Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Measures
2.2.1. Attention-Deficit/Hyperactivity Disorder (ADHD) Symptom Change
2.2.2. Clinical Global Impression (CGI)
2.2.3. Remission
2.3. Analyses
3. Results
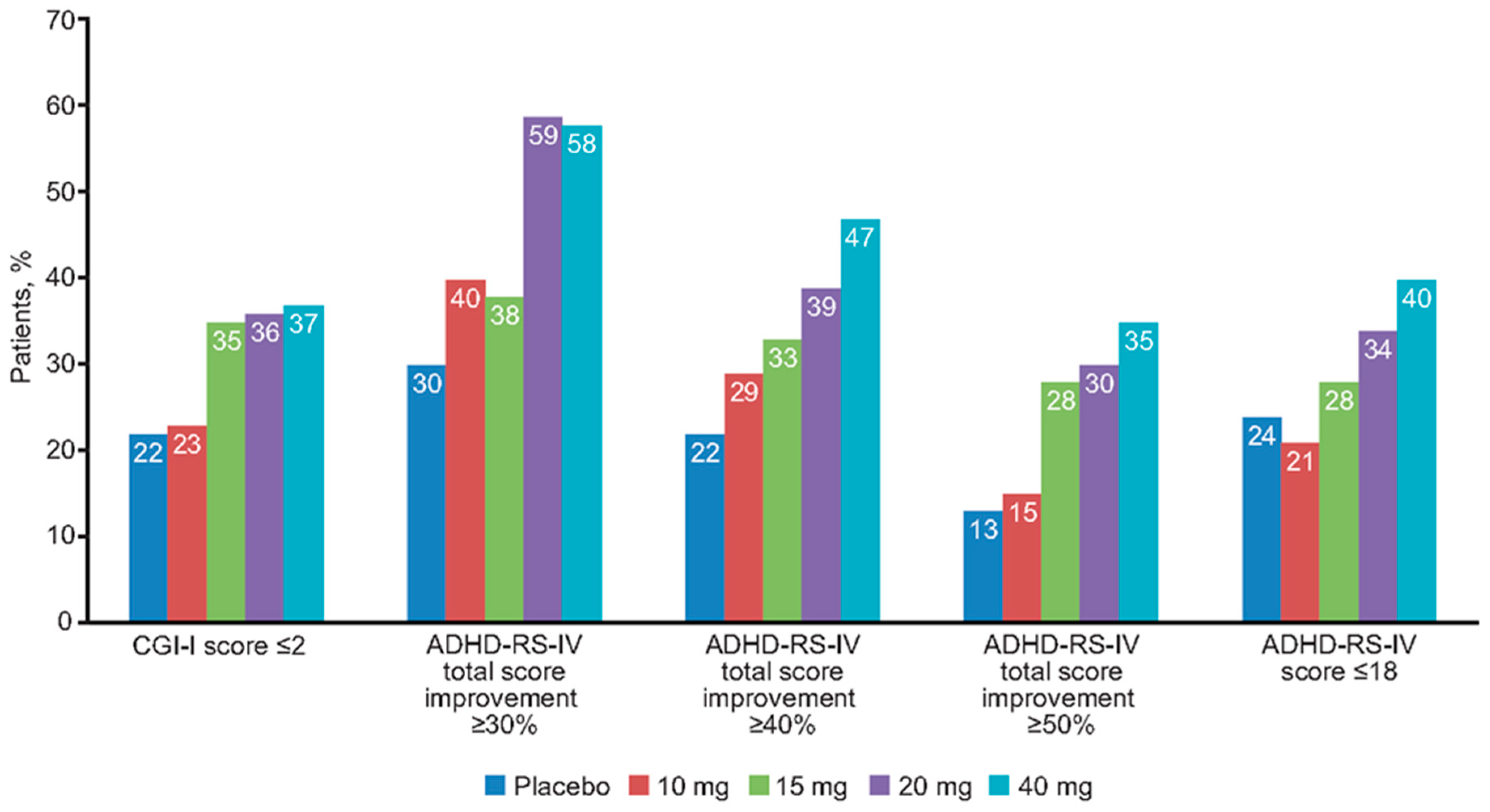
3.1. Response to Treatment
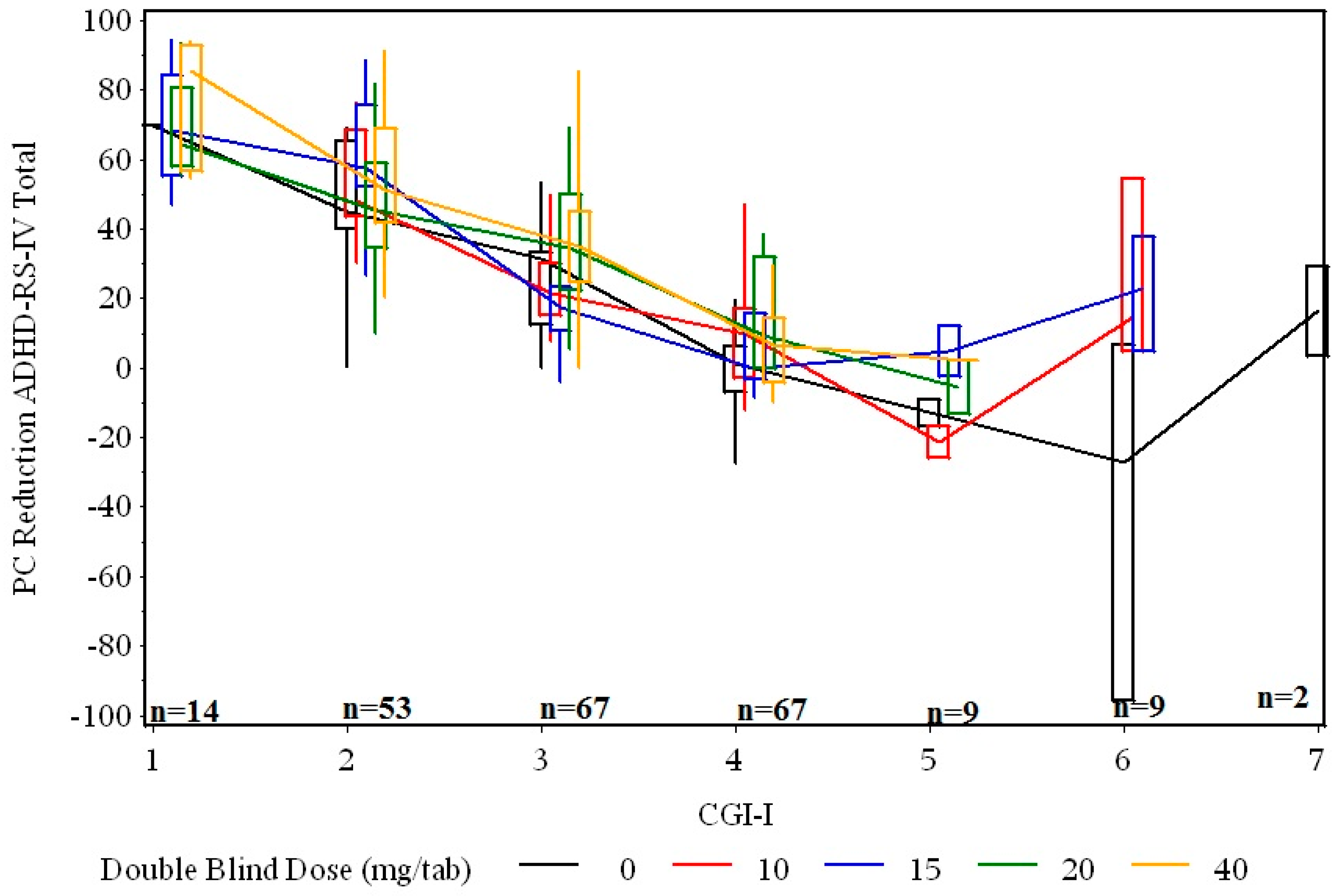
3.2. Relationship between Percent Change in Symptoms and Clinical Global Improvement at End of Double-Blind Treatment
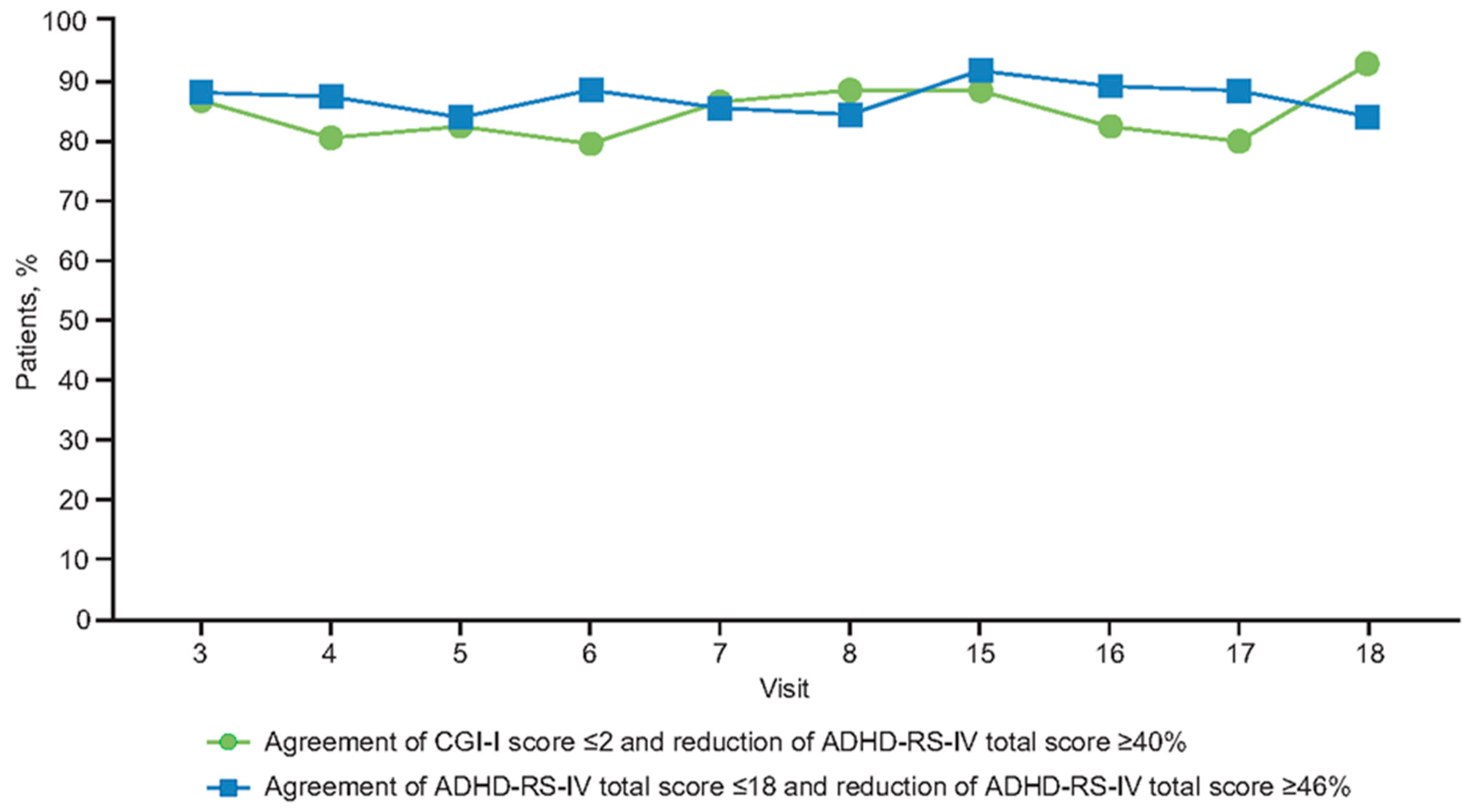
3.3. Relationship between Percent Change in Symptoms and Remission Outcome at End of Double-Blind Treatment
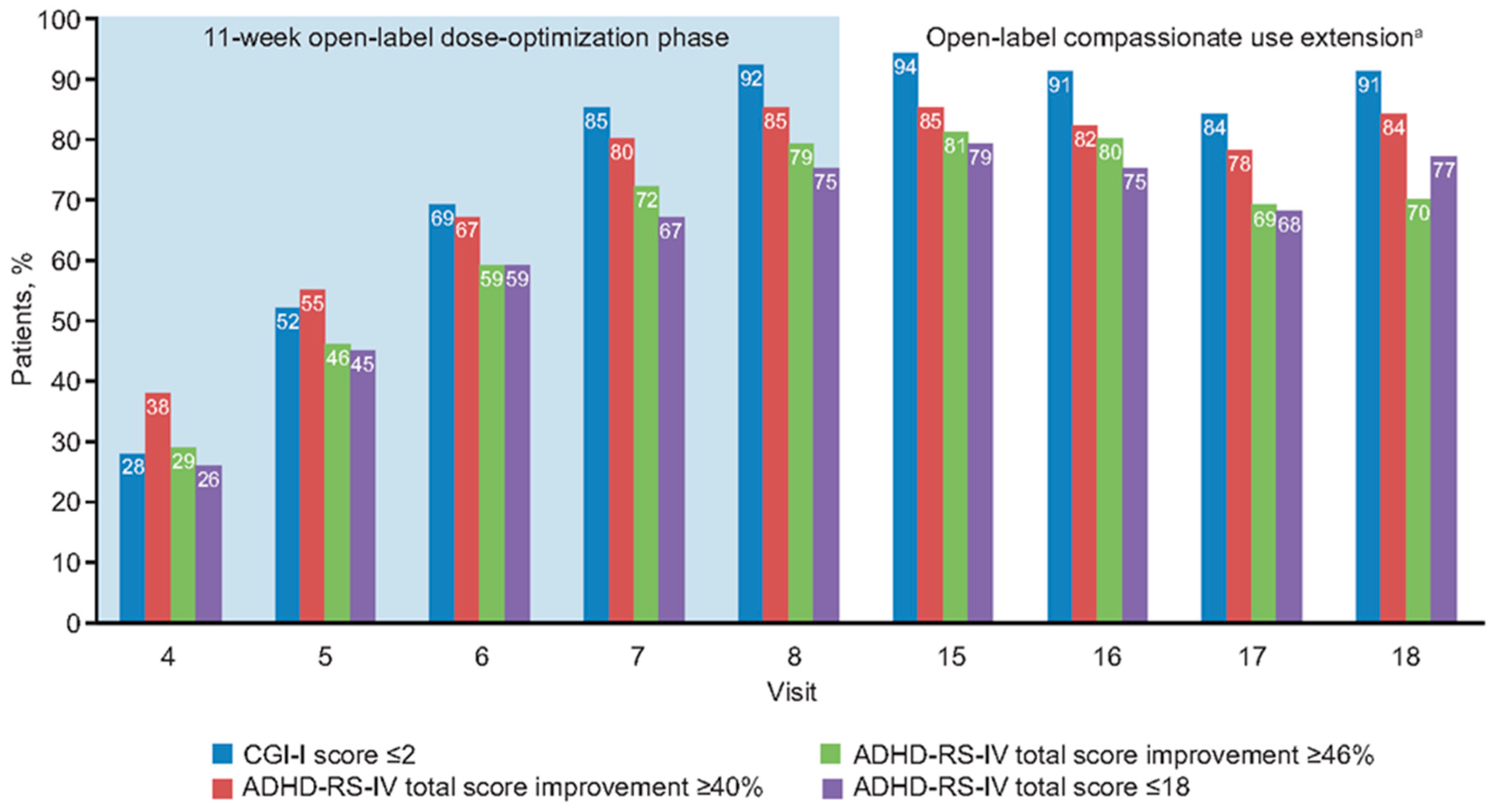
3.4. Relationships at End of Open-Label Dose Optimization Phase
3.5. Relationships During Open-Label Compassionate Use Extension
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 1999, 56, 1073–1086. [Google Scholar] [CrossRef]
- Swanson, J.M.; Kraemer, H.C.; Hinshaw, S.P.; Arnold, L.E.; Conners, C.K.; Abikoff, H.B.; Clevenger, W.; Davies, M.; Elliott, G.R.; Greenhill, L.L.; et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Mick, E.; Faraone, S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am. J. Psychiatry 2000, 157, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Wolraich, M.L.; Lambert, W.; Doffing, M.A.; Bickman, L.; Simmons, T.; Worley, K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr. Psychol. 