Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Identification and Intervention
2.2. Outcomes and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Adhikari, N.K.; Fowler, R.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef]
- Epstein, L. Varying Estimates of Sepsis Mortality Using Death Certificates and Administrative Codes — United States, 1999–2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; West, T.E.; Limmathurotsakul, D.; Peacock, S.J. Strategies to Reduce Mortality from Bacterial Sepsis in Adults in Developing Countries. PLOS Med. 2008, 5, e175. [Google Scholar] [CrossRef]
- Baelani, I.; Jochberger, S.; Laimer, T.; Otieno, D.; Kabutu, J.; Wilson, I.; Baker, T.; Dünser, M.W. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: A self-reported, continent-wide survey of anaesthesia providers. Crit. Care 2011, 15, R10. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Moskowitz, A.; Andersen, L.W.; Huang, D.T.; Berg, K.M.; Grossestreuer, A.V.; Marik, P.E.; Sherwin, R.L.; Hou, P.C.; Becker, L.B.; Cocchi, M.N.; et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: A review of the biologic rationale and the present state of clinical evaluation. Crit. Care 2018, 22, 283. [Google Scholar] [CrossRef]
- The Centers for Medicare & Medicaid Services, the Joint Commission. Severe sepsis and septic shock (SEP). In Specifications Manual for National Hospital Inpatient Quality Measures: Discharges 01-01-18 (1Q18) through 06-30-18 (2Q18); Version 5.3; The Joint Commission: Oakbrook Terrace, IL, USA, 2015; Available online: https://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx (accessed on 27 August 2018).
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.; Draper, E.; Wagner, D.P.; Zimmerman, J. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Kramer, A.A.; McNair, D.S.; Malila, F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit. Care Med. 2006, 34, 1297–1310. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensiv. Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Gilbert, D.N.; Chambers, H.F.; Eliopoulos, G.M.; Saag, M.S.; Pavia, A. (Eds.) Sanford Guide to Antimicrobial Therapy 2018, 48th ed.; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2018. [Google Scholar]
- Shin, T.G.; Kim, Y.J.; Ryoo, S.M.; Hwang, S.Y.; Jo, I.J.; Chung, S.P.; Choi, S.H.; Suh, G.J.; Kim, W.Y. Early vitamin C and thiamine administration to patients with septic shock in emergency departments: Propensity score-based analysis of a before-and-after cohort study. J. Clin. Med. 2019, 8, 102. [Google Scholar] [CrossRef]
