Feasibility and Effectiveness of Electrochemical Dermal Conductance Measurement for the Screening of Diabetic Neuropathy in Primary Care. Decoding Study (Dermal Electrochemical Conductance in Diabetic Neuropathy)
Abstract
1. Introduction
2. Experimental Section
2.1. Hypothesis and Objectives
2.1.1. Main Objective
2.1.2. Specific Objectives
2.2. Material and Methods
2.2.1. Design
2.2.2. Sites
2.2.3. Study Subjects
2.2.4. Sample Size
2.2.5. Variables and Data Collection
First Visit: In Primary Care
Second Visit: In Hospital
Third Visit: In Primary Care
2.2.6. Statistical Analysis
2.2.7. Ethics Approval and Consent to Participate
2.2.8. Registry
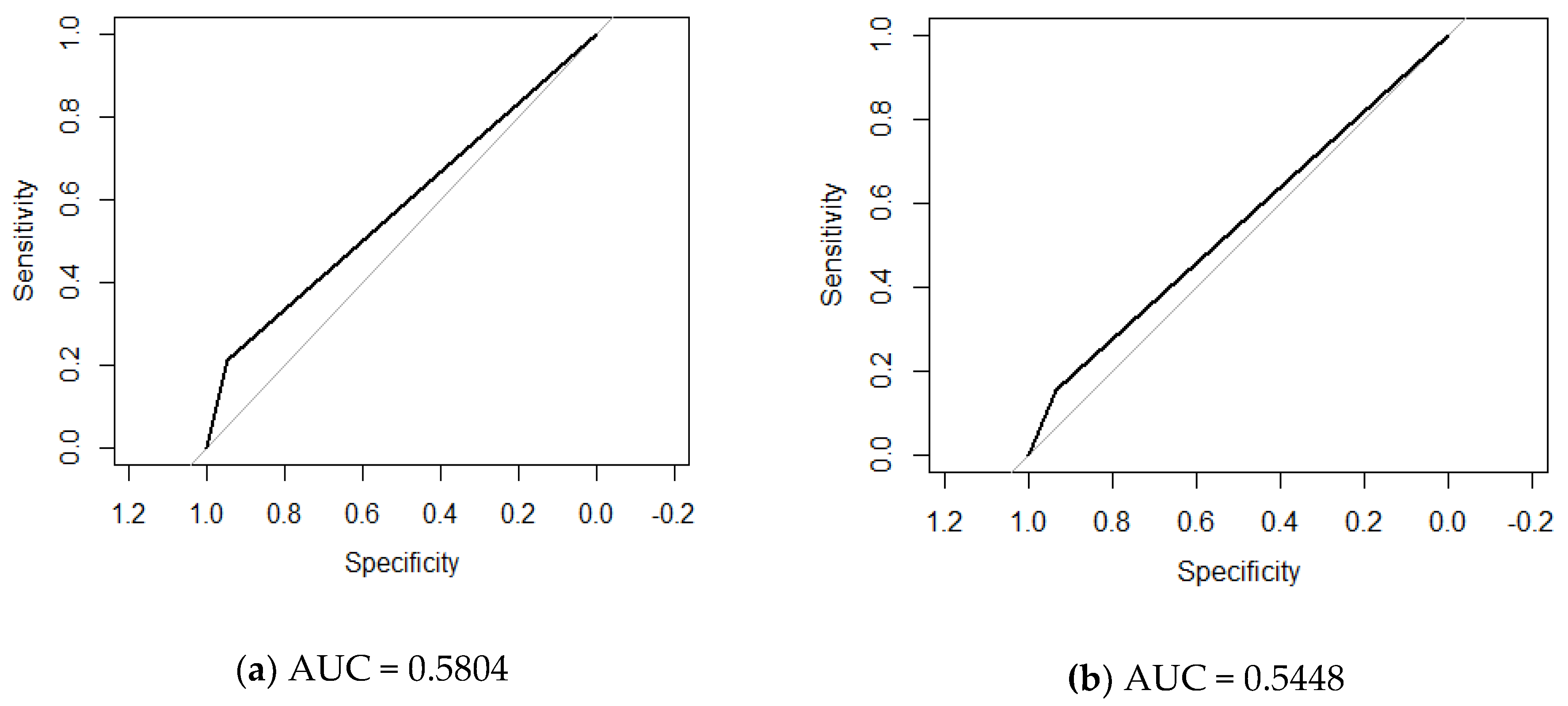
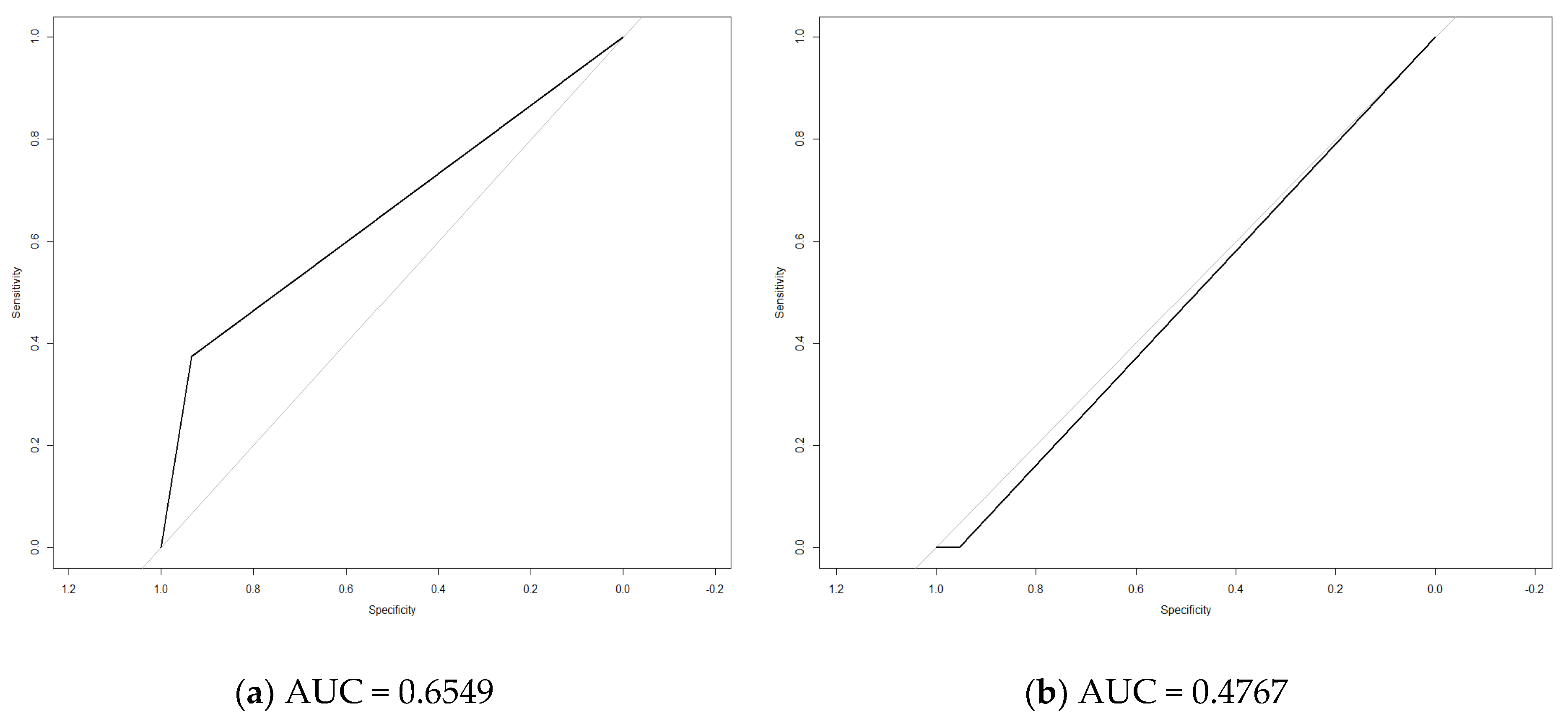
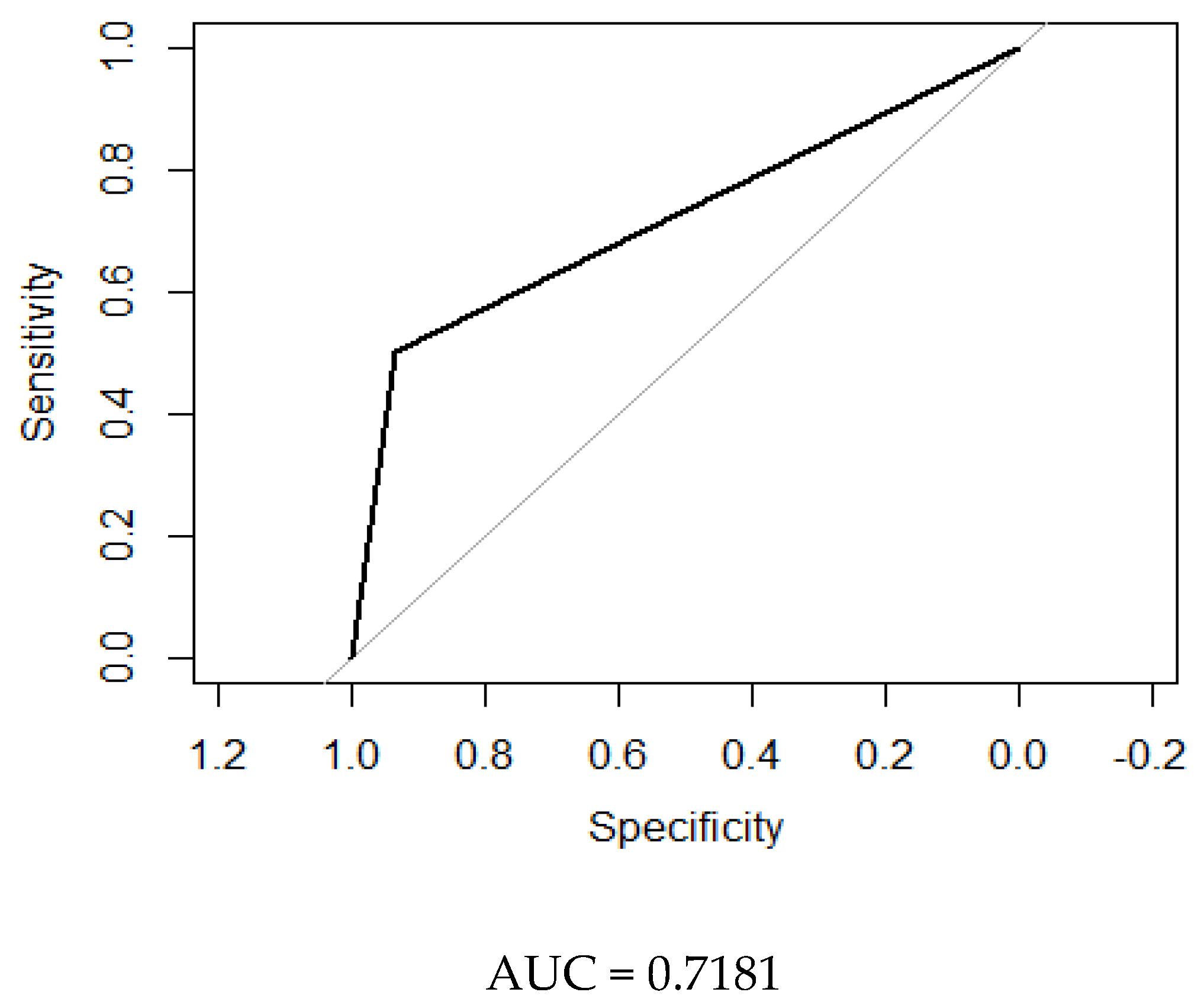
3. Results
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2012, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Said, G. Diabetic neuropathy—A review. Nat. Clin Pract. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bernal, D.S.; Tabasco, M.M.; Riera, M.H.; Pedrola, M.S. Etiología y manejo de la neuropatía diabética dolorosa. Rev. Soc. Esp. Dolor. 2010, 17, 286–296. (In Spanish) [Google Scholar] [CrossRef]
- Casellini, C.M.; Parson, H.K.; Richardson, M.S.; Nevoret, M.L.; Vinik, A.I. Sudoscan, a Noninvasive Tool for Detecting Diabetic Small Fiber Neuropathy and Autonomic Dysfunction. Diabetes Technol. Ther. 2013, 15, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Brandenburg, N.A.; Dukes, E.; Hoffman, D.L.; Tai, K.S.; Stacey, B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manag. 