Genetic Predispositions of Glucocorticoid Resistance and Therapeutic Outcomes in Polymyalgia Rheumatica and Giant Cell Arteritis
Abstract
1. Introduction
1.1. Etiology and Pathogenesis
1.2. Glucocorticoid Treatment of Polymyalgia Rheumatica and Giant Cell Arteritis
1.3. Relapsing Diseases
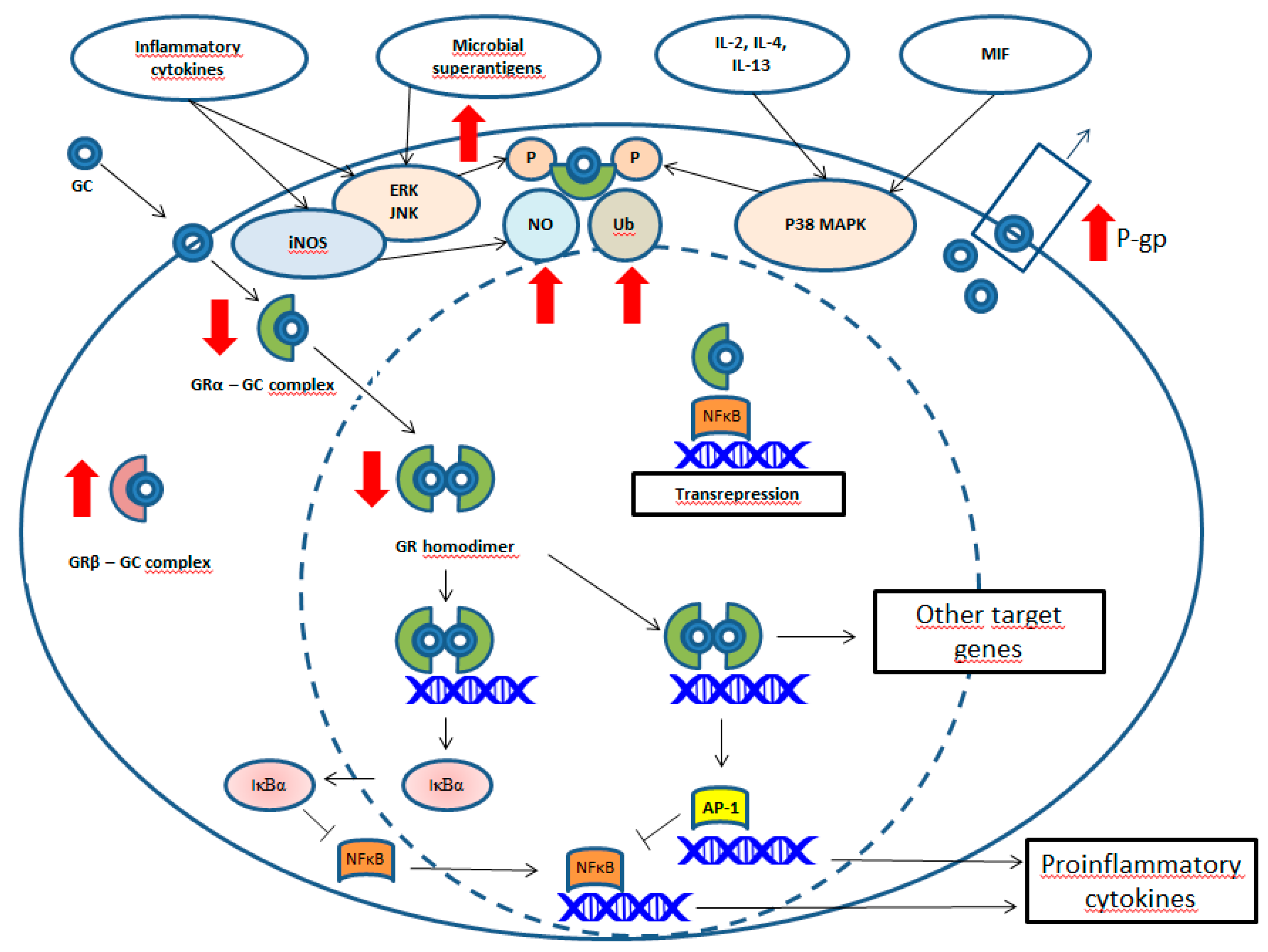
2. Glucocorticoid Resistance
3. Methods
4. Genetic Studies of GCA and PMR with Relevance of GC Treatment Outcome
4.1. Human Leukocyte Antigen (HLA)
4.2. Intercellular Adhesion Molecule-1
4.3. Interleukin 6
4.4. RANTES and CC Chemokine Receptor 5
4.5. Monocyte Chemoattractant Protein-1
4.6. Interferon Gamma
4.7. Interleukin 2 and Interleukin 21
4.8. Interleukin 1 Receptor Antagonist and Interleukin 10
4.9. Corticotropin-Releasing Hormone
4.10. Toll-like Receptor 4 and 9
4.11. Nuclear Factor of κB1
4.12. Protein Tyrosine Phosphatase Non-Receptor 22
4.13. Vascular Endothelial Growth Factor
4.14. Platelet Glycoprotein IIIa
4.15. Interleukin 17A
4.16. Other Genetic Associations
4.17. Deregulated Cell Signaling Pathways in PMR and GCA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP1 | Activator protein 1 |
| CCR5 | CC chemokine receptor 5 |
| BANK1 | B cell scaffold protein with ankyrin repeats 1 |
| BLK | B-lymphoid kinase |
| CRH | Corticotropin-releasing hormone |
| CRP | C-reactive protein |
| ERK | Extracellular signal-regulated kinase |
| ESR | Erythrocyte sedimentation rate |
| GCA | Giant cell arteritis |
| GCs | Glucocorticoids |
| GPIIIa | platelet glycoprotein IIIa |
| GR | Glucocorticoid receptor |
| HLA | Human leukocyte antigen |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IFN-γ | Interferon gamma |
| INFG | IFN-γ gene |
| IL | Interleukin |
| IL-1Ra | IL-1 receptor antagonist |
| IRF | IFN regulatory factor |
| ITGAM | Integrin alpha M |
| IκBα | Inhibitor kappa B alpha |
| JNK | c-Jun N-terminal kinase |
| MAP | Mitogen-activated protein |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MHC | Major histocompatibility complex |
| MIF | Macrophage migration inhibitory factor |
| MPO | myeloperoxidase |
| NFκB1 | Nuclear factor of κ-light polypeptide gene enhancer in B cells 1 |
| PET | positron emission tomography |
| PMR | Polymyalgia rheumatica |
| PTPN22 | Protein tyrosine phosphatase non-receptor 22 |
| RA | rheumatoid arthritis |
| RANTES | Regulated upon activation, normal T cell expressed and presumably secreted |
| SNP | Single nucleotide polymorphism |
| STAT | Signal transduction-activated transcription factor |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TNFAIP3 | AIP3, Tumor necrosis factor-α-induced protein 3 |
| TRAF | TNF receptor-associated factor |
| VNTRs | Variable number of tandem-repeats |
| VEGF | Vascular endothelial growth factor |
References
- Patel, M.; Forsyth, D.R. Polymyalgia rheumatica and giant cell arteritis. J. Clin. Gerontol. Geriatr. 2011, 2, 7–12. [Google Scholar] [CrossRef]
- Rooney, P.J.; Rooney, J.; Balint, G.; Balint, P. Polymyalgia rheumatica: 125 years of epidemiological progress? Scott. Med. J. 2015, 60, 50–57. [Google Scholar] [CrossRef]
- Nesher, G.; Breuer, G.S. Giant Cell Arteritis and Polymyalgia Rheumatica: 2016 Update. Rambam. Maimonides Med. J. 2016, 7. [Google Scholar] [CrossRef]
- Blockmans, D.; De Ceuninck, L.; Vanderschueren, S.; Knockaert, D.; Mortelmans, L.; Bobbaers, H. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: A prospective study in 35 patients. Rheumatology (Oxford) 2007, 46, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Moosig, F.; Czech, N.; Mehl, C.; Henze, E.; Zeuner, R.A.; Kneba, M.; Schroder, J.O. Correlation between 18-fluorodeoxyglucose accumulation in large vessels and serological markers of inflammation in polymyalgia rheumatica: A quantitative PET study. Ann. Rheum. Dis. 2004, 63, 870–873. [Google Scholar] [CrossRef]
- Cantini, F.; Niccoli, L.; Storri, L.; Nannini, C.; Olivieri, I.; Padula, A.; Boiardi, L.; Salvarani, C. Are polymyalgia rheumatica and giant cell arteritis the same disease? Semin. Arthritis. Rheum. 2004, 33, 294–301. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Matteson, E.L.; Castaneda, S. Polymyalgia rheumatica. Lancet 2017, 390, 1700–1712. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Amoli, M.M.; Garcia-Porrua, C.; Ollier, W.E. Genetic markers of disease susceptibility and severity in giant cell arteritis and polymyalgia rheumatica. Semin. Arthritis. Rheum. 2003, 33, 38–48. [Google Scholar] [CrossRef]
- Hellmann, D.B. Giant cell arteritis, polymyalgia rheumatica and Takayasu’s arteritis. In Kelley’s Textbook of Rheumatology, 9th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., Mcinnes, I.B., O’Dell, J.R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; Volume 2, pp. 1461–1480. [Google Scholar]
- Carroll, S.C.; Gaskin, B.J.; Danesh-Meyer, H.V. Giant cell arteritis. Clin. Exp. Ophthalmol. 2006, 34, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodriguez, J.; Segarra, M.; Vilardell, C.; Sanchez, M.; Garcia-Martinez, A.; Esteban, M.J.; Grau, J.M.; Urbano-Marquez, A.; Colomer, D.; Kleinman, H.K.; et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant-cell arteritis: Angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation 2003, 107, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Audia, S.; Fraszczak, J.; Trad, M.; Ornetti, P.; Lakomy, D.; Ciudad, M.; Leguy, V.; Berthier, S.; Vinit, J.; et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis. Rheum. 2012, 64, 3788–3798. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Mata, C.; Marin, M.J.; Calvo-Alen, J.; Blanco, R.; Aurrecoechea, E.; Ruiz-Soto, M.; Martinez-Taboada, V.M. Circulating cytokines in active polymyalgia rheumatica. Ann. Rheum. Dis. 2010, 69, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Hicok, K.C.; Hunder, G.G.; Goronzy, J.J. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann. Intern. Med. 1994, 121, 484–491. [Google Scholar] [CrossRef]
