The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: A Systemic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy (Literature Search)
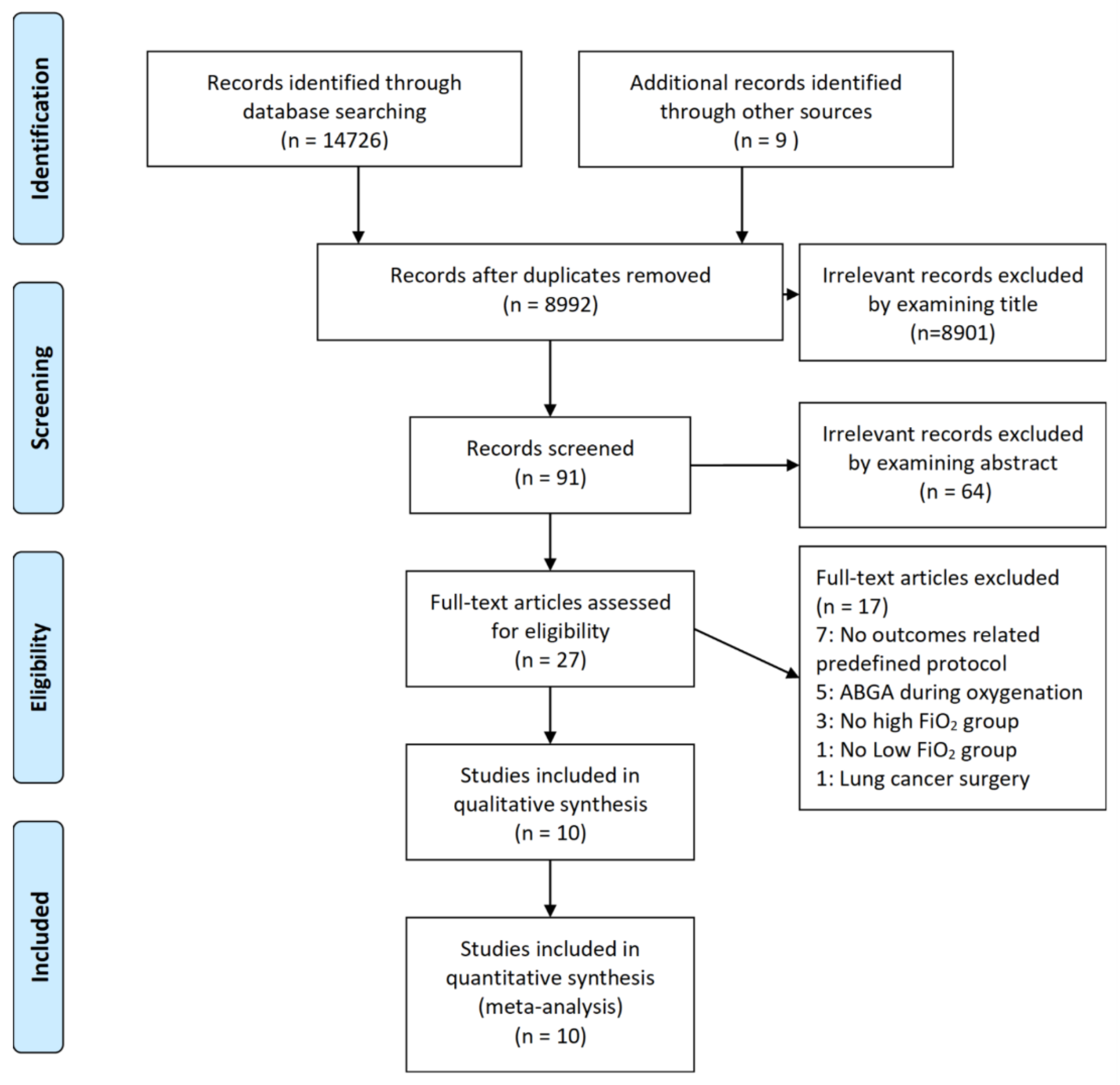
2.2. Study Selection
2.3. Data Extraction and Collection
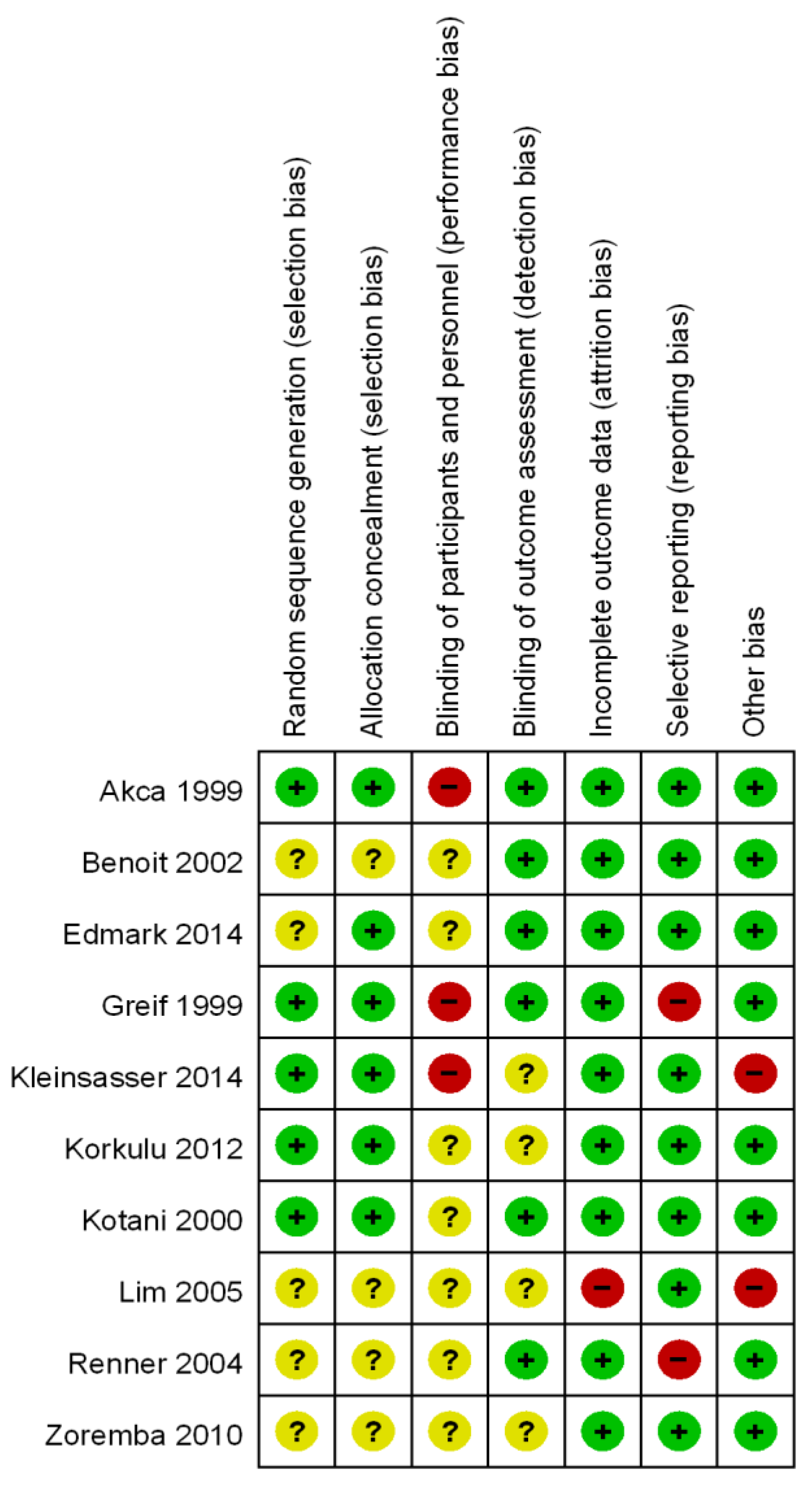
2.4. Methodological Quality and Risk of Bias Assessment
2.5. Type of Interventions and Outcomes
2.6. Data Synthesis and Statistical Analyses (Meta-Analysis)
2.7. Assessment of Heterogeneity
3. Results
3.1. Characteristic of Trials and Patients
3.2. Methodological Quality and Risk of Bias
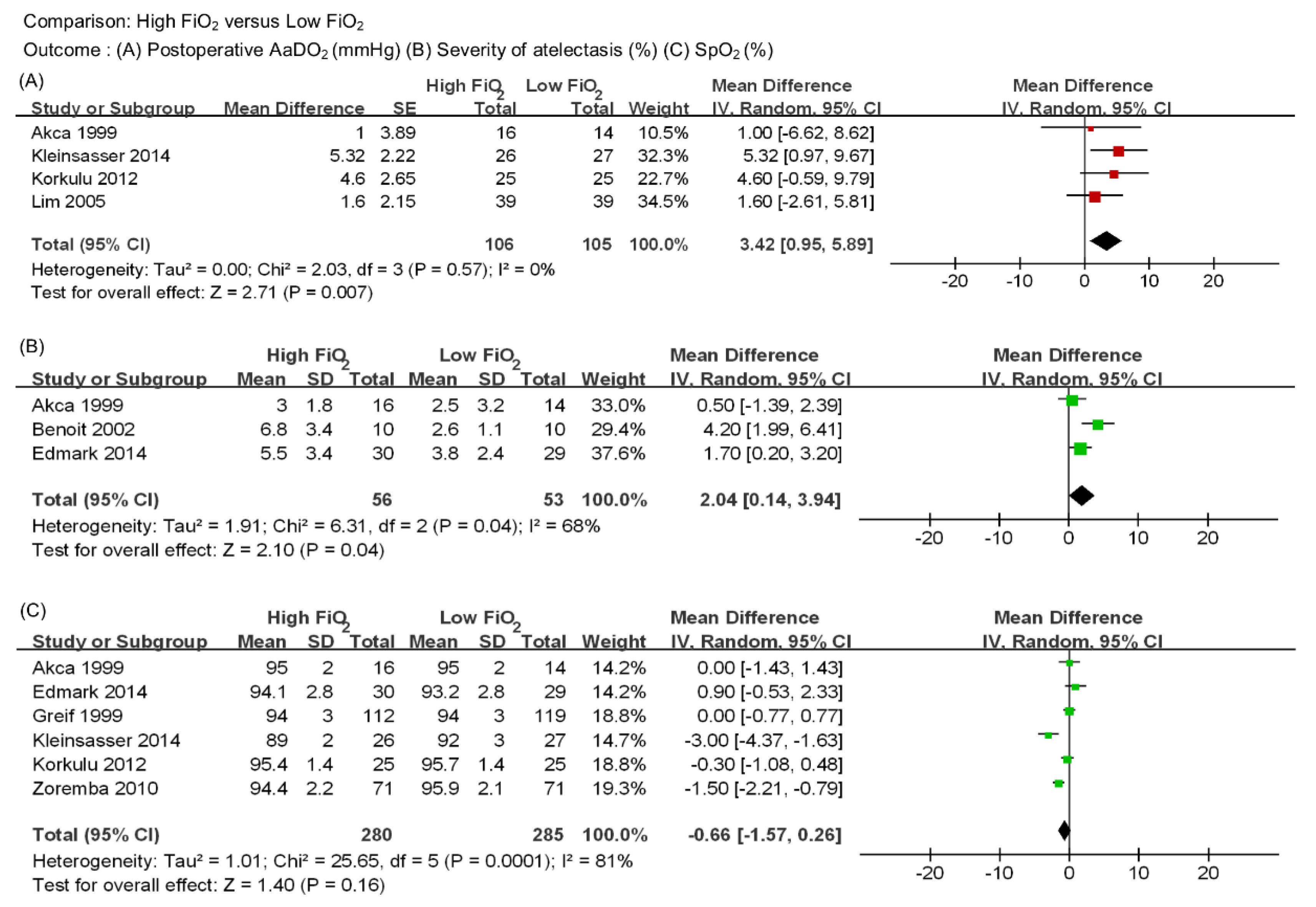
3.3. Outcome Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, U.; Salem, M.R.; Crystal, G.J. Preoxygenation: Physiologic Basis, Benefits, and Potential Risks. Anesth. Analg. 2017, 124, 507–517. [Google Scholar] [CrossRef]
- Neubauer, R.A.; James, P. Cerebral oxygenation and the recoverable brain. Neurol. Res. 1998, 20, S33–S36. [Google Scholar] [CrossRef]
- Gurdol, F.; Cimsit, M.; Oner-Iyidogan, Y.; Kocak, H.; Sengun, S.; Yalcinkaya-Demirsoz, S. Collagen synthesis, nitric oxide and asymmetric dimethylarginine in diabetic subjects undergoing hyperbaric oxygen therapy. Physiol. Res. 2010, 59, 423–429. [Google Scholar]
- Koksal, G.M.; Dikmen, Y.; Erbabacan, E.; Aydin, S.; Çakatay, U.; Sitar, M.E.; Altindas, F. Hyperoxic oxidative stress during abdominal surgery: A randomized trial. J. Anesth. 2016, 30, 610–619. [Google Scholar] [PubMed]
- Martin, D.S.; Grocott, M.P. Oxygen therapy and anaesthesia: Too much of a good thing? Anaesthesia 2015, 70, 522–527. [Google Scholar] [CrossRef]
