survival | NCKD mice tumor volumes were 33% smaller than WD mice (rank-sum, p = 0.009). No differences in tumor volume were observed between LFD and NCKD mice with the latter having the longest survival. |
| Narita [16] | 2008 | BALB/c-nu/nu | LNCap xenograft | HF (56.7%) vs. LF (10.2%) | Tumor volume, PSA | Tumor volume and serum PSA levels were significantly higher in the HFD group than in the LFD group. |
| Mavropoulos [21] | 2009 | SCID | LNCaP xenograft | NCKD (83% fat) vs. LFD (12% fat) vs. WD (40% fat) | Tumor growth, survival | Tumor volumes in the WD group remained significantly larger than tumor volumes in the LFD and NCKD groups. Survival was significantly prolonged for the LF (hazard ratio, 0.49; 95% confidence interval, 0.29–0.79; p = 0.004) and NCKD groups (hazard ratio, 0.59; 95% confidence interval, 0.37–0.93; p = 0.02). |
| Tamura [22] | 2009 | Nude mice | LNCaP xenograft | HFD (14%) vs LFD (6%) | Tumor growth | LNCaP-Mock cells did not reveal any significantgrowth promotion by breeding with HFD. HFD breeding significantly promoted the growth of LNCaP-ELOVL7-1 cells in vivo (p = 0.0081). |
| Kalaany [23] | 2009 | Prostate-specific Pten deletion mouse | Ad libitum vs. CR | Percentage of proliferation and apoptosis | CR did not affect a PTEN-null mouse model of prostate cancer but significantly decreased tumor burden in a mouse model of lung cancer lacking constitutive PI3K signaling. | |
| Bushemeyer [24] | 2010 | SCID | LAPC-4 xenograft | 7 types of diet | Tumor growth, survival | No significant differences in tumor volume were observed among the various groups at any time point. Overall, the treatment group was not significantly related to survival. |
| Llaverias [25] | 2010 | TRAMP mouse | WD (21.2%) vs. chow (4.5%) | Prostate tumor incidence and progression | TRAMP mice fed a WD were shown to develop larger tumors compared to mice fed a chow diet. 67% (6 of 9 mice) of TRAMP mice fed a WD exhibited at least one metastatic focus, whereas 43% (3 of 7 mice) of mice fed a chow diet exhibited the same. | |
| Lloyd [26] | 2010 | SCID | LAPC-4 xenograft | WD (40%) vs. chow (12%) | Tumor growth, survival | No difference in tumor growth or survival between chow and WD was observed. |
| Aronson [27] | 2010 | LNCaP cultured with human serum | PCa men with LF, high-fiber, soy protein-supplemented diet or WD for 4 weeks | Cell growth | LF, high-fiber, soy protein-supplement diet decreased LNCaP cancer cell growth. | |
| Masko [28] | 2010 | SCID CB17 | LAPC-4 xenograft | NCKD (84% fat), 10% carbohydrate diet (74% fat), or 20% carbohydrate diet (64% fat). | Tumor volume, PSA, survival | Tumors were significantly larger in the 10% carbohydrate group on days 52 and 59 (p < 0.05) and at no other point during the study. Diet did not affect survival (p = 0.34). |
| Akinsete [29] | 2012 | C3 (1) Tag transgenic mouse | High ω-6 vs. ω-3 diet | Tumor progression, apoptosis | Slower progression of tumorigenesis and enhanced apoptosis was observed in dorsalateral prostate of high ω-3 diet mice than in high ω-6 diet mice. | |
| Mao [30] | 2012 | Homozygous prostate-specific RXRα knockout mouse | NWD (higher fat content, reduced calcium, vitamin D, and fiber) or AIN-76A | A significant joint effect of NWD and RXRα status in developing mPIN, but interaction was not significant owing to the small sample size. | ||
| Bonorden [31] | 2012 | a) TRAMP mouse, b) C57/BL6 | b) TRAMP-C2 allograft | LFD (AIN-93M) vs. AIN-93M-HFD (33%) | a) tumor differentiation, percentage of metastasis, b) tumor weight and volume | No difference in the prostates of TRAMP mice. TRAMP-C2 cells grew faster when the mice were fed a HFD. |
