GLP-1 Limits Adipocyte Inflammation and Its Low Circulating Pre-Operative Concentrations Predict Worse Type 2 Diabetes Remission after Bariatric Surgery in Obese Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Analytical Procedures
2.3. Oral Glucose Tolerance Test
2.4. RNA Extraction and Real-Time PCR
2.5. Adipocyte Culture
2.6. Statistical Analysis
3. Results
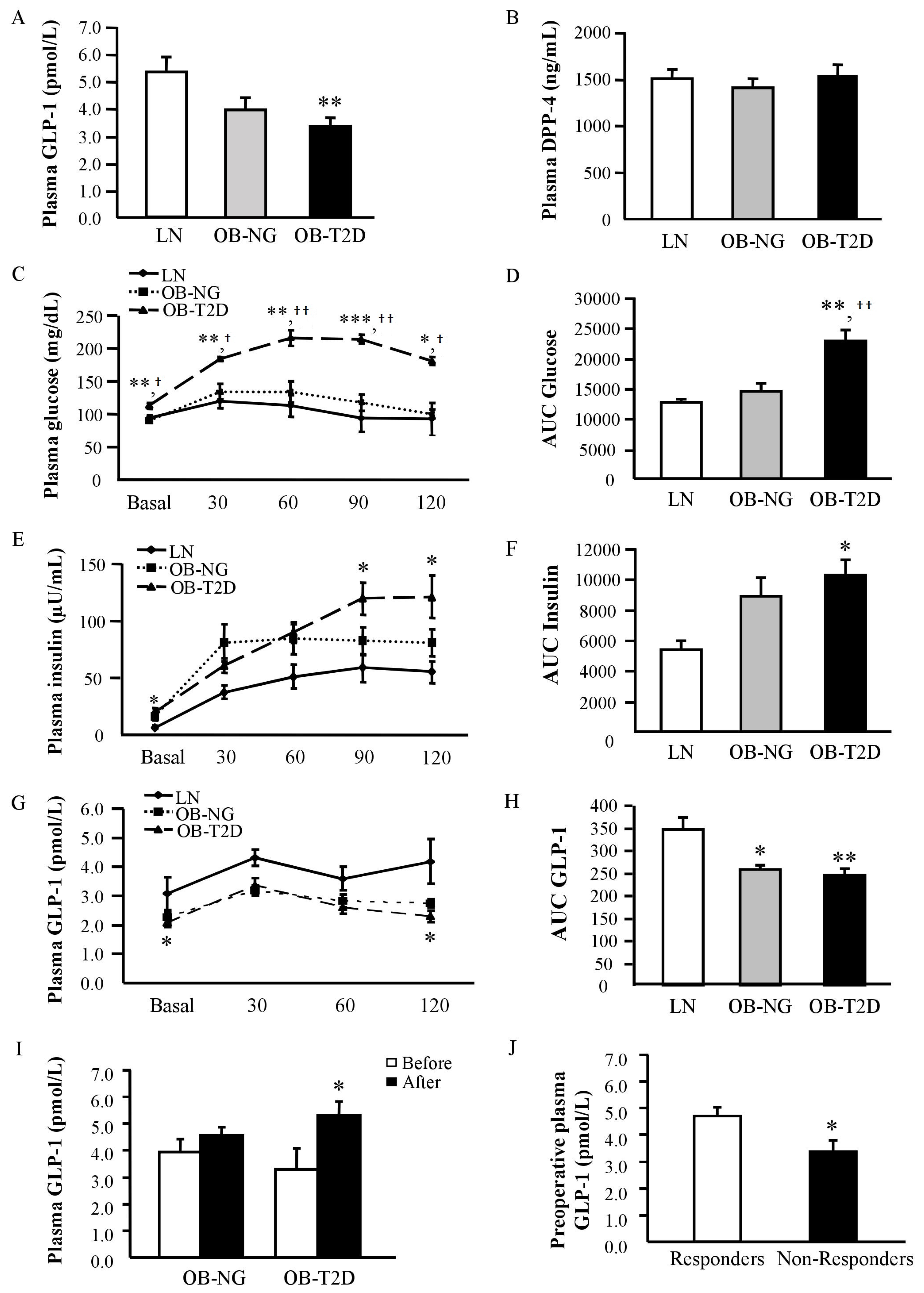
3.1. Decreased GLP-1 Circulating Levels in Human Obesity-Associated T2D
3.2. GLP-1 Circulating Levels in Patients with T2D Increase after RYGB
3.3. Increased Pre-Operative GLP-1 Circulating Levels in Patients with T2D Remission
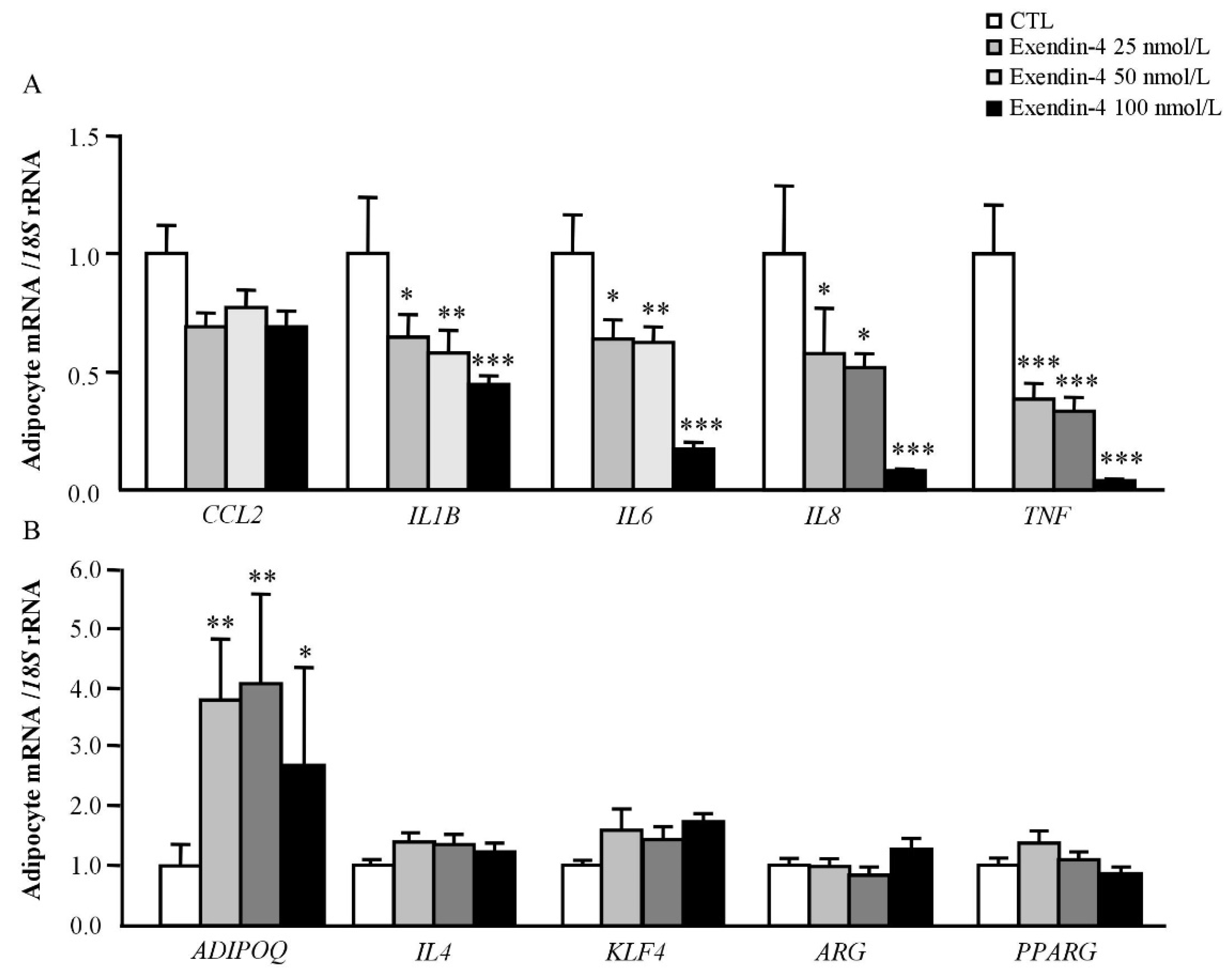
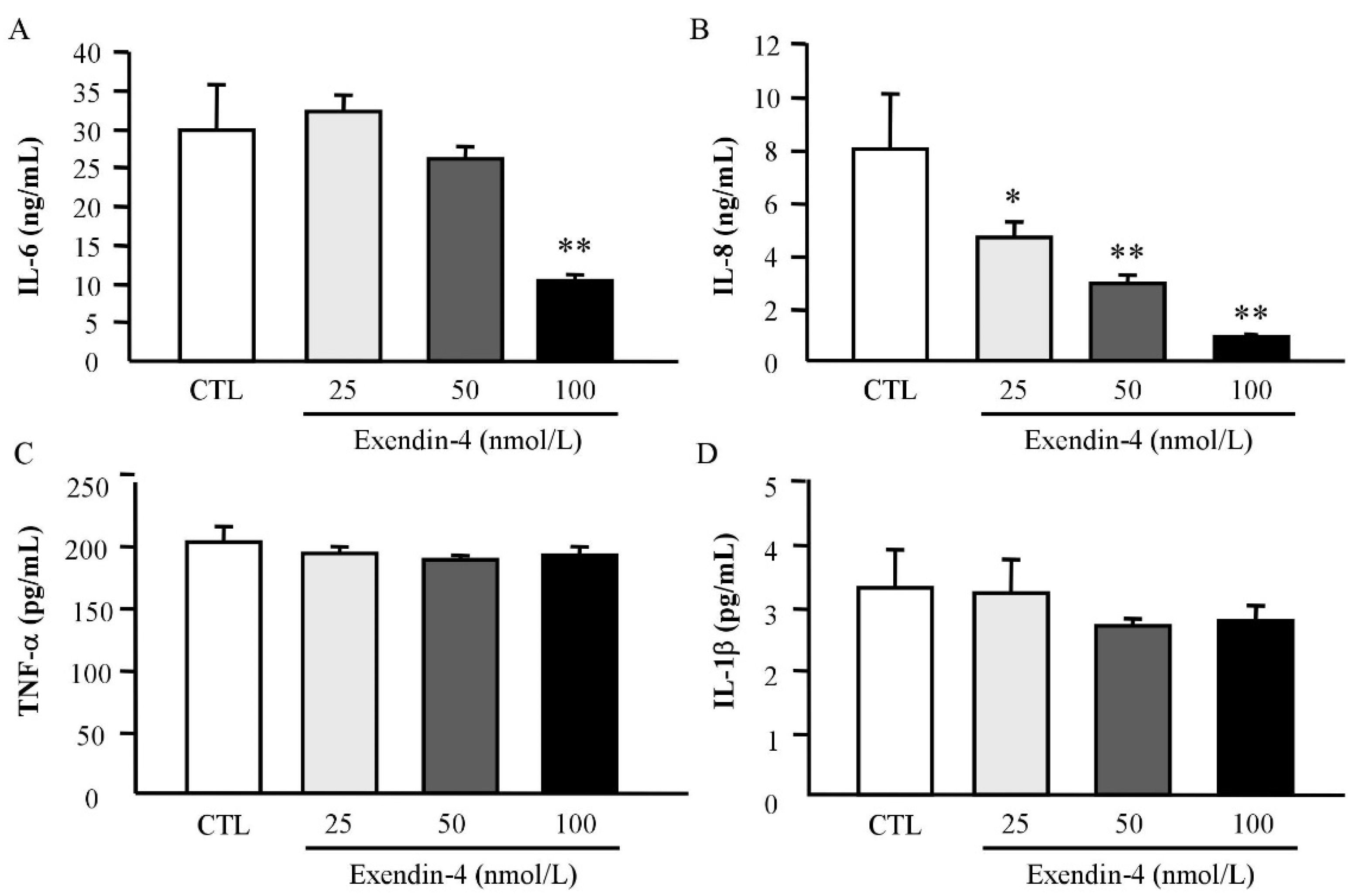
3.4. Exendin-4 Downregulates the Expression of Pro-Inflammatory Markers in Human Visceral Adipocytes
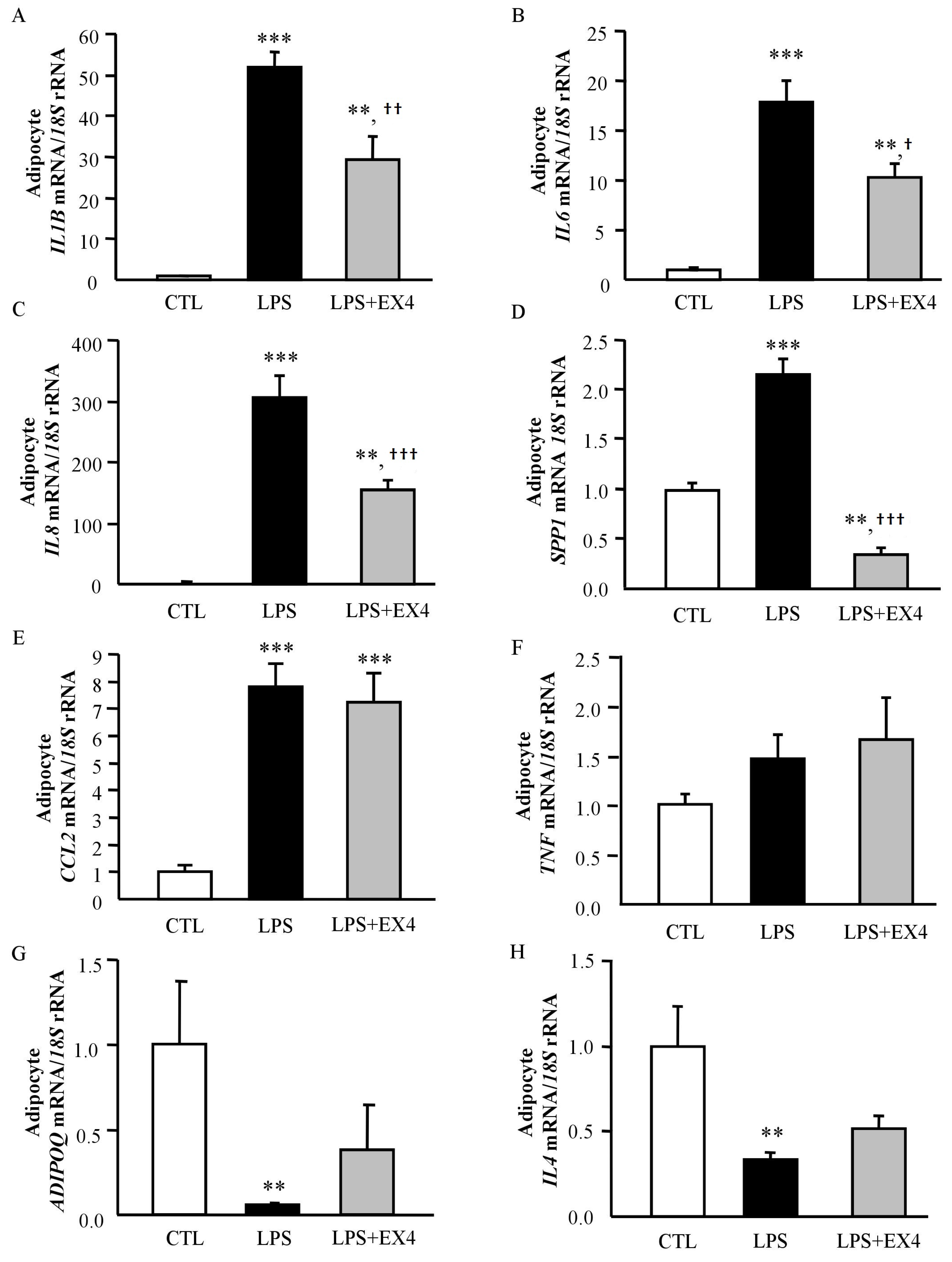
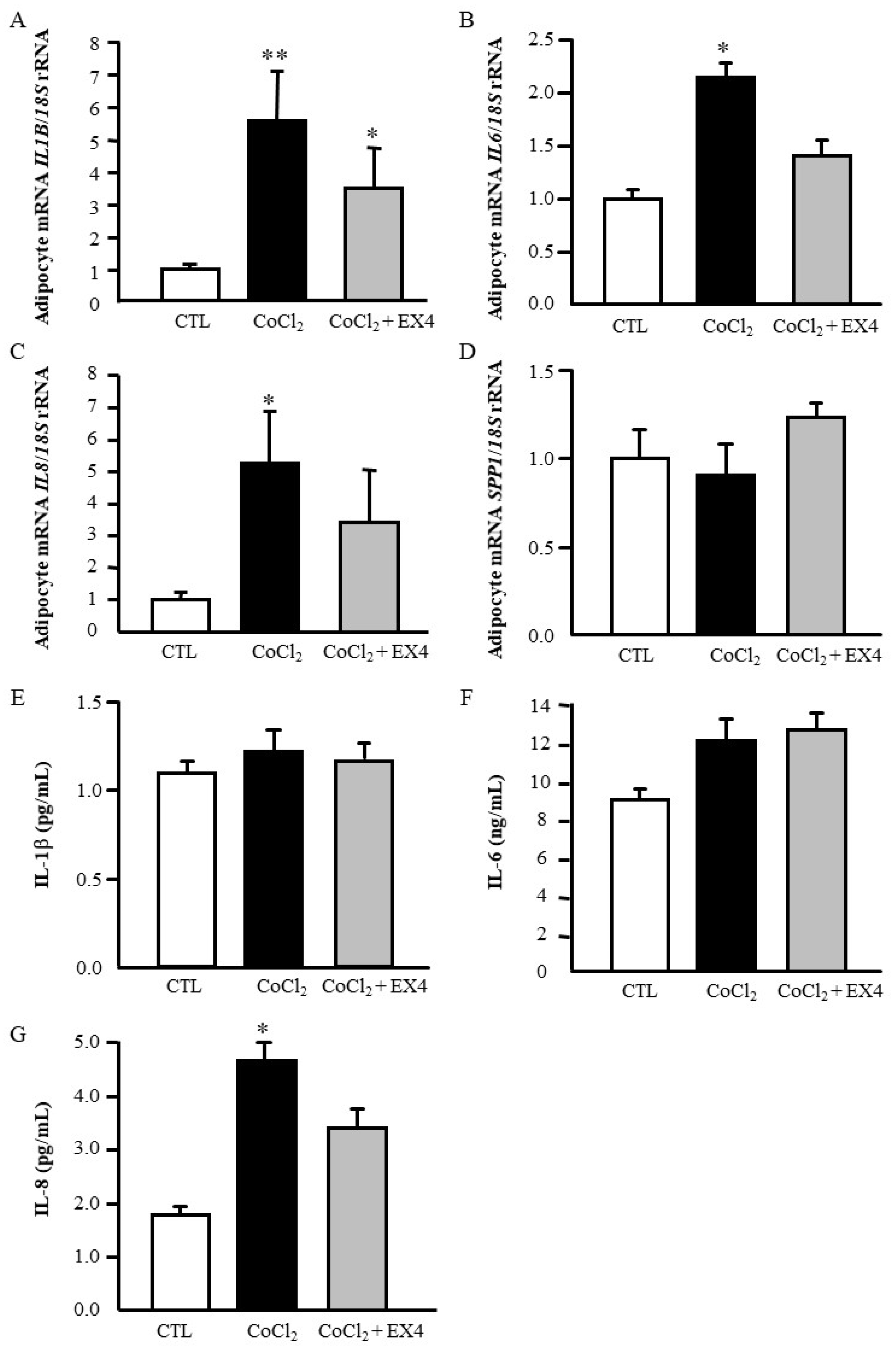
3.5. Exendin-4 Inhibits LPS- and Hypoxia-Induced Inflammation in Human Visceral Adipocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIOPQ | adiponectin |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| ARG1 | arginase 1 |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CCL2 | monocyte chemoattractant protein-1 |
| CRP | C-reactive protein |
| DBP | diastolic blood pressure |
| γ-GT | γ-glutamyltransferase |
| GLP-1 | glucagon-like peptide-1 |
| HOMA | homeostasis model assessment |
| IL | interleukin |
| KLF4 | kruppel-like factor 4 |
| LPS | lipopolysaccharide |
| NG | normoglycemic |
| OGTT | oral glucose tolerance test |
| PARG | peroxisome proliferator-activated receptor-γ |
| QUICKI | quantitative insulin sensitivity check index |
| RYGB | Roux-en-Y gastric bypass |
| SBP | systolic blood pressure; |
| SVFC | stroma-vascular fraction cells; |
| TNF | tumour necrosis factor-α |
| T2D | type 2 diabetes |
| VAT | visceral adipose tissue |
References