2005, 30, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The EURODIAB IDDM Complications Study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef]
- Dyck, P.J.; Kratz, K.M.; Lehman, K.A.; Karnes, J.L.; Melton, L.J.; O’Brien, P.C.; Litchy, W.J.; Windebank, A.J.; Smith, B.E.; Low, P.A.; et al. The Rochester Diabetic Neuropathy Study: Design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology 1991, 41, 799–807. [Google Scholar] [CrossRef]
- Pirart, J. Diabetes mellitus and its degenerative complications: A prospective study of 4400 patients observed between 1947 and 1973. Diabetes Care 1978, 1, 168–188. [Google Scholar] [CrossRef]
- Cabezas-Cerrato, J.; Neuropathy Spanish Study Group of the Spanish Diabetes Society. The prevalence of clinical diabetic polyneuropathy in Spain: A study in primary care and hospital clinic groups. Diabetologia 1998, 41, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.L.; Aguirre, D.R.; Rodríguez, M.C. Neuropatía periférica de los miembros inferiores en diabéticos tipo 2 de diagnóstico reciente. Av Endocrinol 2006, 22, 149. (In Spanish) [Google Scholar]
- Samur, J.A.A.; Rodríguez, M.Z.C.; Olmos, A.I.; Bárcena, D.G. Prevalencia de neuropatía periférica en diabetes mellitus. Acta Médica Grupo Ángeles 2006, 4, 13. (In Spanish) [Google Scholar]