- Amrein, K.; Straaten, H.M.O.-V.; Berger, M.M. Vitamin therapy in critically ill patients: Focus on thiamine, vitamin C, and vitamin D. Intensiv. Care Med. 2018, 44, 1940–1944. [Google Scholar] [CrossRef]
- Long, C.; Maull, K.; Krishnan, R.; Laws, H.; Geiger, J.; Borghesi, L.; Franks, W.; Lawson, T.; Sauberlich, H. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003, 109, 144–148. [Google Scholar] [CrossRef]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Syed, A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; Dewilde, C.; Farthing, C.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- De Grooth, H.J.; Manubulu-Choo, W.P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin C pharmacokinetics in critically ill patients: A randomized trial of four IV regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Donnino, M.W.; Andersen, L.W.; Chase, M.; Berg, K.M.; Tidswell, M.; Giberson, T.; Wolfe, R.; Moskowitz, A.; Smithline, H.; Ngo, L.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit. Care Med. 2016, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.L.; Vincent, J.-L.; Levy, M.M.; Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensiv. Care Med. 2004, 30, 536–555. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Rees, D.D.; Cellek, S.; Palmer, R.M.; Moncada, S. Dexamethasone prevents the induction by endotoxin or a nitric oxide synthase and the associated effects on vascular tone: An insight into endotoxin shock. Biochem. Biophys. Res. Commun. 1990, 173, 541–547. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L. Glucocorticoids and vascular reactivity. Curr. Vasc. Pharmcol. 2004, 2, 1–12. [Google Scholar] [CrossRef]
- De Kruif, M.D.; Lemaire, L.C.; Giebelen, I.A.; van Zoelen, M.A.; Pater, J.M.; van den Pangaart, P.S.; Groot, A.P.; de Vos, A.F.; Elliott, P.J.; Meijers, J.C.; et al. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. J. Immunol. 2007, 178, 1845–1851. [Google Scholar] [CrossRef]
- Siraux, V.; De Backer, D.; Yalavatti, G.; Melot, C.; Gervy, C.; Mockel, J.; Vincent, J.-L. Relative adrenal insufficiency in patients with septic shock: Comparison of low-dose and conventional corticotropin tests. Crit. Care Med. 2005, 33, 2479–2486. [Google Scholar] [CrossRef]
- Barabutis, N.; Khangoora, V.; Marik, P.E.; Catravas, J.D. Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest 2017, 152, 954–962. [Google Scholar] [CrossRef]
- Sidhu, H.; Gupta, S.K.; Thind, R.N. Oxalate metabolism in thiamine-deficient rats. Ann. Nutr. Metab. 1987, 31, 354–361. [Google Scholar] [CrossRef]
- Annane, D.; Sebille, V.; Charpentier, C.; Bollaert, P.E.; François, B.; Korach, J.M.; Capellier, G.; Cohen, Y.; Azoulay, E.; Troché, G.; et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002, 288, 862–871. [Google Scholar] [CrossRef]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef]