- James, W.P.T. Obesity: A global public health challenge. Clin. Chem. 2018, 64, 24–29. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Eng, C.; Kramer, C.K.; Zinman, B.; Retnakaran, R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: A systematic review and meta-analysis. Lancet 2014, 384, 2228–2234. [Google Scholar] [CrossRef]
- Hutch, C.R.; Sandoval, D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology 2017, 158, 4139–4151. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Holst, A.G.; Vilsboll, T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2017, 376, 891–892. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar]
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef]
- Frühbeck, G.; Nogueiras, R. GLP-1: The oracle for gastric bypass? Diabetes 2014, 63, 399–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habegger, K.M.; Heppner, K.M.; Amburgy, S.E.; Ottaway, N.; Holland, J.; Raver, C.; Bartley, E.; Muller, T.D.; Pfluger, P.T.; Berger, J.; et al. GLP-1r responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes 2014, 63, 505–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nannipieri, M.; Baldi, S.; Mari, A.; Colligiani, D.; Guarino, D.; Camastra, S.; Barsotti, E.; Berta, R.; Moriconi, D.; Bellini, R.; et al. Roux-en-y gastric bypass and sleeve gastrectomy: Mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab. 2013, 98, 4391–4399. [Google Scholar] [CrossRef]
- Jimenez, A.; Casamitjana, R.; Flores, L.; Delgado, S.; Lacy, A.; Vidal, J. Glp-1 and the long-term outcome of type 2 diabetes mellitus after roux-en-y gastric bypass surgery in morbidly obese subjects. Ann. Surg. 2013, 257, 894–899. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jun, H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinflammatory, and antioxidant action in type 2 diabetes. Diabetes Care 2014, 37, 1938–1943. [Google Scholar] [CrossRef]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Ferns, G.A. Treatment with GLP1 receptor agonists reduce serum crp concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2017, 31, 1237–1242. [Google Scholar] [CrossRef]