- Dejaco, C.; Singh, Y.P.; Perel, P.; Hutchings, A.; Camellino, D.; Mackie, S.; Abril, A.; Bachta, A.; Balint, P.; Barraclough, K.; et al. 2015 Recommendations for the management of polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2015, 74, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Guillevin, L.; Cid, M.C.; Dasgupta, B.; de Groot, K.; Gross, W.; Hauser, T.; Hellmich, B.; Jayne, D.; Kallenberg, C.G.; et al. EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2009, 68, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Cantini, F.; Niccoli, L.; Macchioni, P.; Consonni, D.; Bajocchi, G.; Vinceti, M.; Catanoso, M.G.; Pulsatelli, L.; Meliconi, R.; et al. Acute-phase reactants and the risk of relapse/recurrence in polymyalgia rheumatica: A prospective followup study. Arthritis. Rheum. 2005, 53, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kremers, H.M.; Reinalda, M.S.; Crowson, C.S.; Zinsmeister, A.R.; Hunder, G.G.; Gabriel, S.E. Relapse in a population based cohort of patients with polymyalgia rheumatica. J. Rheumatol. 2005, 32, 65–73. [Google Scholar]
- Pulsatelli, L.; Boiardi, L.; Pignotti, E.; Dolzani, P.; Silvestri, T.; Macchioni, P.; Cantini, F.; Salvarani, C.; Facchini, A.; Meliconi, R. Serum interleukin-6 receptor in polymyalgia rheumatica: A potential marker of relapse/recurrence risk. Arthritis. Rheum. 2008, 59, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Fulbright, J.W.; Evans, J.M.; Hunder, G.G.; Goronzy, J.J. Corticosteroid requirements in polymyalgia rheumatica. Arch. Intern. Med. 1999, 159, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, S.T.; Kim, J.S.; Yoon, B.Y.; Kwok, S.K.; Kim, H.S.; Kim, Y.S.; Song, J.S.; Lee, S.H.; Kim, H.R. Clinical characteristics and prognostic factors for relapse in patients with polymyalgia rheumatica (PMR). Rheumatol. Int. 2013, 33, 1475–1480. [Google Scholar] [CrossRef]
- Narvaez, J.; Nolla-Sole, J.M.; Clavaguera, M.T.; Valverde-Garcia, J.; Roig-Escofet, D. Longterm therapy in polymyalgia rheumatica: Effect of coexistent temporal arteritis. J. Rheumatol. 1999, 26, 1945–1952. [Google Scholar] [PubMed]
- Schreiber, S.; Buyse, M. The CRP initial response to treatment as prognostic factor in patients with polymyalgia rheumatica. Clin. Rheumatol. 1995, 14, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Salvarani, C.; Olivieri, I.; Macchioni, L.; Ranzi, A.; Niccoli, L.; Padula, A.; Boiardi, L. Erythrocyte sedimentation rate and C-reactive protein in the evaluation of disease activity and severity in polymyalgia rheumatica: A prospective follow-up study. Semin. Arthritis. Rheum. 2000, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Behn, A.R.; Perera, T.; Myles, A.B. Polymyalgia rheumatica and corticosteroids: How much for how long? Ann. Rheum. Dis. 1983, 42, 374–378. [Google Scholar] [CrossRef]
- Ayoub, W.T.; Franklin, C.M.; Torretti, D. Polymyalgia rheumatica. Duration of therapy and long-term outcome. Am. J. Med. 1985, 79, 309–315. [Google Scholar] [CrossRef]
- Kyle, V.; Hazleman, B.L. The clinical and laboratory course of polymyalgia rheumatica/giant cell arteritis after the first two months of treatment. Ann. Rheum Dis 1993, 52, 847–850. [Google Scholar] [CrossRef]
- Cimmino, M.A.; Parodi, M.; Montecucco, C.; Caporali, R. The correct prednisone starting dose in polymyalgia rheumatica is related to body weight but not to disease severity. BMC Musculoskelet. Disord. 2011, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, G.; Boiardi, L.; Cavazza, A.; Catanoso, M.; Macchioni, P.; Muratore, F.; Cimino, L.; Aldigeri, R.; Crescentini, F.; Pipitone, N.; et al. Flares in Biopsy-Proven Giant Cell Arteritis in Northern Italy: Characteristics and Predictors in a Long-Term Follow-Up Study. Medicine (Baltimore) 2016, 95, e3524. [Google Scholar] [CrossRef] [PubMed]
- Alba, M.A.; Garcia-Martinez, A.; Prieto-Gonzalez, S.; Tavera-Bahillo, I.; Corbera-Bellalta, M.; Planas-Rigol, E.; Espigol-Frigole, G.; Butjosa, M.; Hernandez-Rodriguez, J.; Cid, M.C. Relapses in patients with giant cell arteritis: Prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014, 93, 194–201. [Google Scholar] [CrossRef]
- Farrell, R.J.; Kelleher, D. Glucocorticoid resistance in inflammatory bowel disease. J. Endocrinol. 2003, 178, 339–346. [Google Scholar] [CrossRef]
- Van den Akker, E.L.; Koper, J.W.; van Rossum, E.F.; Dekker, M.J.; Russcher, H.; de Jong, F.H.; Uitterlinden, A.G.; Hofman, A.; Pols, H.A.; Witteman, J.C.; et al. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch. Intern. Med. 2008, 168, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Manenschijn, L.; van den Akker, E.L.; Lamberts, S.W.; van Rossum, E.F. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann. N. Y. Acad. Sci. 2009, 1179, 179–198. [Google Scholar] [CrossRef] [PubMed]