- Reinius, H.; Jonsson, L.; Gustafsson, S.; Sundbom, M.; Duvernoy, O.; Pelosi, P.; Hedenstierna, G.; Fredén, F. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: A computerized tomography study. Anesthesiology 2009, 111, 979–987. [Google Scholar] [CrossRef]
- Lindberg, P.; Gunnarsson, L.; Tokics, L.; Secher, E.; Lundquist, H.; Brismar, B.; Hedenstierna, G. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol. Scand. 1992, 36, 546–553. [Google Scholar] [CrossRef]
- Magnusson, L.; Zemgulis, V.; Wicky, S.; Tydén, H.; Thelin, S.; Hedenstierna, G. Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: An experimental study. Anesthesiology 1997, 87, 1153–1163. [Google Scholar] [CrossRef]
- Rothen, H.U.; Sporre, B.; Engberg, G.; Wegenius, G.; Hedenstierna, G. Airway closure, atelectasis and gas exchange during general anaesthesia. Br. J. Anaesth. 1998, 81, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Van Kaam, A.H.; Lachmann, R.A.; Herting, E.; De Jaegere, A.; van Iwaarden, F.; Noorduyn, L.A.; Kok, J.H.; Haitsma, J.J.; Lachmann, B. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am. J. Respir. Crit. Care Med. 2004, 169, 1046–1053. [Google Scholar] [CrossRef]
- Ray, K.; Bodenham, A.; Paramasivam, E. Pulmonary atelectasis in anaesthesia and critical care. Contin. Educ. Anaesth. Critical Care Pain 2014, 14, 236–245. [Google Scholar] [CrossRef][Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hovaguimian, F.; Lysakowski, C.; Elia, N.; Tramer, M.R. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: Systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 303–316. [Google Scholar] [CrossRef]