2003, 28, 559–567. [Google Scholar] [CrossRef] [PubMed]
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale–IV: Checklist, Norms, and Clinical Interpretation; Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Steele, M.; Jensen, P.S.; Quinn, D.M. Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin. Ther. 2006, 28, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.A.; Maki, E.; Gibbins, C.; Gutkin, S.W.; Turgay, A.; Weiss, M.D. Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/hyperactivity disorder: Analysis of Canadian open-label studies. Child Adolesc. Psychiatry Ment. Health 2011, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, R.W.; Cardo, E.; Nagy, P.; Anderson, C.S.; Adeyi, B.; Caballero, B.; Hodgkins, P.; Civil, R.; Coghill, D.R. Treatment response and remission in a double-blind, randomized, head-to-head study of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs 2014, 28, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Hazell, P.L.; Kohn, M.R.; Dickson, R.; Walton, R.J.; Granger, R.E.; Wyk, G.W. Core ADHD symptom improvement with atomoxetine versus methylphenidate: A direct comparison meta-analysis. J. Atten. Disord. 2011, 15, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Newcorn, J.H.; Sutton, V.K.; Weiss, M.D.; Sumner, C.R. Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: The Integrated Data Exploratory Analysis (IDEA) study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Soutullo, C.; Banaschewski, T.; Lecendreux, M.; Johnson, M.; Zuddas, A.; Anderson, C.; Civil, R.; Higgins, N.; Bloomfield, R.; Squires, L.A.; et al. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs 2013, 27, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Wigal, S.B.; Nordbrock, E.; Adjei, A.; Childress, A.; Kupper, R.J.; Greenhill, L. Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR™) in children and adolescents with attention-deficit/hyperactivity disorder: A phase III, randomized, double-blind study. CNS Drugs 2015, 29, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Busner, J.; Targum, S.D. The Clinical Global Impressions Scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar] [PubMed]