- De Iudicibus, S.; Franca, R.; Martelossi, S.; Ventura, A.; Decorti, G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Dejaco, C.; Matteson, E.L.; Dasgupta, B. Polymyalgia Rheumatica and Giant Cell Arteritis: A Systematic Review. JAMA 2016, 315, 2442–2458. [Google Scholar] [CrossRef]
- Schirmer, M.; Muratore, F.; Salvarani, C. Tocilizumab for the treatment of giant cell arteritis. Expert. Rev. Clin. Immunol. 2018, 14, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Quax, R.A.; Manenschijn, L.; Koper, J.W.; Hazes, J.M.; Lamberts, S.W.; van Rossum, E.F.; Feelders, R.A. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 2013, 9, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodriguez, J.; Segarra, M.; Vilardell, C.; Sanchez, M.; Garcia-Martinez, A.; Esteban, M.J.; Queralt, C.; Grau, J.M.; Urbano-Marquez, A.; Palacin, A.; et al. Tissue production of pro-inflammatory cytokines (IL-1beta, TNFalpha and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology (Oxford) 2004, 43, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Fritz, P.; Rieth, A.; Schroth, W.; Kimmel, M.; Biegger, D.; Zakim, D.; Alscher, M.D. Predictors for treatment success and expression of glucocorticoid receptor in giant cell arteritis and polymyalgia rheumatica. J. Rheumatol. 2009, 36, 2269–2276. [Google Scholar] [CrossRef]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic. Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Rauzy, O.; Fort, M.; Nourhashemi, F.; Alric, L.; Juchet, H.; Ecoiffier, M.; Abbal, M.; Adoue, D. Relation between HLA DRB1 alleles and corticosteroid resistance in giant cell arteritis. Ann. Rheum. Dis. 1998, 57, 380–382. [Google Scholar] [CrossRef]
- Dababneh, A.; Gonzalez-Gay, M.A.; Garcia-Porrua, C.; Hajeer, A.; Thomson, W.; Ollier, W. Giant cell arteritis and polymyalgia rheumatica can be differentiated by distinct patterns of HLA class II association. J. Rheumatol. 1998, 25, 2140–2145. [Google Scholar]
- Combe, B.; Sany, J.; Le Quellec, A.; Clot, J.; Eliaou, J.F. Distribution of HLA-DRB1 alleles of patients with polymyalgia rheumatica and giant cell arteritis in a Mediterranean population. J. Rheumatol. 1998, 25, 94–98. [Google Scholar] [PubMed]
- Salvarani, C.; Boiardi, L.; Mantovani, V.; Ranzi, A.; Cantini, F.; Olivieri, I.; Viggiani, M.; Bragliani, M.; Macchioni, P. HLA-DRB1, DQA1, and DQB1 alleles associated with giant cell arteritis in northern Italy. J. Rheumatol. 1999, 26, 2395–2399. [Google Scholar] [PubMed]
- Martinez-Taboda, V.M.; Bartolome, M.J.; Lopez-Hoyos, M.; Blanco, R.; Mata, C.; Calvo, J.; Corrales, A.; Rodriguez-Valverde, V. HLA-DRB1 allele distribution in polymyalgia rheumatica and giant cell arteritis: Influence on clinical subgroups and prognosis. Semin. Arthritis. Rheum. 2004, 34, 454–464. [Google Scholar] [CrossRef]
- Jacobsen, S.; Baslund, B.; Madsen, H.O.; Tvede, N.; Svejgaard, A.; Garred, P. Mannose-binding lectin variant alleles and HLA-DR4 alleles are associated with giant cell arteritis. J. Rheumatol. 2002, 29, 2148–2153. [Google Scholar]
- Rodriguez-Rodriguez, L.; Castaneda, S.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Gomez-Vaquero, C.; Mari-Alfonso, B.; Miranda-Filloy, J.A.; Narvaez, J.; Ortego-Centeno, N.; Vicente, E.F.; et al. Role of the rs6822844 gene polymorphism at the IL2-IL21 region in biopsy-proven giant cell arteritis. Clin. Exp. Rheumatol. 2011, 29, S12–S16. [Google Scholar]
- Gonzalez-Gay, M.A.; Oliver, J.; Orozco, G.; Garcia-Porrua, C.; Lopez-Nevot, M.A.; Martin, J. Lack of association of a functional single nucleotide polymorphism of PTPN22, encoding lymphoid protein phosphatase, with susceptibility to biopsy-proven giant cell arteritis. J. Rheumatol. 2005, 32, 1510–1512. [Google Scholar] [PubMed]
- Amoli, M.M.; Shelley, E.; Mattey, D.L.; Garcia-Porrua, C.; Thomson, W.; Hajeer, A.H.; Ollier, W.E.; Gonzalez-Gay, M.A. Lack of association between intercellular adhesion molecule-1 gene polymorphisms and giant cell arteritis. J. Rheumatol. 2001, 28, 1600–1604. [Google Scholar] [PubMed]
- Martin, J.; Perez-Armengol, C.; Miranda-Filloy, J.A.; Vilchez, J.R.; Lopez-Nevot, M.A.; Garcia-Porrua, C.; Gonzalez-Gay, M.A. Lack of association of a functional -94ins/delATTG NFKB1 promoter polymorphism with susceptibility and clinical expression of biopsy-proven giant cell arteritis in northwest Spain. J. Rheumatol. 2006, 33, 285–288. [Google Scholar]
- Salvarani, C.; Casali, B.; Farnetti, E.; Pipitone, N.; Nicoli, D.; Macchioni, P.; Cimino, L.; Bajocchi, G.; Catanoso, M.G.; Boiardi, L. Interleukin-6 promoter polymorphism at position -174 in giant cell arteritis. J. Rheumatol. 2005, 32, 2173–2177. [Google Scholar] [PubMed]
- Gonzalez-Gay, M.A.; Hajeer, A.H.; Dababneh, A.; Garcia-Porrua, C.; Mattey, D.L.; Amoli, M.M.; Thomson, W.; Ollier, W.E. IL-6 promoter polymorphism at position -174 modulates the phenotypic expression of polymyalgia rheumatica in biopsy-proven giant cell arteritis. Clin. Exp. Rheumatol. 2002, 20, 179–184. [Google Scholar] [PubMed]
- Torres, O.; Palomino-Morales, R.; Vazquez-Rodriguez, T.; Castaneda, S.; Morado, I.C.; Miranda-Filloy, J.A.; Amigo-Diaz, E.; Callejas-Rubio, J.L.; Fernandez-Gutierrez, B.; Martin, J.; et al. Lack of association between IFNGR1 gene polymorphisms and biopsy-proven giant cell arteritis. Clin. Exp. Rheumatol. 2010, 28, 31–34. [Google Scholar] [PubMed]