- Akca, O.; Podolsky, A.; Eisenhuber, E.; Panzer, O.; Hetz, H.; Lampl, K.; Lackner, F.X.; Wittmann, K.; Grabenwoeger, F.; Kurz, A.; et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and 2 hours after colon resection. Anesthesiology 1999, 91, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Benoit, Z.; Wicky, S.; Fischer, J.F.; Frascarolo, P.; Chapuis, C.; Spahn, D.R.; Magnusson, L. The effect of increased FiO2 before tracheal extubation on postoperative atelectasis. Anesth. Analg. 2002, 95, 1777–1781. [Google Scholar] [CrossRef]
- Edmark, L.; Auner, U.; Lindback, J.; Enlund, M.; Hedenstierna, G. Post-operative atelectasis—A randomized trial investigating a ventilator strategy and low oxygen fraction during recovery. Acta Anaesthesiol. Scand. 2014, 58, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Greif, R.; Laciny, S.; Rapf, B.; Hickle, R.S.; Sessler, D.I. Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology 1999, 91, 1246–1252. [Google Scholar] [CrossRef]
- Kleinsasser, A.T.; Pircher, I.; Truebsbach, S.; Knotzer, H.; Loeckinger, A.; Treml, B. Pulmonary function after emergence on 100% oxygen in patients with chronic obstructive pulmonary disease: A randomized, controlled trial. Anesthesiology 2014, 120, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Korkulu, F.; Uysal, H.Y.; Acar, H.V.; Eruyar, S.; Dikmen, B. The effect of different oxygen concentrations used for the induction and maintenance of anesthesia on gas exchange in the lungs. Turk. J. Anaesthesiol Reanim. 2012, 40, 11–19. [Google Scholar] [CrossRef]
- Kotani, N.; Hashimoto, H.; Sessler, D.I.; Muraoka, M.; Hashiba, E.; Kubota, T.; Matsuki, A. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology 2000, 93, 15–25. [Google Scholar] [CrossRef]