- Daousi, C.; Pinkney, J.H.; Cleator, J.; Wilding, J.P.; Ranganath, L.R. Acute peripheral administration of synthetic human glp-1 (7-36 amide) decreases circulating il-6 in obese patients with type 2 diabetes mellitus: A potential role for GLP-1 in modulation of the diabetic pro-inflammatory state? Regul. Pept. 2013, 183, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, 13–27. [Google Scholar] [CrossRef]
- Still, C.D.; Wood, G.C.; Benotti, P.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Ibele, A.; Seiler, J.; Irving, B.A.; Celaya, M.P.; et al. Preoperative prediction of type 2 diabetes remission after roux-en-y gastric bypass surgery: A retrospective cohort study. Lancet Diabetes Endocrinol. 2014, 2, 38–45. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Rodríguez, A.; Salvador, J.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Validation of endogenous control genes in human adipose tissue: Relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm. Metab. Res. 2007, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Rotellar, F.; Valentí, V.; Silva, C.; Mugueta, C.; Pulido, M.R.; Vazquez, R.; Salvador, J.; et al. The ghrelin o-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 2012, 55, 3038–3050. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbaek, A.; et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The addition-pro study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef]
- Alssema, M.; Rijkelijkhuizen, J.M.; Holst, J.J.; Teerlink, T.; Scheffer, P.G.; Eekhoff, E.M.; Gastaldelli, A.; Mari, A.; Hart, L.M.; Nijpels, G.; et al. Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and alt. Eur. J. Endocrinol. 2013, 169, 421–430. [Google Scholar] [CrossRef]
- Cavin, J.B.; Couvelard, A.; Lebtahi, R.; Ducroc, R.; Arapis, K.; Voitellier, E.; Cluzeaud, F.; Gillard, L.; Hourseau, M.; Mikail, N.; et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after roux-en-y gastric bypass vs sleeve gastrectomy. Gastroenterology 2016, 150, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Laferrere, B. Diabetes remission after bariatric surgery: Is it just the incretins? Int. J. Obes. 2011, 35, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; Bagnol, D.; Woods, S.C.; D’Alessio, D.A.; Seeley, R.J. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008, 57, 2046–2054. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Ferno, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic ampk. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.D.; Larsen, M.O.; Jelic, K.; Lindgren, O.; Vikman, J.; Holst, J.J.; Deacon, C.F.; Ahren, B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J. Clin. Endocrinol. Metab. 2010, 95, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Varin, E.M.; Mulvihill, E.E.; Beaudry, J.L.; Pujadas, G.; Fuchs, S.; Tanti, J.F.; Fazio, S.; Kaur, K.; Cao, X.; Baggio, L.L.; et al. Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic dpp4 inhibition. Cell Metab. 2018. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.; et al. GLP-1r agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1r. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Kim Chung le, T.; Hosaka, T.; Yoshida, M.; Harada, N.; Sakaue, H.; Sakai, T.; Nakaya, Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase a pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009, 390, 613–618. [Google Scholar] [CrossRef]
- Vendrell, J.; El Bekay, R.; Peral, B.; Garcia-Fuentes, E.; Megia, A.; Macias-Gonzalez, M.; Fernandez Real, J.; Jimenez-Gomez, Y.; Escote, X.; Pachon, G.; et al. Study of the potential association of adipose tissue glp-1 receptor with obesity and insulin resistance. Endocrinology 2011, 152, 4072–4079. [Google Scholar] [CrossRef]
- Fletcher, M.M.; Halls, M.L.; Christopoulos, A.; Sexton, P.M.; Wootten, D. The complexity of signalling mediated by the glucagon-like peptide-1 receptor. Biochem. Soc. Trans. 2016, 44, 582–588. [Google Scholar] [CrossRef]