- Gonzalez-Gay, M.A.; Hajeer, A.H.; Dababneh, A.; Garcia-Porrua, C.; Amoli, M.M.; Llorca, J.; Ollier, W.E. Interferon-gamma gene microsatellite polymorphisms in patients with biopsy-proven giant cell arteritis and isolated polymyalgia rheumatica. Clin. Exp. Rheumatol. 2004, 22, S18–S20. [Google Scholar] [PubMed]
- Rueda, B.; Lopez-Nevot, M.A.; Lopez-Diaz, M.J.; Garcia-Porrua, C.; Martin, J.; Gonzalez-Gay, M.A. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteritis. J. Rheumatol. 2005, 32, 1737–1741. [Google Scholar]
- Alvarez-Rodriguez, L.; Carrasco-Marin, E.; Lopez-Hoyos, M.; Mata, C.; Fernandez-Prieto, L.; Ruiz-Soto, M.; Calvo, J.; Rodriguez-Valverde, V.; Ruiz, T.; Blanco, R.; et al. Interleukin-1RN gene polymorphisms in elderly patients with rheumatic inflammatory chronic conditions: Association of IL-1RN*2/2 genotype with polymyalgia rheumatica. Hum. Immunol. 2009, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Amoli, M.M.; Salway, F.; Zeggini, E.; Ollier, W.E.; Gonzalez-Gay, M.A. MCP-1 gene haplotype association in biopsy proven giant cell arteritis. J. Rheumatol. 2005, 32, 507–510. [Google Scholar] [PubMed]
- Rueda, B.; Roibas, B.; Martin, J.; Gonzalez-Gay, M.A. Influence of interleukin 10 promoter polymorphisms in susceptibility to giant cell arteritis in Northwestern Spain. J. Rheumatol. 2007, 34, 1535–1539. [Google Scholar] [PubMed]
- Boiardi, L.; Casali, B.; Farnetti, E.; Pipitone, N.; Nicoli, D.; Macchioni, P.; Cimino, L.; Bajocchi, G.; Catanoso, M.G.; Pattacini, L.; et al. Interleukin-10 promoter polymorphisms in giant cell arteritis. Arthritis. Rheum. 2006, 54, 4011–4017. [Google Scholar] [CrossRef] [PubMed]
- Rueda, B.; Miranda-Filloy, J.A.; Martin, J.; Gonzalez-Gay, M.A. Association of CD24 gene polymorphisms with susceptibility to biopsy-proven giant cell arteritis. J. Rheumatol. 2008, 35, 850–854. [Google Scholar]
- Boiardi, L.; Casali, B.; Farnetti, E.; Pipitone, N.; Nicoli, D.; Macchioni, P.; Cimino, L.; Bajocchi, G.L.; Catanoso, M.G.; Pattacini, L.; et al. Toll-like receptor 4 (TLR4) gene polymorphisms in giant cell arteritis. Clin. Exp. Rheumatol. 2009, 27, S40–S44. [Google Scholar]
- Palomino-Morales, R.; Torres, O.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Castaneda, S.; Callejas-Rubio, J.L.; Miranda-Filloy, J.A.; Fernandez-Gutierrez, B.; Martin, J.; Gonzalez-Gay, M.A. Association between toll-like receptor 4 gene polymorphism and biopsy-proven giant cell arteritis. J. Rheumatol. 2009, 36, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Beares, I.; Munoz Cacho, P.; Mata, C.; Calvo-Alen, J.; Corrales, A.; Tripathi, G.; Blanco, R.; Garcia-Unzueta, M.; et al. Lack of association between Toll-like receptor 4 gene polymorphisms and giant cell arteritis. Rheumatology (Oxford) 2011, 50, 1562–1568. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Beares, I.; Calvo-Alen, J.; Ruiz, T.; Villa, I.; Martinez-Taboada, V.M. Toll-like receptor 9 gene polymorphisms in polymyalgia rheumatica and giant cell arteritis. Scand. J. Rheumatol. 2012, 41, 487–489. [Google Scholar] [CrossRef]
- Salvarani, C.; Casali, B.; Farnetti, E.; Pipitone, N.; Formisano, D.; Nicoli, D.; Macchioni, P.; Cimino, L.; Bajocchi, G.; Grazia Catanoso, M.; et al. PlA1/A2 polymorphism of the platelet glycoprotein receptor IIIA and risk of cranial ischemic complications in giant cell arteritis. Arthritis. Rheum. 2007, 56, 3502–3508. [Google Scholar] [CrossRef]
- Salvarani, C.; Boiardi, L.; Mantovani, V.; Ranzi, A.; Cantini, F.; Olivieri, I.; Bragliani, M.; Collina, E.; Macchioni, P. HLA-DRB1 alleles associated with polymyalgia rheumatica in northern Italy: Correlation with disease severity. Ann. Rheum. Dis. 1999, 58, 303–308. [Google Scholar] [CrossRef]
- Boiardi, L.; Casali, B.; Farnetti, E.; Pipitone, N.; Nicoli, D.; Cantini, F.; Macchioni, P.; Bajocchi, G.; Catanoso, M.G.; Pulsatelli, L.; et al. Relationship between interleukin 6 promoter polymorphism at position -174, IL-6 serum levels, and the risk of relapse/recurrence in polymyalgia rheumatica. J. Rheumatol. 2006, 33, 703–708. [Google Scholar]
- Alvarez-Rodriguez, L.; Lopez-Hoyos, M.; Beares, I.; Mata, C.; Garcia-Unzueta, M.; Calvo-Alen, J.; Blanco, R.; Aurrecoechea, E.; Tripathi, G.; Martinez-Taboada, V.M. Toll-like receptor 4 gene polymorphisms in polymyalgia rheumatica and elderly-onset rheumatoid arthritis. Clin. Exp. Rheumatol. 2011, 29, 795–800. [Google Scholar] [PubMed]
- Salvarani, C.; Boiardi, L.; Timms, J.M.; Silvestri, T.; Ranzi, A.; Macchioni, P.L.; Pulsatelli, L.; di Giovine, F.S. Absence of the association with CC chemokine receptor 5 polymorphism in polymyalgia rheumatica. Clin. Exp. Rheumatol. 2000, 18, 591–595. [Google Scholar] [PubMed]
- Boiardi, L.; Salvarani, C.; Timms, J.M.; Silvestri, T.; Macchioni, P.L.; di Giovine, F.S. Interleukin-1 cluster and tumor necrosis factor-alpha gene polymorphisms in polymyalgia rheumatica. Clin. Exp. Rheumatol. 2000, 18, 675–681. [Google Scholar]
- Amoli, M.M.; Shelley, E.; Mattey, D.L.; Garcia-Porrua, C.; Thomson, W.; Hajeer, A.H.; Ollier, W.E.; Gonzalez-Gay, M.A. Intercellular adhesion molecule-1 gene polymorphisms in isolated polymyalgia rheumatica. J. Rheumatol. 2002, 29, 502–504. [Google Scholar] [PubMed]
- Salvarani, C.; Casali, B.; Boiardi, L.; Ranzi, A.; Macchioni, P.; Nicoli, D.; Farnetti, E.; Brini, M.; Portioli, I. Intercellular adhesion molecule 1 gene polymorphisms in polymyalgia rheumatica/giant cell arteritis: Association with disease risk and severity. J. Rheumatol. 2000, 27, 1215–1221. [Google Scholar]