- Franklin, G.M.; Shetterly, S.M.; Cohen, J.A.; Baxter, J.; Hamman, R.F. Risk factors for distal symmetric neuropathy in NIDDM: The San Luis Valley Diabetes Study. Diabetes Care 1994, 17, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Anglin, R.E.; Hunt, D.L.; Panju, A. Does this patient with diabetes have large-fiber peripheral neuropathy? JAMA 2010, 303, 1526–1532. [Google Scholar] [CrossRef]
- González, C.P. Monofilamento de Semmes-Weinstein. Diabetes Práctica Actual Habilidades En Aten Primaria 2010, 1, 8–19. (In Spanish) [Google Scholar]
- Feng, Y.; Schlösser, F.J.; Sumpio, B.E. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J. Vasc. Surg. 2009, 50, 675–682. [Google Scholar] [CrossRef]
- Gordon Smith, A.; Lessard, M.; Reyna, S.; Doudova, M.; Robinson Singleton, J. The Diagnostic Utility of Sudoscan for Distal Symmetric Peripheral Neuropathy. J. Diabetes Complicat. 2014. Available online: http://www.sciencedirect.com/science/article/pii/S1056872714000555 (accessed on 7 February 2018). [CrossRef]
- Raisanen, A.; Eklund, J.; Calvet, J.H.; Tuomilehto, J. Sudomotor Function as a Tool for Cardiorespiratory Fitness Level Evaluation: Comparison with Maximal Exercise Capacity. Int. J. Environ. Res. Public Health 2014, 11, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Kantikar, V.V.; Pande, A.J.; Deslypere, J.P. Quick and Simple Evaluation of Sudomotor Function for Screening of Diabetic Neuropathy. ISRN Endocrinol. 2012, 2012, 103714. Available online: http://downloads.hindawi.com/journals/isrn.endocrinology/2012/103714.pdf (accessed on 25 January 2018). [CrossRef] [PubMed]