| Variables | Triple Therapy (n = 47) | Standard Care (n = 47) | p |
|---|---|---|---|
| Age, mean ± SD, year | 58.3 ± 17.0 | 60.1 ± 14.0 | 0.589 |
| Weight, mean ± SD, kg | 82.1 ± 32.6 | 80.7 ± 22.5 | 0.812 |
| Sex, male, No (%) | 28 (59.6) | 29 (61.7) | 0.833 |
| Comorbidities, No (%) | |||
| None/Unknown | 4 (8.5) | 3 (6.4) | 1.000 |
| Diabetes | 14 (29.8) | 10 (21.3) | 0.344 |
| Hypertension | 18 (38.3) | 21 (44.7) | 0.53 |
| CAD/MI | 7 (14.9) | 4 (8.5) | 0.336 |
| Heart failure | 5 (10.6) | 4 (8.5) | 0.503 |
| Malignancy | 8 (17.0) | 12 (25.5) | 0.313 |
| COPD | 2 (4.3) | 7 (14.9) | 0.158 |
| Cirrhosis | 5 (10.6) | 11 (23.4) | 0.100 |
| CVA | 5 (10.6) | 1 (2.1) | 0.203 |
| CKD | 4 (8.5) | 10 (21.3) | 0.082 |
| Immunocompromised | 3 (6.4) | 6 (12.8) | 0.486 |
| Drug addiction | 4 (8.5) | 5 (10.6) | 1.000 |
| Primary diagnosis No (%) | |||
| Pneumonia | 22 (46.8) | 18 (38.3) | 0.404 |
| Urosepsis | 4 (8.5) | 8 (17.0) | 0.216 |
| Primary bacteremia | 3 (6.4) | 3 (6.4) | 1.000 |
| GI/biliary | 10 (21.3) | 15 (31.9) | 0.243 |
| Other (meningitis, TSS, unknown, patient deceased before cultures, necrotizing fasciitis) | 7 (14.9) | 4 (8.5) | 0.336 |
| Unknown | 1 (2.1) | 0 (0.0) | 1.000 |
| Mechanical ventilation, No (%) | 43 (91.5) | 39 (83.0) | 0.216 |
| Vasopressors, No (%) | 47 (100) | 47 (100) | 1.000 |
| Acute kidney injury, No (%) | 38 (80.9) | 32 (68.1) | 0.156 |
| Positive blood cultures, No (%) | 16 (34.0) | 18 (38.3) | 0.668 |
| Lab values | |||
| WBC, mean ± SD, ×109 (excluding immunosuppressed patients) | 16.6 ± 13.0 | 16.1 ± 11.8 | 0.834 |
| Lactate, median (IQR), mmol/L | 2.7 (1.5–5.5) | 2.9 (1.5–4.2) | 0.708 |
| Creatinine, median (IQR), mg/dL (excluding CKD) | 1.4 (0.9–2.2) | 1.4 (0.9–2.5) | 0.988 |
| Procalcitonin, median (IQR), mcg/mL | 7.3 (0.7–52.1) | 4.3 (1.4–13.3) | 0.534 |
| Treatment timing and duration | |||
| Fluids within 3 h of culture, No (%) | 31 (66.0) | 36 (76.6) | 0.254 |
| Fluids at least 30 mL/kg (within 3 h), No (%) | 21 (67.7) | 23 (63.9) | 0.740 |
| Antibiotics within 3 h of culture, No (%) | 32 (68.1) | 26 (55.3) | 0.203 |
| Appropriate antibiotics (overall), No (%) | 37 (78.7) | 33 (70.2) | 0.344 |
| Number of vitamin C doses, mean ± SD | 12.3 ± 6.3 | 0 | – |
| Duration of vitamin C therapy, median (IQR), h | 90.0 (45.0–96.0) | 0 | – |
| Number of thiamine doses, mean ± SD | 6.6 ± 3.2 | 0 | – |
| Duration of thiamine therapy, median (IQR), h | 96.0 (48.0–96.0) | 0 | – |
| Receipt of hydrocortisone, No (%) | 47 (100) | 19 (40.4) | <0.05 |