- Lim, Y.J.; Seo, Y.J.; Jung, S.M.; Yang, H.S. The Effect of Increased FiO2 before Removal of the Laryngeal Mask Airway on Postanesthetic Arterial Partial Oxygen Pressure. Korean J. Anesthesiol. 2005, 48, 576–581. [Google Scholar] [CrossRef]
- Renner, M.; Hohlrieder, M.; Wolk, T.; Pühringer, F.; Kleinsasser, A.T.; Keller, C.; Benzer, A. Administration of 100% oxygen before removal of the laryngeal mask airway does not affect postanesthetic arterial partial pressure of oxygen. Anesth. Analg. 2004, 98, 257–259. [Google Scholar] [CrossRef]
- Zoremba, M.; Dette, F.; Hunecke, T.; Braunecker, S.; Wulf, H. The influence of perioperative oxygen concentration on postoperative lung function in moderately obese adults. Eur. J. Anaesthesiol. 2010, 27, 501–507. [Google Scholar] [CrossRef]
- Maity, A.; Saha, D.; Swaika, S.; Maulik, S.G.; Choudhury, B.; Sutradhar, M. Detection of hypoxia in the early postoperative period. Anesth. Essays Res. 2012, 6, 34–37. [Google Scholar] [CrossRef]
- Morosin, M.; Vignati, C.; Novi, A.; Salvioni, E.; Veglia, F.; Alimento, M.; Merli, G.; Sciomer, S.; Sinagra, G.; Agostoni, P. The alveolar to arterial oxygen partial pressure difference is associated with pulmonary diffusing capacity in heart failure patients. Respir. Physiol. Neurobiol. 2016, 233, 1–6. [Google Scholar] [CrossRef]
- Sarkar, M.; Niranjan, N.; Banyal, P.K. Mechanism of hypoxemia. Lung India 2017, 34, 47–60. [Google Scholar] [CrossRef]
- Tusman, G.; Bohm, S.H.; Suarez-Sipmann, F. Advanced Uses of Pulse Oximetry for Monitoring Mechanically Ventilated Patients. Anesth. Analg. 2017, 124, 62–71. [Google Scholar] [CrossRef]