- Eranki, V.; Santosh, R.; Rajitha, K.; Pillai, A.; Sowmya, P.; Dupin, J.; Calvet, J. Sudomotor function assessment as a screening tool for microvascular complications in type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 101, e11–e13. [Google Scholar] [CrossRef]
- Luk, A.O.Y.; Fu, W.-C.; Li, X.; Ozaki, R.; Chung, H.H.Y.; Wong, R.Y.M.; So, W.-Y.; Chow, F.C.C.; Chan, J.C.N. The Clinical Utility of SUDOSCAN in Chronic Kidney Disease in Chinese Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0134981. [Google Scholar] [CrossRef]
- Ramachandran, A.; Moses, A.; Shetty, S.; Thirupurasundari, C.J.; Seeli, A.C.; Snehalatha, C.; Singvi, S.; Deslypere, J.-P. A new non-invasive technology to screen for dysglycaemia including diabetes. Diabetes Res. Clin. Pract. 2010, 88, 302–306. [Google Scholar] [CrossRef]
- Selvarajah, D.; Cash, T.; Davies, J.; Sankar, A.; Rao, G.; Grieg, M.; Pallai, S.; Gandhi, R.; Wilkinson, I.D.; Tesfaye, S. SUDOSCAN: A Simple, Rapid, and Objective Method with Potential for Screening for Diabetic Peripheral Neuropathy. PLoS ONE 2015, 10, 0138224. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Portillo, R.; Lira, D.; Quiñónez, M. Evaluación Neurofisiológica y Clínica en Pacientes con Diabetes Mellitus. 2005, pp. 11–18. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1025-55832005000100003 (accessed on 27 February 2018).