- Steele, M.; Weiss, M.; Swanson, J.; Wang, J.; Prinzo, R.S.; Binder, C.E. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can. J. Clin. Pharmacol. 2006, 13, e50–e62. [Google Scholar] [PubMed]
- Cutler, A.J.; Brams, M.; Bukstein, O.; Mattingly, G.; McBurnett, K.; White, C.; Rubin, J. Response/remission with guanfacine extended-release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Findling, R.L.; Adeyi, B.; Chen, G.; Dirks, B.; Babcock, T.; Scheckner, B.; Lasser, R.; Pucci, M.; Abdullah, H.; McGough, J. Clinical response and symptomatic remission in children treated with lisdexamfetamine dimesylate for attention-deficit/hyperactivity disorder. CNS Spectr. 2010, 15, 559–568. [Google Scholar] [CrossRef]
- Mattingly, G.W.; Weisler, R.H.; Young, J.; Adeyi, B.; Dirks, B.; Babcock, T.; Lasser, R.; Scheckner, B.; Goodman, D.W. Clinical response and symptomatic remission in short- and long-term trials of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
| Characteristic, n (%) | MPH-MLR (n = 183) | Placebo (n = 47) |
|---|---|---|
| Age group, y | ||
| 6–8 | 49 (26.8) | 11 (23.4) |
| 9–11 | 56 (30.6) | 20 (42.6) |
| 12–14 | 55 (30.1) | 8 (17.0) |
| 15–18 | 23 (12.6) | 8 (17.0) |
| Female | 59 (32.2) | 17 (36.2) |
| White | 125 (68.3) | 33 (70.2) |