| Daily dose of hydrocortisone, mean ± SD, mg | 176.7 ± 42.0 | 177.5 ± 42.5 | 0.941 |
| Duration of hydrocortisone therapy, median (IQR), h | 96.0 (48.0–156.0) | 104.5 (77.3–188.0) | 0.220 |
| Critical illness scores and predicted mortality | |||
| Day 1 SOFA, mean ± SD | 10.6 ± 10.6 | 9.7 ± 10.0 | 0.211 |
| APACHE II, mean ± SD | 21.5 ± 8.0 | 20.0 ± 7.4 | 0.739 |
| APACHE IV, mean ± SD | 88.6 ± 29.1 | 84.1 ± 25.4 | 0.455 |
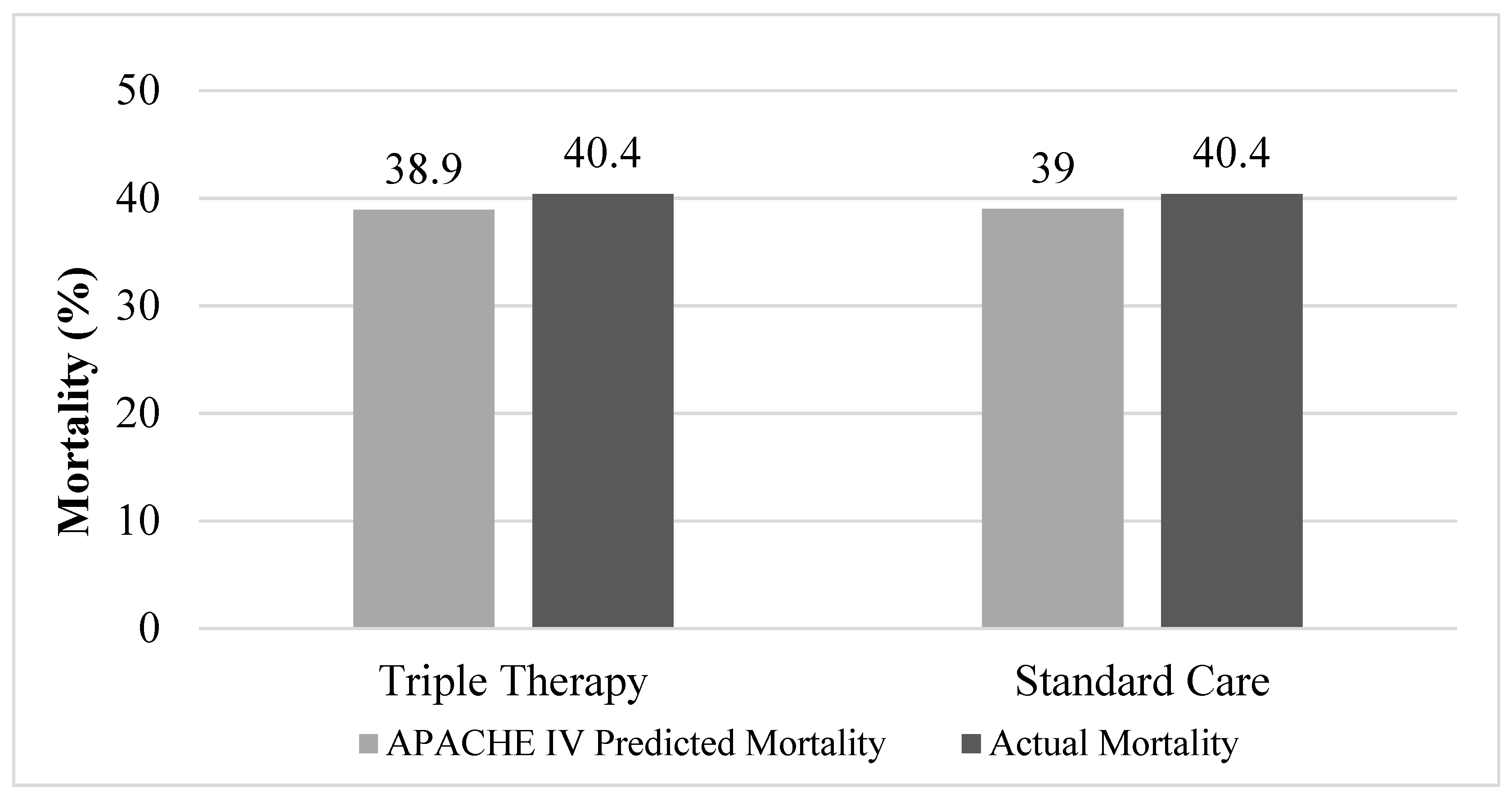
| APACHE IV Predicted mortality, mean ± SD | 38.9 ± 27.2 | 39.0 ± 23.6 | 0.991 |
| Variables | Triple Therapy (n = 47) | Standard Care (n = 47) | p |
|---|---|---|---|
| Hospital mortality, No. (%) | 19 (40.4) | 19 (40.4) | 1.000 |
| ICU mortality, No (%) | 17 (36.2) | 18 (38.3) | 0.831 |
| RRT for AKI, No. (%) | 11 of 38 (28.9) | 11 of 32 (34.4) | 0.626 |
| ICU LOS, median (IQR), days | 11.0 (7.0–19.0) | 10.0 (5.0–17.0) | 0.491 |
| Hospital LOS, median (IQR), days | 19.0 (9.0–26.0) | 14.0 (8.0–23.0) | 0.346 |
| Duration of vasopressors, median (IQR), h | 84.2 (37.0–169.3) | 62.5 (32.6–105.9) | 0.324 |
| ΔSOFA score in 72 h, mean ± SD | 1.3 ± 4.1 | 0.1 ± 4.7 | 0.390 |
| ΔPCT in 72 h, median (IQR), ng/mL | 0.1 (−55.0–9.1) | 2.5 (−3.2–4.4) | 0.268 |
| Variables | Triple Therapy (n = 20) | Standard Care (n = 47) | p |
|---|---|---|---|
| Hospital mortality, No. (%) | 7 (35.0) | 19 (40.4) | 0.677 |
| ICU mortality, No (%) | 6 (30.0) | 18 (38.3) | 0.517 |
| RRT for AKI, No. (%) | 5 of 16 (31.3) | 11 of 32 (34.4) | 0.829 |
| ICU LOS, median (IQR), days | 14.5 (7.5–22.0) | 10.0 (5.0–17.0) | 0.204 |
| Hospital LOS, median (IQR), days | 19.0 (9.3–23.5) | 14.0 (8.0–23.0) | 0.376 |
| Duration of vasopressors, median (IQR), h | 85.1 (42.8–176.2) | 62.5 (32.6–105.9) | 0.250 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litwak, J.J.; Cho, N.; Nguyen, H.B.; Moussavi, K.; Bushell, T. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. J. Clin. Med. 2019, 8, 478. https://doi.org/10.3390/jcm8040478
Litwak JJ, Cho N, Nguyen HB, Moussavi K, Bushell T. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. Journal of Clinical Medicine. 2019; 8(4):478. https://doi.org/10.3390/jcm8040478
Chicago/Turabian StyleLitwak, Jane J., Nam Cho, H. Bryant Nguyen, Kayvan Moussavi, and Thomas Bushell. 2019. "Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application" Journal of Clinical Medicine 8, no. 4: 478. https://doi.org/10.3390/jcm8040478
APA StyleLitwak, J. J., Cho, N., Nguyen, H. B., Moussavi, K., & Bushell, T. (2019). Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. Journal of Clinical Medicine, 8(4), 478. https://doi.org/10.3390/jcm8040478