- Velmurugan, K.; Balamurugan, A.N.; Loganathan, G.; Ahmad, A.; Hering, B.J.; Pugazhenthi, S. Antiapoptotic actions of exendin-4 against hypoxia and cytokines are augmented by CREB. Endocrinology 2012, 153, 1116–1128. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Salvador, J.; Rotellar, F.; Silva, C.; Catalán, V.; Rodríguez, A.; Gil, M.J.; Frühbeck, G. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes. Surg. 2006, 16, 262–269. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Salvador, J.; Páramo, J.A.; Orbe, J.; de Irala, J.; Diez-Caballero, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin. Biochem. 2002, 35, 315–320. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin. Endocrinol. 2008, 68, 213–219. [Google Scholar] [CrossRef]
- Gallego-Escuredo, J.M.; Gómez-Ambrosi, J.; Catalán, V.; Domingo, P.; Giralt, M.; Frühbeck, G.; Villarroya, F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 2015, 39, 121–129. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef]
| Lean | Obese NG | Obese T2D | |
|---|---|---|---|
| n (males, females) | 30 (12, 18) | 30 (12, 18) | 33 (16, 17) |
| Age (years) | 46 ± 2 | 39 ± 2 | 51 ± 2 † |
| BMI (kg/m2) | 23.0 ± 0.2 | 46.0 ± 1.6 *** | 43.1 ± 1.2 *** |
| Body fat (%) | 29.1 ± 1.8 | 52.1 ± 1.1 *** | 48.7 ± 1.4 *** |
| Waist (cm) | 83 ± 1 | 130 ± 3 *** | 127 ± 2 *** |
| Waist-to-hip ratio | 0.87 ± 0.01 | 0.96 ± 0.02 *** | 1.00 ± 0.01 *** |
| SBP (mm Hg) | 109 ± 2 | 126 ± 3 *** | 129 ± 3 *** |
| DBP (mm Hg) | 67 ± 1 | 80 ± 2 *** | 80 ± 2 *** |
| Fasting glucose (mg/dL) | 92 ± 2 | 90 ± 2 | 151 ± 11 ***,††† |
| Glucose 2-h after OGTT (mg/dL) | 98 ± 8 | 119 ± 4 | 220 ± 21 ***,††† |
| Fasting insulin (μU/mL) | 6.1 ± 0.8 | 16.8 ± 3.8 | 28.2 ± 6.1 ** |
| HOMA | 1.4 ± 0.2 | 3.8 ± 0.9 | 8.2 ± 1.0 ***,††† |
| QUICKI | 0.375 ± 0.010 | 0.340 ± 0.010 | 0.296 ± 0.006 ***,††† |
| Triacylglycerol (mg/dL) | 80 ± 7 | 101 ± 6 | 156 ± 11 ***,†† |
| Total cholesterol (mg/dL) | 180 ± 6 | 199 ± 9 | 179 ± 6 |
| LDL-cholesterol (mg/dL) | 103 ± 5 | 128 ± 7 * | 104 ± 6 † |
| HDL-cholesterol (mg/dL) | 59 ± 2 | 50 ± 3 * | 44 ± 2 *** |
| Leptin (ng/mL) | 11.4 ± 3.0 | 50.2 ± 4.0 *** | 44.7 ± 5.2 *** |