| ADHD diagnosis subtype | ||
| Combined | 111 (60.7) | 29 (61.7) |
| Predominantly inattentive | 62 (33.9) | 13 (27.7) |
| Predominantly hyperactive/impulsive | 5 (2.7) | 1 (2.1) |
| Not reported | 5 (2.7) | 4 (8.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, M.; Childress, A.; Nordbrock, E.; Adjei, A.L.; Kupper, R.J.; Mattingly, G. Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release. J. Clin. Med. 2019, 8, 461. https://doi.org/10.3390/jcm8040461
Weiss M, Childress A, Nordbrock E, Adjei AL, Kupper RJ, Mattingly G. Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release. Journal of Clinical Medicine. 2019; 8(4):461. https://doi.org/10.3390/jcm8040461
Chicago/Turabian StyleWeiss, Margaret, Ann Childress, Earl Nordbrock, Akwete L. Adjei, Robert J. Kupper, and Greg Mattingly. 2019. "Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release" Journal of Clinical Medicine 8, no. 4: 461. https://doi.org/10.3390/jcm8040461
APA StyleWeiss, M., Childress, A., Nordbrock, E., Adjei, A. L., Kupper, R. J., & Mattingly, G. (2019). Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release. Journal of Clinical Medicine, 8(4), 461. https://doi.org/10.3390/jcm8040461