- Alvarez-Rodriguez, L.; Carrasco-Marin, E.; Tripathi, G.; Munoz-Cacho, P.; Lopez-Hoyos, M.; Mata, C.; Calvo-Alen, J.; Garcia-Unzueta, M.; Aurrecoechea, E.; Alvarez-Dominguez, C.; et al. Influence of interleukin 10 promoter polymorphisms in polymyalgia rheumatica: Disease susceptibility and functional consequences. Clin. Exp. Rheumatol. 2014, 32, 484–489. [Google Scholar] [PubMed]
- Loffers, C.; Heilig, B.; Hecker, M. T-786C single nucleotide polymorphism of the endothelial nitric oxide synthase gene as a risk factor for endothelial dysfunction in polymyalgia rheumatica. Clin. Exp. Rheumatol. 2015, 33, 726–730. [Google Scholar]
- Gonzalez-Gay, M.A.; Hajeer, A.H.; Dababneh, A.; Makki, R.; Garcia-Porrua, C.; Thomson, W.; Ollier, W. Seronegative rheumatoid arthritis in elderly and polymyalgia rheumatica have similar patterns of HLA association. J. Rheumatol. 2001, 28, 122–125. [Google Scholar]
- Dasgupta, B.; Panayi, G.S. Interleukin-6 in serum of patients with polymyalgia rheumatica and giant cell arteritis. Br. J. Rheumatol. 1990, 29, 456–458. [Google Scholar] [CrossRef]
- Samson, M.; Libert, F.; Doranz, B.J.; Rucker, J.; Liesnard, C.; Farber, C.M.; Saragosti, S.; Lapoumeroulie, C.; Cognaux, J.; Forceille, C.; et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996, 382, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Cid, M.C.; Hoffman, M.P.; Hernandez-Rodriguez, J.; Segarra, M.; Elkin, M.; Sanchez, M.; Vilardell, C.; Garcia-Martinez, A.; Pla-Campo, M.; Grau, J.M.; et al. Association between increased CCL2 (MCP-1) expression in lesions and persistence of disease activity in giant-cell arteritis. Rheumatology (Oxford) 2006, 45, 1356–1363. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Hajeer, A.H.; Dababneh, A.; Garcia-Porrua, C.; Amoli, M.M.; Thomson, W.; Ollier, W.E. Corticotropin releasing hormone promoter polymorphisms in giant cell arteritis and polymyalgia rheumatica. Clin. Exp. Rheumatol. 2002, 20, 133–138. [Google Scholar] [PubMed]
- Serrano, A.; Marquez, A.; Mackie, S.L.; Carmona, F.D.; Solans, R.; Miranda-Filloy, J.A.; Hernandez-Rodriguez, J.; Cid, M.C.; Castaneda, S.; Morado, I.C.; et al. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Ann. Rheum. Dis. 2013, 72, 1882–1886. [Google Scholar] [CrossRef]
- Meliconi, R.; Pulsatelli, L.; Dolzani, P.; Boiardi, L.; Macchioni, P.; Salvarani, C.; Silvestri, T.; Frizziero, L.; Facchini, A. Vascular endothelial growth factor production in polymyalgia rheumatica. Arthritis. Rheum. 2000, 43, 2472–2480. [Google Scholar] [CrossRef]
- Boiardi, L.; Casali, B.; Nicoli, D.; Farnetti, E.; Chen, Q.; Macchioni, P.; Catanoso, M.G.; Pulsatelli, L.; Meliconi, R.; Salvarani, C. Vascular endothelial growth factor gene polymorphisms in giant cell arteritis. J. Rheumatol. 2003, 30, 2160–2164. [Google Scholar]
- Carmona, F.D.; Martin, J.; Gonzalez-Gay, M.A. New insights into the pathogenesis of giant cell arteritis and hopes for the clinic. Expert Rev. Clin. Immunol. 2016, 12, 57–66. [Google Scholar] [CrossRef]
- Marquez, A.; Hernandez-Rodriguez, J.; Cid, M.C.; Solans, R.; Castaneda, S.; Fernandez-Contreras, M.E.; Ramentol, M.; Morado, I.C.; Narvaez, J.; Gomez-Vaquero, C.; et al. Influence of the IL17A locus in giant cell arteritis susceptibility. Ann. Rheum. Dis. 2014, 73, 1742–1745. [Google Scholar] [CrossRef]
- Deng, J.; Younge, B.R.; Olshen, R.A.; Goronzy, J.J.; Weyand, C.M. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation 2010, 121, 906–915. [Google Scholar] [CrossRef]
- Espigol-Frigole, G.; Corbera-Bellalta, M.; Planas-Rigol, E.; Lozano, E.; Segarra, M.; Garcia-Martinez, A.; Prieto-Gonzalez, S.; Hernandez-Rodriguez, J.; Grau, J.M.; Rahman, M.U.; et al. Increased IL-17A expression in temporal artery lesions is a predictor of sustained response to glucocorticoid treatment in patients with giant-cell arteritis. Ann. Rheum. Dis. 2013, 72, 1481–1487. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immune mechanisms in medium and large-vessel vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Rizzo, A.; Guggino, G.; Cavazza, A.; Alessandro, R.; Maugeri, R.; Cannizzaro, A.; Boiardi, L.; Iacopino, D.G.; Salvarani, C.; et al. Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford) 2015, 54, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.D.; Serrano, A.; Rodriguez-Rodriguez, L.; Castaneda, S.; Miranda-Filloy, J.A.; Morado, I.C.; Narvaez, J.; Solans, R.; Sopena, B.; Mari-Alfonso, B.; et al. A nonsynonymous functional variant of the ITGAM gene is not involved in biopsy-proven giant cell arteritis. J. Rheumatol. 2011, 38, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Morales, R.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Castaneda, S.; Ortego-Centeno, N.; Miranda-Filloy, J.A.; Lamas, J.R.; Martin, J.; Gonzalez-Gay, M.A. Lack of association between STAT4 gene polymorphism and biopsy-proven giant cell arteritis. J. Rheumatol. 2009, 36, 1021–1025. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, L.; Castaneda, S.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Mari-Alfonso, B.; Gomez-Vaquero, C.; Miranda-Filloy, J.A.; Ortego-Centeno, N.; Narvaez, J.; Blanco, R.; et al. Influence of IL2RA rs2104286 polymorphism in the risk of biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 2331–2333. [Google Scholar] [CrossRef]