- Lu, B.; Hu, J.; Wen, J.; Zhang, Z.; Zhou, L.; Li, Y.; Hu, R. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes–ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS ONE 2013, 8, e61053. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Rathmann, W.; Dickhaus, T.; Meisinger, C.; Mielck, A. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy. Diabetes Care 2008, 31, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Teruel, C.M.; Fernández-Real, J.M.; Ricart, W.; Valent, R.F.; Vallés, M.P. Prevalence of diabetic retinopathy in the region of Girona. Study of related factors. Arch. Soc. Espanola Oftalmol. 2005, 80, 85–91. [Google Scholar]
- Cabré, J.J.; Mur, T.; Costa, B.; Barrio, F.; López-Moya, C.; Sagarra, R.; García-Barco, M.; Vizcaíno, J.; Bonaventura, I.; Ortiz, N.; et al. Feasibility and effectiveness of electrochemical dermal conductance measurement for the screening of diabetic neuropathy in primary care. DECODING Study (Dermal Electrochemical Conductance in Diabetic Neuropathy). Rationale and design. Medicine 2018, 97, e10750. [Google Scholar] [CrossRef]
- Meijer, J.W.; Van Sonderen, E.; Blaauwwiekel, E.E.; Smit, A.J.; Groothoff, J.W.; Eisma, W.H.; Links, T.P. Diabetic neuropathy examination: A hierarchical scoring system to diagnose distal polyneuropathy in diabetes. Diabetes Care 2000, 23, 750–753. [Google Scholar] [CrossRef]
- Krieger, S.M.; Reimann, M.; Haase, R.; Henkel, E.; Hanefeld, M.; Ziemssen, T. Sudomotor Testing of Diabetes Polyneuropathy. Front. Neurol. 2018, 9, 803. [Google Scholar] [CrossRef]
- Binns-Hall, O.; Selvarajah, D.; Sanger, D.; Walker, J.; Scott, A.; Tesfaye, S. One-stop microvascular screening service: An effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet. Med. 2018, 35, 887–894. [Google Scholar] [CrossRef]
- Mao, F.; Liu, S.; Qiao, X.; Zheng, H.; Xiong, Q.; Wen, J.; Zhang, S.; Zhang, Z.; Ye, H.; Shi, H.; et al. SUDOSCAN, an effective tool for screening chronic kidney disease in patients with type 2 diabetes. Exp. Ther. Med. 2017, 14, 1343–1350. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Yu, J.; Liu, S.; Zhang, R.; Ma, X.; Yang, Y.; Wu, P. Diagnostic Accuracy of Monofilament Tests for Detecting Diabetic Peripheral Neuropathy: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2017, 2017, 8787261. [Google Scholar] [CrossRef]
- Veresiu, A.I.; Bondor, C.I.; Florea, B.; Vinik, E.J.; Vinik, A.I.; Gavan, N.A. Detection of undisclosed neuropathy and assessment of its impact on quality of life: A survey in 25,000 Romanian patients with diabetes. J. Diabetes Its Complicat. 2015, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Sudoscan Use Instructions. Available online: https://www.sudoscan.com/for-physicians/research-articles/ (accessed on 18 March 2019).