- Sun, Z.; Sessler, D.I.; Dalton, J.E.; Devereaux, P.J.; Shahinyan, A.; Naylor, A.J.; Hutcherson, M.T.; Finnegan, P.S.; Tandon, V.; Darvish-Kazem, S.; et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth. Analg. 2015, 121, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Brimacombe, J. Bronchial mucus transport velocity in paralyzed anesthetized patients: A comparison of the laryngeal mask airway and cuffed tracheal tube. Anesth. Analg. 1998, 86, 1280–1282. [Google Scholar] [PubMed]
- Konrad, F.; Schreiber, T.; Brecht-Kraus, D.; Georgieff, M. Mucociliary transport in ICU patients. Chest 1994, 105, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analysis of randomized controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]

| Author | Year | Design | No. of Patients (High FiO2/Low FiO2) | Language | Age | FiO2 | Airway Device | Maintenance Anesthetics | Intervention Period | Type of Surgery | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High FiO2 | Low FiO2 | ||||||||||
| Akca | 1999 | RCT | 30 (16/14) | English | 49 ± 14 | 41 ± 10 | 0.8/0.3 | ETT | IA | Intraoperative and postoperative 2 hour | Colon resection |
| Benoit | 2002 | RCT | 20 (10/10) | English | 40 ± 14 | 32 ± 11 | 1.0/0.4 | ETT | IV | Emergence | Surgery of the extremities |
| Edmark | 2014 | RCT | 59 (30/29) | English | 53 ± 12 | 53 ± 11 | 1.0/0.3 | LMA | IV | Emergence | Orthopedic surgery |
| Greif | 1999 | RCT | 231 (112/119) | English | 59 ± 14 | 60 ± 13 | 0.8/0.3 | ETT | IA | Intraoperative and postoperative 2 hour | Colon or rectum resection |
| Kleinsasser | 2014 | RCT | 53 (26/27) | English | 68 ± 13 | 65 ± 10 | 1.0/0.3 | ETT | IV | Emergence | Carotid endarterectomy |