| Adiponectin (µg/mL) | 13.1 ± 1.9 | 11.6 ± 1.6 | 6.7 ± 0.6 ***,†† |
| Uric acid (mg/dL) | 4.5 ± 0.2 | 5.7 ± 0.3 * | 5.8 ± 0.3 ** |
| Creatinine (mg/dL) | 0.8 ± 0.03 | 0.78 ± 0.03 | 0.82 ± 0.03 |
| CRP (mg/L) | 4.81 ± 1.74 | 9.23 ± 1.82 * | 8.27 ± 1.43 * |
| Fibrinogen (mg/dL) | 279 ± 18 | 394 ± 17 ** | 370 ± 20 * |
| von Willebrand factor (%) | 108 ± 4 | 115.1 ± 11.4 | 148.5 ± 9.2 |
| Homocysteine (μmol/L) | 9.7 ± 2.4 | 10.2 ± 1.7 | 10.4 ± 1.0 |
| AST (IU/L) | 14 ± 1 | 17 ± 3 | 16 ± 1 |
| ALT (IU/L) | 17 ± 2 | 24 ± 3 * | 23 ± 2 |
| AST/ALT | 0.96 ± 0.08 | 0.76 ± 0.06 * | 0.73 ± 0.03 * |
| ALP (IU/L) | 82 ± 6 | 75 ± 6 | 67 ± 6 |
| γ-GT (IU/L) | 14 ± 1 | 17 ± 2 | 33 ± 5 ***,†† |
| Obese NG Before Surgery | Obese NG After Surgery | Obese T2D Before Surgery | Obese T2D After Surgery | |
|---|---|---|---|---|
| n (males, females) | 22 (6, 16) | 22 (6, 16) | 55 (22, 33) | 55 (22, 33) |
| BMI (kg/m2) | 44.0 ± 1.9 | 30.8 ± 1.7 *** | 45.7 ± 1.0 | 35.2 ± 1.0 *** |
| Body fat (%) | 52.3 ± 1.0 | 38.9 ± 2.6 *** | 51.6 ± 1.0 | 43.0 ± 1.5 *** |
| Waist (cm) | 123 ± 4 | 98 ± 4 *** | 130 ± 2 | 110 ± 2 *** |
| Waist-to-hip ratio | 0.94 ± 0.02 | 0.90 ± 0.02 * | 0.99 ± 0.01 | 0.96 ± 0.01 ** |
| SBP (mm Hg) | 123 ± 3 | 111 ± 3 *** | 130 ± 2 | 118 ± 2 ** |
| DBP (mm Hg) | 78 ± 2 | 70 ± 2 ** | 82 ± 1 | 73 ± 1 * |
| Fasting glucose (mg/dL) | 91 ± 2 | 87 ± 2 * | 122 ± 5 | 104 ± 5 ** |
| Fasting insulin (μU/mL) | 15.1 ± 5.2 | 6.8 ± 1.3 | 22.2 ± 1.8 | 10.2 ± 1.0 ** |
| HOMA | 3.7 ± 1.3 | 1.6 ± 0.3 | 6.5 ± 0.5 | 2.5 ± 0.3 *** |
| QUICKI | 0.35 ± 0.01 | 0.39 ± 0.01 ** | 0.30 ± 0.01 | 0.35 ± 0.01 *** |
| HbA1c (%) | - | - | 7.9 ± 0.4 | 6.7 ± 0.3 ** |
| Triacylglycerol (mg/dL) | 101 ± 9 | 72 ± 7 ** | 142 ± 13 | 94 ± 8 *** |
| Total cholesterol (mg/dL) | 201 ± 12 | 152 ± 7 *** | 185 ± 7 | 157 ± 5 ** |
| LDL-cholesterol (mg/dL) | 129 ± 10 | 83 ± 6 *** | 110 ± 6 | 88 ± 4 ** |
| HDL-cholesterol (mg/dL) | 51 ± 6 | 55 ± 3 | 46 ± 2 | 51 ± 2 * |
| Leptin (ng/mL) | 49.1 ± 5.2 | 12.5 ± 3.1 *** | 48.7 ± 4.6 | 17.7 ± 1.9 *** |