- Palomino-Morales, R.; Torres, O.; Vazquez-Rodriguez, T.R.; Castaneda, S.; Morado, I.C.; Miranda-Filloy, J.A.; Amigo-Diaz, E.; Callejas-Rubio, J.L.; Fernandez-Gutierrez, B.; Martin, J.; et al. Lack of association between the rs6920220 (G/A) polymorphism of the 6q23 region and biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 1020–1023. [Google Scholar] [CrossRef]
- Torres, O.; Palomino-Morales, R.; Vazquez-Rodriguez, T.R.; Castaneda, S.; Morado, I.C.; Miranda-Filloy, J.A.; Ortego-Centeno, N.; Gonzalez-Alvaro, I.; Fernandez-Gutierrez, B.; Martin, J.; et al. Lack of association between TRAF1/C5 gene polymorphisms and biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.D.; Serrano, A.; Rodriguez-Rodriguez, L.; Callejas, J.L.; Simeon, C.P.; Carreira, P.; Castaneda, S.; Solans, R.; Blanco, R.; Spanish Scleroderma, G.; et al. Evaluation of a shared autoimmune disease-associated polymorphism of TRAF6 in systemic sclerosis and giant cell arteritis. J. Rheumatol. 2012, 39, 1275–1279. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, L.; Carmona, F.D.; Castaneda, S.; Miranda-Filloy, J.A.; Morado, I.C.; Narvaez, J.; Mari-Alfonso, B.; Gomez-Vaquero, C.; Amigo-Diaz, E.; Rios-Fernandez, R.; et al. Role of rs1343151 IL23R and rs3790567 IL12RB2 polymorphisms in biopsy-proven giant cell arteritis. J. Rheumatol. 2011, 38, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Carmona, F.D.; Miranda-Filloy, J.A.; Castaneda, S.; Rodriguez-Rodriguez, L.; Morado, I.C.; Gomez-Vaquero, C.; Solans, R.; Sopena, B.; Blanco, R.; et al. Autoimmune disease-associated CD226 gene variants are not involved in giant cell arteritis susceptibility in the Spanish population. Clin. Exp. Rheumatol. 2012, 30, S29–S33. [Google Scholar] [PubMed]
- Salvarani, C.; Casali, B.; Farnetti, E.; Pipitone, N.; Nicoli, D.; Macchioni, P.L.; Cimino, L.; Bajocchi, G.L.; Catanoso, M.G.; Pattacini, L.; et al. -463 G/A myeloperoxidase promoter polymorphism in giant cell arteritis. Ann. Rheum. Dis. 2008, 67, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Morales, R.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Martin, J.; Gonzalez-Gay, M.A. C-reactive protein gene polymorphisms in biopsy-proven giant cell arteritis from Northwestern Spain. J. Rheumatol. 2009, 36, 341–346. [Google Scholar] [CrossRef]
- Serrano, A.; Carmona, F.D.; Castaneda, S.; Miranda-Filloy, J.A.; Morado, I.C.; Gomez-Vaquero, C.; Solans, R.; Sopena, B.; Blanco, R.; Unzurrunzaga, A.; et al. A case-control study suggests that the CCR6 locus is not involved in the susceptibility to giant cell arteritis. Clin. Exp. Rheumatol. 2013, 31, S5–S8. [Google Scholar]
- Torres, O.; Palomino-Morales, R.; Vazquez-Rodriguez, T.R.; Castaneda, S.; Morado, I.C.; Miranda-Filloy, J.A.; Valero, F.; Callejas-Rubio, J.L.; Fernandez-Gutierrez, B.; Martin, J.; et al. Lack of association between IRF5 gene polymorphisms and biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 136–140. [Google Scholar] [CrossRef]
- Torres, O.; Palomino-Morales, R.; Castaneda, S.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Miranda-Filloy, J.A.; Amigo-Diaz, E.; Vicente, E.F.; Ortego-Centeno, N.; Fernandez-Gutierrez, B.; et al. Role of BANK1 gene polymorphisms in biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 1502–1504. [Google Scholar] [CrossRef]
- Salvarani, C.; Casali, B.; Nicoli, D.; Farnetti, E.; Macchioni, P.; Catanoso, M.G.; Chen, Q.; Bajocchi, G.; Boiardi, L. Endothelial nitric oxide synthase gene polymorphisms in giant cell arteritis. Arthritis. Rheum. 2003, 48, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, L.; Castaneda, S.; Vazquez-Rodriguez, T.R.; Morado, I.C.; Mari-Alfonso, B.; Gomez-Vaquero, C.; Miranda-Filloy, J.A.; Narvaez, J.; Ortego-Centeno, N.; Blanco, R.; et al. Influence of CD40 rs1883832 polymorphism in susceptibility to and clinical manifestations of biopsy-proven giant cell arteritis. J. Rheumatol. 2010, 37, 2076–2080. [Google Scholar] [CrossRef]
- Torres, O.; Palomino-Morales, R.; Vazquez-Rodriguez, T.R.; Castaneda, S.; Morado, I.C.; Miranda-Filloy, J.A.; Ortego-Centeno, N.; Fernandez-Gutierrez, B.; Martin, J.; Gonzalez-Gay, M.A. Role of the C8orf13-BLK region in biopsy-proven giant cell arteritis. Hum. Immunol. 2010, 71, 525–529. [Google Scholar] [CrossRef]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schuler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Nikolaev, A.; Reissig, S.; et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef]
- Saadoun, D.; Garrido, M.; Comarmond, C.; Desbois, A.C.; Domont, F.; Savey, L.; Terrier, B.; Geri, G.; Rosenzwajg, M.; Klatzmann, D.; et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis. Rheumatol. 2015, 67, 1353–1360. [Google Scholar] [CrossRef]

| Genetic Polymorphisms or Mutations of GR |
|---|
| Chrousos syndrome–primary glucocorticoid resistance Defective ligand binding to GR Lower transport GR–GC complex to nucleus Defective transactivation |
| Post-translational modifications of GR |
| Phosphorylation Nitrosylation Ubiquitination |
| Increased GRβ expression |
| Increased activity of pro-inflammatory transcription factors and kinases |
| NFκB, AP1 JNK, STAT5, JAK3 |
| Defective histone acetylation |
| Reduced acetylation of lysine 5 on histone 4 Reduced histone deacetylase 2 |
| Increased oxidative stress |
| Increased phosphoinositide-3-kinase-δ activation |
| Increased P-glycoprotein |
| Increased efflux of steroids |
| Inactivation of glucocorticoids |