| Korkulu | 2012 | RCT | 50 (25/25) | Turkish | 41.7 ± 11.6 | 46.2 ± 11.3 | 1.0/0.4 | ETT | IA | Intraoperative | Laparoscopic cholecystectomy |
| Kotani | 2000 | RCT | 60 (30/30) | English | 49 ± 8 | 48 ± 9 | 1.0/0.3 | ETT | IV | Intraoperative | Orthopedic surgery |
| Lim | 2005 | RCT | 78 (39/39) | Korean | 43.5 ± 14.3 | 41.5 ± 15.1 | 1.0/0.3 | LMA | IV or IA | Emergence | Surgery of extremities |
| Renner | 2004 | RCT | 64 (32/32) | English | 31 ± 7 | 30 ± 7 | 1.0/0.3 | LMA | IV | Emergence | Peripheral musculoskeletal surgery |
| Zoremba | 2010 | RCT | 142 (71/71) | English | 51 ± 11 | 50 ± 12 | 0.8/0.4 | ETT | IV | Intraoperative | Minor peripheral surgery |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, C.-H.; Park, E.Y.; Lee, S.Y.; Ryu, J.-H. The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: A Systemic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 583. https://doi.org/10.3390/jcm8050583
Koo C-H, Park EY, Lee SY, Ryu J-H. The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: A Systemic Review and Meta-Analysis. Journal of Clinical Medicine. 2019; 8(5):583. https://doi.org/10.3390/jcm8050583
Chicago/Turabian StyleKoo, Chang-Hoon, Eun Young Park, Sun Young Lee, and Jung-Hee Ryu. 2019. "The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: A Systemic Review and Meta-Analysis" Journal of Clinical Medicine 8, no. 5: 583. https://doi.org/10.3390/jcm8050583
APA StyleKoo, C.-H., Park, E. Y., Lee, S. Y., & Ryu, J.-H. (2019). The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: A Systemic Review and Meta-Analysis. Journal of Clinical Medicine, 8(5), 583. https://doi.org/10.3390/jcm8050583