| Adiponectin | 11.6 ± 1.3 | 16.9 ± 1.3 *** | 7.2 ± 0.4 | 12.0 ± 0.7 *** |
| Uric acid (mg/dL) | 5.5 ± 0.3 | 4.8 ± 0.3 ** | 5.9 ± 0.2 | 5.3 ± 0.2 * |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.2 * |
| CRP (mg/L) | 10.23 ± 3.20 | 4.96 ± 0.30 * | 7.91 ± 1.99 | 2.48 ± 0.76 * |
| Fibrinogen (mg/dL) | 394 ± 23 | 342 ± 20 ** | 364 ± 17 | 350 ± 16 |
| von Willebrand factor (%) | 97 ± 18 | 91 ± 15 | 155 ± 13 | 127 ± 12 * |
| Homocysteine (μmol/L) | 14.1 ± 5.8 | 10.6 ± 2.8 | 10.1 ± 0.9 | 9.9 ± 0.7 |
| AST (IU/L) | 17 ± 4 | 14 ± 1 | 15 ± 1 | 18 ± 1 |
| ALT (IU/L) | 24 ± 4 | 16 ± 2 | 23 ± 2 | 26 ± 3 |
| AST/ALT | 0.74 ± 0.07 | 0.90 ± 0.04 ** | 0.75 ± 0.04 | 0.80 ± 0.04 |
| ALP (IU/L) | 78 ± 8 | 61 ± 5 ** | 70 ± 5 | 72 ± 5 |
| γ-GT (IU/L) | 17 ± 2 | 10 ± 2 *** | 31 ± 3 | 18 ± 1 *** |
| IL-1β (pg/mL) | 1.57 ± 0.18 | 1.41 ± 0.07 | 1.61 ± 0.10 | 1.42 ± 0.08 |
| IL-6 (pg/mL) | 19.8 ± 8.2 | 8.5 ± 0.4 * | 19.1 ± 2.6 | 11.1 ± 0.8 ** |
| IL-18 (pg/mL) | 126.8 ± 9.2 | 99.4 ± 7.6 * | 122.9 ± 6.7 | 112.5 ± 6.3 * |
| MCP-1 (pg/mL) | 221 ± 32 | 198 ± 21 | 247 ± 25 | 234 ± 20 |
| T2D-Responders | T2D-Non-Responders | |
|---|---|---|
| n (males, females) | 31 (16, 15) | 16 (8, 8) |
| DiaRem Score | 4.05 ± 0.74 | 11.00 ± 1.81 ** |
| Insulin treatment (%) | 6.5% | 31.5% |
| Other T2D treatment (%) | 19.4% | 87.5% |
| Age (years) | 48 ± 2 | 52 ± 2 |
| BMI (kg/m2) | 41.2 ± 1.8 | 39.3 ± 1.8 |
| Body fat (%) | 48.4 ± 1.3 | 44.8 ± 1.8 |
| Waist (cm) | 126 ± 2 | 121 ± 4 |
| Waist-to-hip ratio | 0.99 ± 0.01 | 0.99 ± 0.02 |
| SBP (mm Hg) | 132 ± 3 | 128 ± 4 |
| DBP (mm Hg) | 81 ± 2 | 77 ± 1 |
| HOMA | 7.8 ± 1.3 | 10.9 ± 2.7 |
| QUICKI | 0.296 ± 0.006 | 0.291 ± 0.014 |
| Triacylglycerol (mg/dL) | 126 ± 11 | 228 ± 39 * |
| Total cholesterol (mg/dL) | 184 ± 7 | 207 ± 27 |
| LDL-cholesterol (mg/dL) | 106 ± 7 | 97 ± 6 |
| HDL-cholesterol (mg/dL) | 49 ± 4 | 37 ± 3 |
| Uric acid (mg/dL) | 5.5 ± 0.3 | 6.2 ± 0.8 |
| Creatinine (mg/dL) | 0.82 ± 0.03 | 0.97 ± 0.09 * |
| CRP (mg/L) | 0.8. ± 0.1 | 0.6 ± 0.3 |