| Konijeti [32] | 2012 | SCID | 22Rv1 | HFD (43.3%) + saline, HFD + IGF-1R-Ab, LFD (12.4%) + saline, LFD + IGF-1R-Ab | Tumor volume | No significant differences in final tumor volumes or final tumor weights were observed between the treatment groups. At day 14 of the intervention, the mean tumor volume was significantly lower in the LFD + IGF-1R-Ab group than in the HF group. |
| Huang [33] | 2012 | BALB/c-nu/nu | LNCaP xenograft | HFD (59.9%) vs. HCD (9.5%) vs CD (41.2%) | Tumor volume | The tumor growth of LNCaP xenograft was significantly higher in the HFD group than in the HCD and CD groups. |
| Wang [34] | 2012 | a) nude mice, b) Prostate-specific Pten deletion mouse | a) pten-/- allograft | High ω-6 vs ω-3 diet | a) tumor volume and weight, b) body weight, invasion rate, Ki67 | ω-3 PUFA resulted in slower growth of castration-resistant tumors compared to ω-6 PUFA. |
| Vandelsluis [35] | 2013 | Nu/nu athymic mice | LNCaP xenograft | HFD (23.8%) vs. SD (6.0%) | Tumor volume | The HF with exercise group showed significantly higher tumor growth rates compared to all other groups. The SD with exercise group had significantly lower tumor growth rates of compared to the HFD without exercise group. |
| Pommier [36] | 2013 | C57BL/6 Lxra and Lxrb double knockout mice | Normal or hypercholesterolemic diet | Presence of PIN, number of Ki-67 positive cells | High-cholesterol diet induced proliferation in LXR mutant mouse prostate. | |
| Huang [37] | 2014 | BALB/c-nu/nu | LNCaP xenograft | HFD (59.9%) vs. LFD (9.5%) | Tumor volume | The tumor growth of LNCaP xenograft was significantly higher in the HFD group than the LFD groups. |
| Moiola [38] | 2014 | Swiss nu/nu | PC-3 xenograft | HFD (homemade) vs. CD | Tumor volume | No significant differences in tumor growth were observed in CD-fed mice; however, we found that only 60% of HFD-fed mice inoculated with CtBP1-depleted cells developed a tumor. |
| Chang [39] | 2014 | TRAMP mouse | HFD (45%) vs. CD (10%) | Histophathologica score | Histopathological scores in the dorsal and lateral lobes were higher in the 10-week HFD group than in the 10-week CD group. | |
| Liu [40] | 2015 | Pten haploinsufficientmale mice | High calorie vs. regular diet | mPIN score | High-calorie diet caused neoplastic progression, angiogenesis, inflammation, and epithelial-mesenchymal transition | |
| Cho [41] | 2015 | a) TRAMP, b) C57BL/6J | b) TRAMPC2 allograft | HFD (60%) vs. CD (10%) | Rate of poorly differentiated ca, tumor weight | In TRAMP mice, HFD feeding increased the incidence of poorly differentiated carcinoma. In the allograft model, HFD increased solid tumor growth and the expression of proteins related to proliferation/angiogenesis. |
| Xu [42] | 2015 | TRAMP | HFD (40%) vs. ND (16%) | Tumor formation rate, survival | The mortality of TRAMP mice from HFD group was significantly higher than that of normal diet group (23.81% and 7.14%, p = 0.035). The tumor incidence of HFD TRAMP mice at 20th week was significantly higher than normal diet group (78.57% and 35.71%, p = 0.022) | |
| Xu [43] | 2015 | TRAMP | HF (40%) vs. ND (16%) | Tumor incidence, survival | TRAMP mice in HFD group had significantly higher mortality rates than those in the normal diet group (p = 0.032). The HFD group had a significantly higher tumor formation rate at age 20 weeks than the normal diet group (p = 0.045). | |
| Lo [44] | 2016 | SCID | PDX kidney capsule xenograft | HF (43%) vs LF (6%) | Pathology and biomarker expression | Prostate cancer tumorigenicity is not accelerated in the setting of diet-induced obesity or in the presence of human PPAT. |