| Expression of 11β-hydroxysteroid dehydrogenase 2 |
| Polymorphism | Population | Allele or Genotype | Clinical Features | Allele Freq. (%) | OR (95% CI) | p | R. |
|---|---|---|---|---|---|---|---|
| HLA-DRB1 | 41 GCA patients French patients | *04 | GC resistant vs. GC sensitive patients | 88.9 vs. 37.5 | RR 2.37 (1.43–3.92) | 0.0064 | [41] |
| HLA-DRB1 | 53 GCA patients 145 controls Spanish patients (Lugo region) | *04 | patients with visual manifestations (partial or complete visual loss) vs. healthy controls | 63.6 vs. 26.2 | 4.9 (1.2–21.4) | 0.008 | [42] |
| HLA-DRB1 | 42 GCA patients 79 PMR patients French patients | *04 | steroid dosage in allele positive vs. all patients | NS | [43] | ||
| *04 | number of relapses in allele positive vs. all patients | NS | |||||
| HLA-DRB1 | 39 GCA patients Italian patients (Reggio Emilia) | *04 | patients with vs. without systemic signs and/or symptoms | 34.5 vs. 0 | RR 1.5 (1.2–2.0) | 0.04 | [44] |
| HLA-DRB1 | 44 GCA patients Spanish patients (Cantabria) | *07 | patients with vs. without ischemic complications | 30 vs. 19 | 1.8 (0.7–5.1) | NS | [45] |
| *01 | patients with vs. without ischemic complications | 10 vs. 17.2 | 0.5 (0.1–2.1) | NS | |||
| *13 | patients with vs. without ischemic complications | 6.7 vs. 12.1 | 0.5 (0.1–2.7) | NS | |||
| HLA-DRB1 | 102 GCA/PMR patients Danish patients | *04 | cumulative dose of prednisolone for the first year of treatment in allele positive vs. negative patients | median: 4.5g vs. 3.8g | 0.011 | [46] | |
| MBL A/O | AO + OO vs. AA | cumulative dose of prednisolone for the first year of treatment in patients | 46 vs. 54 | NS | |||
| IL2-IL21 rs6822844 G/T | 272 GCA patients Spanish patients | T | patients with vs. without severe ischemic events * | 12.8 vs. 7.7 | 1.72 (0.97–3.05) | 0.05 0.30 # | [47] |
| T | patients with vs. without jaw claudication | 13.7 vs. 8.2 | 1.76 (1.02–3.04) | 0.04 0.24 # | |||
| T | patients with vs. without permanent visual loss | 17.5 vs. 10 | 2.09 (0.90–4.86) | 0.13 0.78 # | |||
| PTPN22 rs2476601 1858C/T | 96 GCA patients Spanish patients (Lugo region) | C or T | patients with vs. without severe ischemic events * | NS | [48] | ||
| ICAM-1 241 R/G | 58 GCA patients Spanish patients (Lugo region) | R or G | patients with vs. without visual manifestations ** | NS | [49] | ||
| ICAM-1 469 K/E | K or E | NS | |||||
| HLA-DRB1 | *04 | 40 vs. 12.1 | 4.8 (1.3–18) | 0.01 | |||
| NFKB1 −94ins/delATTG | 96 GCA patients Spanish patients (Lugo region) | ins or del | patients with vs. without severe ischemic events * | NS | [50] | ||
| IL-6 −174 G/C | 126 GCA patients Italian patients (Reggio Emilia) | G or C | patients with vs. without ischemic complications *** | NS | [51] | ||
| IL-6 −174 G/C | 62 GCA patients Spanish patients (Lugo region) | G or C | patients with vs. without visual manifestations ** | NS | [52] | ||
| IFNGR1 rs1327474 −611A/G | 216 GCA patients Spanish patients | A or G | patients with vs. without (severe) ischemic events */** | NS | [53] | ||
| INFGR1 rs11914 +189G/C | G or C | NS | |||||
| INFGR1 rs7749390 +95C/T | C or T | NS | |||||
| IFN-γ gene dinucleotide (CA) repeat within the first intron | 59 GCA patients Spanish patients (Lugo region) | *4 (128 bp) | patients with vs. without visual manifestations ** | 17.9 vs. 42.5 | 0.36 (0.13–1.00) | 0.05 | [54] |
| *3 (126 bp) | patients with vs. without visual manifestations ** | 71.4 vs. 44.4 | 3.13 (1.27–7.68) | 0.01 | |||
| VEGF rs1570360 −1154 G/A | 103 GCA patients 226 controls Spanish patients (Lugo region) | G or A | patients with vs. without severe ischemic events * | NS | [55] | ||
| VEGF rs2010963 −634 G/C | G | patients with vs. without severe ischemic events * | 75.5 vs. 60 | 2.05 (1.13–3.71) | 0.017 0.034 # | ||
| G | patients with severe ischemic events * vs. healthy controls | 75.5 vs. 63.7 | 1.75 (1.08–2.88) | 0.021 0.042 # | |||
| GG + GC vs. CC | patients with vs. without severe ischemic events * | 5.26 (1.39–19.98) | 0.009 0.018 # | ||||
| IL-1RN 86 bp VNTRs within the second intron | 69 GCA patients 437 controls Spanish patients (Cantabria) | *1 (four repeats) or *2 (two repeats) | relapses/recurrences, number of relapses, duration of corticosteroid therapy, cumulative prednisone dose, presence of ischemic manifestations *** | NS | [56] | ||
| MCP-1 rs2857657 intron 1 (G/C) | 79 GCA patients 99 controls Spanish patients (Lugo region) | G or C | patients with vs. without visual manifestations ** or controls | NS | [57] | ||
| MCP-1 rs4586 exon 2 (T/C) | T or C | NS | |||||
| MCP-1 rs13900 3′UTR (C/T) | C or T | NS | |||||
| IL-10 rs1800872 −592C/A | 103 GCA patients 232 controls Spanish patients (Lugo region) | C or A | severe ischemic complications * | NS | [58] | ||
| IL-10 rs1800896 −1082G/A | G or A | NS | |||||
| IL-10 rs1800872 −592C/A | 140 GCA patients 200 controls Italian patients (Reggio Emilia) | C or A | severe ischemic complications *** | NS | [59] | ||
| IL-10 rs1800896 −1082G/A | G or A | NS | |||||
| CD24 rs3838646 P1527 TG/del | 120 GCA patients 195 controls Spanish patients (Lugo region) | TG or del | severe ischemic complications * | NS | [60] | ||
| CD24 rs8734 C52T | C or T | NS | |||||
| TLR4 rs4986790 +896A/G | 155 GCA patients 210 controls Italian patients (Reggio Emilia) | A or G | patients with vs. without visual loss and/or cerebrovascular accidents | NS | [61] | ||