| Variable | Participants with T2D (n = 100) | Participants with Prediabetes (n = 50) | Participants with Normal Glucose Tolerance (n = 47) | p |
|---|---|---|---|---|
| Age (mean ± SD) | 64.65 ± 7.5 | 62.7 ± 6.8 | 63.47 ± 7.4 | 0.30 |
| Gender (% men) | 50 | 38 | 38.3 | 0.33 |
| Origin country (% Spain) | 93 | 100 | 93.6 | 0.70 |
| Family history of diabetes (%) | 83 (83) | 26 (50.2) | 33 (70.2) | <0.001 |
| Current smoker; n (%) | 8 (8) | 2 (4) | 3 (6.4) | 0.99 |
| Alcohol consumption; n (%) | 59 (59) | 34 (68) | 30 (63.8) | 0.70 |
| T2D evolution (mean ± SD, year) | 10.6 ± 7.52 | - | - | - |
| T2D complications (%) | ||||
| Cardiopathy | 4 | 1 | 0 | - |
| retinopathy | 6 | 0 | 0 | - |
| peripheral vascular disease | 7 | 0 | 0 | - |
| stroke | 3 | 0 | 1 | - |
| nephropathy | 6 | 0 | 0 | - |
| NIAD treatment (n) | 152 | - | - | - |
| Insulin treatment (n) | 29 | - | - | - |
| Antihypertensive treatment; n (%) | 74 (74) | 28 (56) | 25 (53.1) | 0.02 |
| Cholesterol-lowering agents; n (%) | 62 (62) | 18 (36) | 15 (31.9) | 0.001 |
| Antiaggregant treatment; n (%) | 21 (21) | 8 (16) | 6 (12.7) | 0.33 |
| Height (mean ± SD, cm) | 162.4 ± 9.7 | 162.7 ± 8.8 | 160.8 ± 7.5 | 0.54 |
| Weight (mean ±SD, kg) | 79.6 ± 13.6 | 85.3 ± 18.3 | 74.9 ± 14.9 | 0.004 |
| BMI (mean ±SD, kg/m2) | 30.3 ± 4.5 | 33.28 ± 10.8 | 28.6 ± 4.6 | 0.003 |
| Waist circumference (mean ± SD, cm) | 105.0 ± 11.2 | 108,0 ± 15.3 | 109.5 ± 11.4 | 0.016 |
| SBP (mean ± SD, mmHg) | 136.5 ± 14.1 | 132.3 ± 13.6 | 125.8 ± 15.2 | <0.001 |
| DBP (mean ± SD, mmHg) | 78.2 ± 10.4 | 79.8 ± 7.9 | 75.3 ± 9.5 | 0.06 |
| HbA1c (mean ± SD, %) | 7.2 ± 1.2 | 5.9 ± 0,3 | 5.3 ± 0.4 | 0.02 |
| Glomerular Filtration Rate (mean ± SD, mL/min/1.73 m2) | 67.3 ± 14.4 | 87.0 ± 7.3 | 72.9 ± 19.3 | <0.001 |
| Total cholesterol (mean ± SD, mg/mL) | 191.9 ± 39.4 | 196.8 ± 33.9 | 190.9 ± 30.9 | 0.67 |
| HDL-chol (mean ± SD, mg/dL) | 48.7 ± 11.0 | 58.9 ± 14.5 | 54.9 ± 12.2 | <0.001 |
| LDL-chol (mean ± SD, mg/dL) | 111.7 ± 33.3 | 114.4 ± 32.0 | 116.5 ± 28.0 | 0.69 |
| Triglycerides (mean ± SD, mg/dL) | 159.6 ± 90.0 | 120.7 ± 49.2 | 117.0 ± 44.7 | <0.001 |
| DN4 questionnaire (% score = 0) | 61 | 70 | 74.5 | <0.001 |
| Gold Standard | ||||
|---|---|---|---|---|
| EMG | MFT | NDS | DN4 | |
| DEC | Sen = 21.2% | Sen = 15.3% | Sen = 33.3% | Sen = 21.4% |
| Spe = 94.8% | Spe = 93.5% | Spe = 94% | Spe = 93.4% | |
| PPV = 46.6% | PPV =26.6% | PPV = 26.6% | PPV = 20% | |
| NPV = 81.3% | NPV = 87.9% | NPV = 95.6% | NPV = 93.4% | |
| LR+ = 4.13 | LR+ = 2.35 | LR+ = 5.55 | LR+ = 3.24 | |
| LR− = 0.83 | LR− = 0.90 | LR− = 0.71 | LR− = 0.77 | |
| Gold Standard | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFT | EMG | NDS | DN4 | ||||||||||