| Liang [45] | 2016 | Immunocompetent FVB mice | MycCap alloraft | High ω-6 vs. ω-3 diet | Tumor volume | Tumor volumes were significantly smaller in the ω-3 than in the ω-6 group (p = 0.048). |
| Huang [46] | 2016 | BALB/c-nu/nu | LNCap xenograft | HFD (59.9%) vs LF (9.5%) | Intratumoral AKT and Extracellular Signal-regulated Kinase (ERK) activation, AMPK inactivation | HFD resulted in AKT and ERK activation and AMPK inactivation. |
| Labbe [47] | 2016 | Prostate specific Pten and Ptpn1 deletion mouse | HFD vs. chow | Microinvasive rate | PCa in Pten-/-Ptpn1-/- mice was characterized by increased cell proliferation and Akt activation, interpreted to reflect a heightened sensitivity to IGF-1 stimulation upon HFD feeding | |
| Kwon [48] | 2016 | 14K-creER PTEN (K14-CreER;Ptenfl/fl;mTmG (K14-Pten-mTmG) triple transgenic mice | HFD vs. RD | PIN 3/4 rate | HFD increased the number of PIN. | |
| Zhang [49] | 2016 | C57BL6 | RM1 mouse prostate cancer alloglaft | HFD (58%) vs. chow | Tumor growth | CXCL1 chemokine gradient was required for the obesity-dependent tumor ASC recruitment, vascularization and tumor growth promotion |
| Chang [50] | 2017 | C57BL6 | HFD (45%) vs. chow | Cav-1 secretion from adipose tissue | Cav-1 secretion was evident in adipose tissues and were substantially promoted in HFD-fed mice. | |
| Kim [51] | 2017 | SCID | PC-3 xenograft | 10%, 45%, or 60% fat | Tumor size, tumor weight | The 45% and 60% fat diets significantly promoted the growth of xenografts comparison to the 10% fat diet |
| Nara [52] | 2017 | a) BALB/c-nu/nu | a) LNCap xenograft, b) PC-3 and DU145 cultured with mice serum | HFD (59.9%) vs. CD (9.5%) | a) Tumor volume, b) cell proliferation | The tumor growth of prostate cancer LNCaP xenograft was significantly higher in the HFD group than in the CD groups. Cells cultured with HFD mouse serum had higher proliferation. |
| Huang [53] | 2017 | BALB/c-nu/nu | Intraperitoneal injection PC-3M-luc-C6 | HFD (59.9%) vs. LF (9.5%) | Luciferase activity (IVIS), number of metastasis | HFD and PrSC increased luciferase activity and number of metastasis. |
| Hayashi [54] | 2018 | Prostate-specific Pten deletion mouse | HFD (62.2%) vs. CD (12.5%) | Tumor growth | HFD accelerated tumor growth alogn with the inflammatory response. | |
| Massillo [55] | 2018 | C57BL/6J | TRAMP C1 allograft | HFD (37%) vs. CD (5%) | Tumor volume | HFD significantly increased tumor growth and serum estradiol in mice. |
| Chen [56] | 2018 | Prostate specific Pten and Pml deletion mouse | HFD (60%) vs. chow (17%) | Rate of mice having metastases | A HFD-derived metastatic progression and increases lipid abundance in prostate tumors | |
| Hu [57] | 2018 | TRAMP | HFD (40%) vs. CD (16%) | Proportion of poor tumor differentiation and tumor metastasis | A trend toward poorer PCa differentiation was observed in HFD-fed mice, while no statistical significance was detected. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. J. Clin. Med. 2019, 8, 597. https://doi.org/10.3390/jcm8050597
Narita S, Nara T, Sato H, Koizumi A, Huang M, Inoue T, Habuchi T. Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. Journal of Clinical Medicine. 2019; 8(5):597. https://doi.org/10.3390/jcm8050597
Chicago/Turabian StyleNarita, Shintaro, Taketoshi Nara, Hiromi Sato, Atsushi Koizumi, Mingguo Huang, Takamitsu Inoue, and Tomonori Habuchi. 2019. "Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression" Journal of Clinical Medicine 8, no. 5: 597. https://doi.org/10.3390/jcm8050597
APA StyleNarita, S., Nara, T., Sato, H., Koizumi, A., Huang, M., Inoue, T., & Habuchi, T. (2019). Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. Journal of Clinical Medicine, 8(5), 597. https://doi.org/10.3390/jcm8050597