| TLR4 rs4986790 +896A/G | 210 GCA patients 678 controls Spanish patients (Lugo region, Madrid, Granada) | A or G | patients with vs. without visual ischemic complications **/severe ischemic manifestations * | NS | [62] | ||
| patients with visual ischemic complications vs. healthy controls **/severe ischemic manifestations * | NS | ||||||
| TLR4 rs4986790 +896A/G | 72 GCA patients 126 controls Spanish patients (Cantabria) | A or G | at least one relapse/recurrence, number of relapses, duration of GC treatment, cumulative prednisone dose; ischemic manifestations *** | NS | [63] | ||
| TLR4 rs4986791 +1196C/T | C or T | NS | |||||
| TLR9 rs187084 −1486T/C | 97 GCA patients 128 controls Spanish patients | TC vs. TT | a shorter duration of GC therapy | 0.029 | [64] | ||
| TLR9 rs5743836 −1237T/C | CC vs. TT | a higher number of relapses/recurrences | 0.045 | ||||
| GPIIIa rs5918 PlA1/A2 +1565T/C | 140 GCA patients 241 controls Italian patients (Reggio Emilia) | PlA2 | patients with vs. without cranial ischemic complications | 27.4 vs. 15.1 | 2.1 (1.1–4.1) | 0.037 | [65] |
| Polymorphism | Population | Allele or Genotype | Clinical Features | Allele Freq. (%) | OR (95% CI) | p | R. |
|---|---|---|---|---|---|---|---|
| HLA-DRB1 | 89 PMR patients Spanish patients (Cantabria region) | *09 | patients with vs. without relapses | 5.6 vs. 0 | 0.04 | [45] | |
| *0101 | patients with vs. without relapses | 11.1 vs. 8.3 | 1.4 (0.4–5.2) | 0.07 | |||
| *07 | patients with vs. without relapses | 11.1 vs. 4.2 | 2.9 (0.6–13.6) | 0.2 | |||
| HLA-DRB1 | 91 PMR patients Italian patients (Reggio Emilia) | *01 | patients with vs. without relapses/recurrences | RH 1.56 (0.54–4.5) | 0.01 | [66] | |
| *10 | patients with vs. without relapses/recurrences | RH 2.63 (0.23–30.11) | 0.005 | ||||
| IL-6 −174 G/C | 84 iPMR patients Spanish patients (Lugo region) | G or C | patients with vs. without relapses/recurrences | NS | [52] | ||
| IL-6 −174 G/C | 112 iPMR patients Italian patients (Reggio Emilia) | G or C | patients with vs. without relapses/recurrences | NS | [67] | ||
| TLR4 rs4986791 +1196 C/T | 164 PMR patients Spanish patients | CC | cumulative dose of prednisone in PMR patients | 5.6 ± 4.8 vs. 2.1 ± 0.5g | 0.031 | [68] | |
| CC | duration of prednisone treatment in PMR patients | 41.8 ± 39.3 vs. 16.0 ± 10.4 months | 0.10 | ||||
| CC | a number of relapses in PMR patients | 0.9 ± 1.3 vs. 0.0 ± 0.0 | 0.07 | ||||
| CCR5Δ32 | 88 PMR patients 86 controls Italian patients (Reggio Emilia) | CCR5 or CCR5Δ32 | initial and cumulative dose of prednisone, duration of therapy, relapse/recurrence in PMR patients vs. healthy controls | NS | [69] | ||
| IL-1RN 86 bp VNTRs within the second intron | 139 iPMR patients 437 controls Spanish patients (Cantabria region) | *1 (four repeats) or *2 (two repeats) | relapses/recurrences, number of relapses, duration of corticosteroid therapy, cumulative prednisone dose | NS | [56] | ||
| IL-1A +4845 C/T | 92 PMR patients 79 controls Italian patients (Reggio Emilia) | C or T | relapses/recurrences, duration of corticosteroid therapy, cumulative prednisone dose | NS | [70] | ||
| IL-1B −511 | *1 or *2 | NS | |||||
| IL-1B +3954 | *1 or *2 | NS | |||||
| TNFA −308 | *1 or *2 | NS | |||||
| IL-1RN Intron 2 | *1 or *2 | NS | |||||
| ICAM-1 241 R/G | 72 iPMR patients Spanish patients (Lugo region) | R or G | patients with vs. without relapse | NS | [71] | ||
| ICAM-1 469 K/E | K or E | patients with vs. without relapse | NS | ||||
| HLA-DRB1 | *0401 | patients with vs. without relapse | 7.2 (1.5–35.5) | 0.01 | |||
| HLA-DRB1 and ICAM-1 241 | *0401 and GG | patients with vs. without relapse | 15.2 (2.3–99.5) | 0.005 0.03 # | |||
| ICAM-1 241 R/G | 91 iPMR patients 228 controls Italian patients (Reggio Emilia) | RR + GR vs. GG | relapses/recurrences | 1.6 (1.1–2.4) | 0.01 | [72] | |
| IL-10 −592C/A | 168 iPMR patients 124 controls Spanish patients (Cantabria region) | C or A | relapses/recurrences, number of relapses, duration of corticosteroid therapy, cumulative prednisone dose | NS | [73] | ||
| IL-10 −1082A/G | A or G | NS | |||||
| NOS3 −786T/C | 78 iPMR patients 2061 controls German patients (Heidelberg) | T or C | steroid responsiveness | NS | [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smutny, T.; Barvik, I.; Veleta, T.; Pavek, P.; Soukup, T. Genetic Predispositions of Glucocorticoid Resistance and Therapeutic Outcomes in Polymyalgia Rheumatica and Giant Cell Arteritis. J. Clin. Med. 2019, 8, 582. https://doi.org/10.3390/jcm8050582
Smutny T, Barvik I, Veleta T, Pavek P, Soukup T. Genetic Predispositions of Glucocorticoid Resistance and Therapeutic Outcomes in Polymyalgia Rheumatica and Giant Cell Arteritis. Journal of Clinical Medicine. 2019; 8(5):582. https://doi.org/10.3390/jcm8050582
Chicago/Turabian StyleSmutny, Tomas, Ivan Barvik, Tomas Veleta, Petr Pavek, and Tomas Soukup. 2019. "Genetic Predispositions of Glucocorticoid Resistance and Therapeutic Outcomes in Polymyalgia Rheumatica and Giant Cell Arteritis" Journal of Clinical Medicine 8, no. 5: 582. https://doi.org/10.3390/jcm8050582
APA StyleSmutny, T., Barvik, I., Veleta, T., Pavek, P., & Soukup, T. (2019). Genetic Predispositions of Glucocorticoid Resistance and Therapeutic Outcomes in Polymyalgia Rheumatica and Giant Cell Arteritis. Journal of Clinical Medicine, 8(5), 582. https://doi.org/10.3390/jcm8050582