| DM | No-DM | Pre-DM | DM | No-DM | Pre-DM | DM | No-DM | Pre-DM | DM | No-DM | Pre-DM | ||
| DEC | Sen | 25% | 0% | 14% | 20% | 0% | 5% | 37% | 0% | 1% | 30% | 0 | 0 |
| Spe | 93% | 95% | 93% | 96% | 95% | 93% | 93% | 95% | 93% | 93% | 95.5% | 91% | |
| PPV | 33% | 0% | 25% | 67% | 0% | 25% | 33% | 0% | 25% | 33% | 0 | 0 | |
| NPV | 90% | 84% | 87% | 74% | 98% | 96% | 94.5% | 93% | 1% | 92% | 95.5% | 93% | |
| LR+ | 3.57 | 0 | 2 | 5 | 0 | 0.71 | 5.28 | 0 | 0.14 | 4.28 | 0 | 0 | |
| LR− | 0.80 | 20 | 0.92 | 0.83 | 20 | 1.02 | 0.67 | 20 | 1.06 | 0.75 | 22.2 | 11.1 | |
| Conductance (µS) | ||||
|---|---|---|---|---|
| Normal Tolerance | Pre-DM | DM | ||
| DEC | H-DEC (hands) | 70.3 ± 12.7 | 63.9 ± 19.2 | 51.8 ± 17.2 |
| F-DEC (feet) | 76.8 ± 15.8 | 73.3 ± 22.5 | 71.3 ± 18.0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabré, J.J.; Mur, T.; Costa, B.; Barrio, F.; López-Moya, C.; Sagarra, R.; García-Barco, M.; Vizcaíno, J.; Bonaventura, I.; Ortiz, N.; et al. Feasibility and Effectiveness of Electrochemical Dermal Conductance Measurement for the Screening of Diabetic Neuropathy in Primary Care. Decoding Study (Dermal Electrochemical Conductance in Diabetic Neuropathy). J. Clin. Med. 2019, 8, 598. https://doi.org/10.3390/jcm8050598
Cabré JJ, Mur T, Costa B, Barrio F, López-Moya C, Sagarra R, García-Barco M, Vizcaíno J, Bonaventura I, Ortiz N, et al. Feasibility and Effectiveness of Electrochemical Dermal Conductance Measurement for the Screening of Diabetic Neuropathy in Primary Care. Decoding Study (Dermal Electrochemical Conductance in Diabetic Neuropathy). Journal of Clinical Medicine. 2019; 8(5):598. https://doi.org/10.3390/jcm8050598
Chicago/Turabian StyleCabré, Juan J., Teresa Mur, Bernardo Costa, Francisco Barrio, Charo López-Moya, Ramon Sagarra, Montserrat García-Barco, Jesús Vizcaíno, Immaculada Bonaventura, Nicolau Ortiz, and et al. 2019. "Feasibility and Effectiveness of Electrochemical Dermal Conductance Measurement for the Screening of Diabetic Neuropathy in Primary Care. Decoding Study (Dermal Electrochemical Conductance in Diabetic Neuropathy)" Journal of Clinical Medicine 8, no. 5: 598. https://doi.org/10.3390/jcm8050598
APA StyleCabré, J. J., Mur, T., Costa, B., Barrio, F., López-Moya, C., Sagarra, R., García-Barco, M., Vizcaíno, J., Bonaventura, I., Ortiz, N., Flores-Mateo, G., Solà-Morales, O., & the Catalan Diabetes Prevention Research Group. (2019). Feasibility and Effectiveness of Electrochemical Dermal Conductance Measurement for the Screening of Diabetic Neuropathy in Primary Care. Decoding Study (Dermal Electrochemical Conductance in Diabetic Neuropathy). Journal of Clinical Medicine, 8(5), 598. https://doi.org/10.3390/jcm8050598