| Fibrinogen (mg/dL) | 391 ± 26 | 340 ± 68 |
| von Willebrand factor (%) | 135 ± 11 | 129 ± 21 |
| AST (UI/L) | 16 ± 1 | 20 ± 4 |
| ALT (UI/L) | 27 ± 3 | 31 ± 7 |
| ALP (UI/L) | 78 ± 9 | 69 ± 14 |
| γ-GT (UI/L) | 24 ± 3 | 48 ± 18 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izaguirre, M.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Valentí, V.; Moncada, R.; Unamuno, X.; Silva, C.; de la Higuera, M.; et al. GLP-1 Limits Adipocyte Inflammation and Its Low Circulating Pre-Operative Concentrations Predict Worse Type 2 Diabetes Remission after Bariatric Surgery in Obese Patients. J. Clin. Med. 2019, 8, 479. https://doi.org/10.3390/jcm8040479
Izaguirre M, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Becerril S, Valentí V, Moncada R, Unamuno X, Silva C, de la Higuera M, et al. GLP-1 Limits Adipocyte Inflammation and Its Low Circulating Pre-Operative Concentrations Predict Worse Type 2 Diabetes Remission after Bariatric Surgery in Obese Patients. Journal of Clinical Medicine. 2019; 8(4):479. https://doi.org/10.3390/jcm8040479
Chicago/Turabian StyleIzaguirre, Maitane, Javier Gómez-Ambrosi, Amaia Rodríguez, Beatriz Ramírez, Sara Becerril, Víctor Valentí, Rafael Moncada, Xabier Unamuno, Camilo Silva, Magdalena de la Higuera, and et al. 2019. "GLP-1 Limits Adipocyte Inflammation and Its Low Circulating Pre-Operative Concentrations Predict Worse Type 2 Diabetes Remission after Bariatric Surgery in Obese Patients" Journal of Clinical Medicine 8, no. 4: 479. https://doi.org/10.3390/jcm8040479
APA StyleIzaguirre, M., Gómez-Ambrosi, J., Rodríguez, A., Ramírez, B., Becerril, S., Valentí, V., Moncada, R., Unamuno, X., Silva, C., de la Higuera, M., Salvador, J., Monreal, I., Frühbeck, G., & Catalán, V. (2019). GLP-1 Limits Adipocyte Inflammation and Its Low Circulating Pre-Operative Concentrations Predict Worse Type 2 Diabetes Remission after Bariatric Surgery in Obese Patients. Journal of Clinical Medicine, 8(4), 479. https://doi.org/10.3390/jcm8040479







